Abstract
Common carp (Cyprinus carpio) is one of the most consumed fish in the world and can be exposed to various forms of pollution, such as potential toxic elements (PTEs). Several studies have been conducted on the concentration of PTEs in common carp fish. The aims of the current study were to meta-analyze the concentration of PTEs in common carp fish and estimate human health risks in consumers. A search was conducted in international databases, including Scopus, PubMed, Science Direct, Web of Science, and Embase to retrieve papers up to January 20, 2024. The non-carcinogenic risk due to PTEs in fish fillets was calculated via the target hazard quotient (THQ), and the carcinogenic risk due to iAs in fish fillets was calculated via cancer risk (CR). The highest concentrations of Cu, methyl-Hg, and Ni were observed in the fillets of common carp fish. The non-carcinogenic risk was lower than 1 in all countries; hence, consuming common carp fish does not pose a non-carcinogenic risk. Adult consumers in Iraq were exposed to an unacceptable carcinogenic due to iAs in common carp fish. Hence, it is recommended that plans be conducted to reduce the concentration of PTEs in common carp fish in Iraq.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Various environmental pollutants, followed by the food chain, have increased consumer health concern [1,2,3,4,5]. These contaminants include potentially toxic elements (PTEs), mycotoxins [6] in doughs [7], maize [8], yogurt [9], and pathogens in milk [10,11,12]. PTEs contamination is a worldwide issue and following with other variables disrupting the ecosystem and leading to serious health risks for organisms [13,14,15,16]. In the World Health Organization classification, low concentrations of essential elements such as copper (Cu), cobalt (Co), manganese (Mn), selenium (Se), and zinc (Zn) are necessary for the natural metabolism of organisms [17,18,19,20]. For example, zinc is involved in more than 300 enzymatic and hormonal activities [21] and has catalytic, structural, and regulatory roles [22]. At the same time, potentially toxic elements (PTEs) such as cadmium (Cd), inorganic arsenic (iAs), lead (Pb), and mercury (Hg) are biologically non-essential elements. PTEs interfere with normal biological functions and exhibit toxicity even at low concentrations [22,23,24,25]. The occurrence of PTEs in aquatic ecosystems can originate from anthropogenic activities, including industry, mining, agriculture, and transportation, or from natural sources, such as erosion, atmospheric precipitation, and geological weathering [26,27,28]. The subsequent presence of environmental pollutants such as Pb, Cd, Hg, and As in the aquatic food chain has become inevitable [29]. Unlike environmental organic pollutants, heavy metals are not degraded by chemical or biological processes and accumulate in ecosystems [30].
Exposure to PTEs and other pollutants can have several adverse effects on human health, depending on the dose, duration of exposure, and the health status of the exposed people [31,32,33,34,35]. Human exposure to Pb can cause intestinal, nervous, hematological, and cardiovascular problems [36]. Cd can exhibit mutagenic, carcinogenic, and teratogenic effects, and it can cause kidney, liver, bone, and reproductive dysfunctions [37]. Hg can cause damage to the central nervous system (CNS), cardiovascular system, skin, lungs, liver, and kidney [38].
Fish at the top of the food web are bioindicators of metal contamination in aquatic ecosystems [39]. The amount of heavy metals uptake and accumulation in fish can be correlated with various physiological, ecological, physical, chemical, and biological conditions [40]. Abiotic and biotic factors, including acidity, temperature, hardness, size, life cycle, history, age, sex, habitat preferences, and feeding habits, can affect the accumulation of the metal in fish tissues [41]. Notably, the levels of PTEs accumulation in fish organs and skin are generally higher than in fish muscle; however, muscles are the most commonly consumed parts by humans [42]. The Food and Drug Administration, World Health Organization, and Environmental Protection Agency warn about exceeding levels of heavy metals in some aquatic organisms. However, they also recognize the importance of omega-3 fatty acids (n-3 FAs), essential metals like selenium, and fat-soluble vitamins found in fish [43,44,45].
The common carp (Cyprinus carpio) is one of the most common species of commercially farmed fish worldwide [46]. Studies have revealed that omnivorous fish species, such as common carp, may exhibit higher concentrations of heavy metals than carnivorous and benthivorous fish species [47, 48]. Majnoni et al. reported that high concentrations of PTEs (Hg et al.) in common carp from the Zarivar River (Iran) could be linked to wastewater discharge. This study suggests that the bioaccumulation and biomagnification of heavy metals in water may lead to increased levels of PTEs in aquatic organisms in the future [49].
The study by Pazooki et al. has shown acceptable Pb, Zn, and Cu levels in the muscle and skin of wild and cultured common carp from the Southeastern Caspian Sea of Iran [50]. In Hosseini Alhashemi’s study, the levels of Cd, Cr, and Cu in the muscle of common Carpio from freshwater wetlands in Iran were found to be higher than in the muscle of other studied fish species such as Barbusgrypus, Barbus luteus, Barbussharpeyi, Liza abu, and Siluriustrisostegus [51]. Heshmati et al. found that the concentration of Pd, Cd, Hg, and Mn was higher in the wild C. carpio fish muscles from the Caspian Sea than in farmed carp samples. However, the estimated daily intake of all examined PTEs was acceptable [52]. Similarly, in Aryaee’s study, the concentrations of Cd, Fe, Cu, Pb, Co, Ni, Zn, and Cr in fish species from the Zabol Chahnimeh Reservoirs of Iran were below levels of concern for human consumption [53]. Several investigations have been performed on the concentration of PTEs in common carp fish (Appendix 1). Therefore, the main aims of this study were to meta-analyze the concentration of PTEs in common carp fish based on the defined subgroups and to calculate the health risks (both non-carcinogenic and carcinogenic risks) for consumers.
Materials and Method
Search Strategy
We systematically searched according to preferred reporting items for systematic reviews and meta-analyses (PRISMA) protocol [54,55,56]. The two authors searched international databases, including Scopus, PubMed, Science Direct, Web of Science, and Embase, to find published papers up to January 20, 2024. The search syntaxes for finding papers were obtained using medical subject headings (MeSH) and published papers. Syntaxes included “Toxic elements,” “Heavy metals,” “Potential toxic elements,” “Potential hazard elements,” “Elements,” “trace metals,” AND “fish” OR “marine foods,” OR “carp fish” OR “common carp” OR “Cyprinus carpio.” The titles and abstracts of retrieved papers were screened, and duplicate papers were excluded [56]. Subsequently, the full text of the papers was downloaded, and after reading the complete text, the required data was extracted. Disagreements between the authors in the selection or exclusion of papers were resolved by the corresponding author, who made the final decision. Duplicate papers were removed and screened using EndNote software version 8.0.
Eligibility Criteria
Our inclusion criteria comprised studies that detected PTEs in common carp, with full-text available in English, employing valid methods of detection and presenting statistical data on PTE levels (such as mean, standard deviation, and/or range). Review papers, letters to editors, thesis, books, conference proceedings, book chapters, and experimental or intervention studies were excluded. The country of study, sample size, statistical information on PTE concentrations (mean, standard deviation), and the method of detection were extracted.
Meta-Analysis of Data
A meta-analysis of PTE levels in common carp fish fillets was conducted using the mean (μg/kg-ww) and standard error (SE). We employed I2 index and chi-square statistics to assess heterogeneity [57, 58]. If the I2 index statistic exceeded 50%, indicating substantial heterogeneity, the random effects model (REM) was employed to calculate a pooled effect size. The meta-analysis of concentration in fillets of common carp fish was conducted using the Stata software (Version 17.0 College Station, TX, USA).
Health Risk Assessment
The daily intake of consumers was calculated by Eq. 1[15, 59,60,61]:
In this equation, CDI is chronic daily intake (mg/kg-day); C, levels of PTEs in fillets of fish (μg/kg-ww); IR, ingestion rate (g/day); ED, exposure duration (year); EF, exposure frequency (350 day/year); AT, mean lifetime (day) and BW, body weight for children and adults is 15 and 70 kg, respectively [62]. ED for children and adults equals 6 and 70 years, respectively. AT for non-cancer risk is 2190 days and 25,550 days for children and adults, respectively, and AT for cancer risk equals 25,550 days for both children and adults. The ingestion rate of common carp fish is shown in Appendix 2.
The non-cancer risk was estimated using the below equation [63]:
In this equation, RfD and TDI are oral reference doses and tolerable daily intake [18]. RfD for Cd, Ni, Cu, and iAs and methyl-Hg equals 0.001, 0.011, 0.04, 0.0003, and 0.0001 mg/kg-d, respectively [64, 65]. TDI for Pb is 0.0036 mg/kg-d [64, 65]. When the target hazard quotient (THQ) ≤ 1, the non-cancer risk is acceptable[63].
The cancer risk of iAs in carp fish was estimated by the below equation [66, 67]:
where CSF is the cancer slope factor, CSF for iAs is 1.5 (mg/kg-d)−1 [65]. CR is classified as ignorable carcinogenic risk: CR < 1.00E − 06; acceptable carcinogenic risk: 1.00E − 06 ≤ CR < 1.00E − 04; and unacceptable carcinogenic risk: CR ≥ 1.00E − 04 [68].
Results and Discussion
Method of Detection
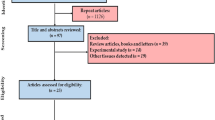
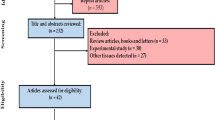
Sixty-eight papers with 148 data reports were included in our study (Fig. 1). Monitoring the amount of PTEs in fish sample matrices is one of the most important research topics in health hazards [69]. Different research laboratories and regulatory agencies employ several analytical techniques for routinely observing, evaluating, and quantifying PTEs from water, air, soil, animal, plant, and food samples. Information about the application range of different analytical techniques may be essential for selecting the appropriate method, which, in turn, guides the sampling and sample preparation processes [70]. In the current study, the rank order of method of detection based on percentage use in detection was AAS (54%) > ICP-OES; ICP-AES, and ICP (25%) > ICP-MS (20%) > XRF (1%) (Fig. 2). Flame or graphite furnace AAS, ICP-OES, ICP-AES, and ICP are the common spectral techniques utilized in the estimation of PTEs in investigated studies. On the other hand, a specific technique like XRF can effectively identify trace levels of metals. It was shown that metals were measured using XRF in only one study [71] (Appendix 1). ICP is commonly used for multi-element analysis at low detection limits, whereas AAS is preferred for analyzing specific elements at higher concentrations [72]. AAS is a widely used and quantitative technique for metal analysis that can identify approximately 70 metals [73]. It was found that more than 50% of articles utilized AAS as the detection method for various PTEs. Numerous nebulizers, such as a graphite furnace and flame, are applied in hydride and mercury cold vapor AAS techniques to identify PTEs [74]. Flame AAS is a suitable technique that can measure heavy metals at concentrations of part per million (ppm) levels with appropriate accuracy [75]. It is a fast and relatively inexpensive procedure, completing the entire analysis within 10–15 s per sample with high accuracy (repeatability) and negligible interferences [76].
Flame AAS is practical for finding PTEs from several sample matrices. Unfortunately, this technique suffers from poor sensitivity compared with graphite furnace AAS or ICP-MS. Hence, flame AAS is inappropriate for identifying arsenic (As) because of the insufficient maximum temperatures required for atomization [73, 76]. In contrast, as an atomization procedure, graphite furnace AAS can distinguish metals in both aqueous and solid samples with great precision at parts per billion (ppb) levels [77]. Graphite furnace AAS is comparable to flame AAS except for the atomization process. This technique includes warming at high temperatures in order to volatilize and atomize the sample [77]. The advantages of graphite furnace AAS include the requirement for smaller samples (20 μ) for analysis than flame AAS and lower detection limits. However, there are drawbacks to graphite furnace AAS, including the expense of the furnace, lower sample throughput, more troublesome operation, low precision, limited range of working, slow analytical processes, and matrix interferences [78]. Following AAS (54%), the following most commonly used techniques were ICP-OES; ICP-AES and ICP collectively accounted for 25% of identifications of heavy metals in common carp (Cyprinus carpio) fish in the literature review (Appendix 1).
ICP is the most sensitive and widely applied analytical method for detecting trace metals in various sample types. In the appendix, it was reported in only 1% of studies for heavy metal measurement.
ICP is the most sensitive and widely applied analytical method for detecting trace metals in various samples [73]. It was reported in 1% of studies for heavy metal measurement (Appendix 1). ICP operates using a plasma where energy transfer to generate and preserve the ionized gas is carried out via electromagnetic induction [79]. Both ICP-OES and ICP-AES are similar analytical techniques used for heavy metal estimation. Approximately 24% of articles examined in the current study demonstrated how heavy metals can be measured using these techniques (Appendix 1).
Since its commercial introduction in the mid-1970s, ICP-OES has rapidly become broadly utilized and acknowledged for numerous applications of metal determination in a wide assortment of samples [80]. Whereas AAS measures the quantity of light absorbed at a specific wavelength as elemental atoms enter an excited state, ICP-OES measures the light elements emit in a sample as they enter an ICP source [77]. Both techniques can detect trace metal concentrations in complex matrices with excellent precision and accuracy. However, ICP-OES offers several advantages over AAS. It can evaluate concentrations of multiple elements in a single sample with a single aspiration. Therefore, this situation leads to significant speed over AAS when the goal is to quantify several elements in a sample[74]. Additionally, ICP-OES has a much broader analytical working range and operates without recalibration. It can measure samples varying in concentration from 1 μg/L to 1 g/L without requiring recalibration, whereas AAS spans only three orders of magnitude, from 1 μg/L to 1 mg/L [77]. ICP-OES is particularly well-suited for detecting trace heavy metals, especially when all elements are consistently present at concentrations above ten ppb.
However, for lower concentrations of metals, especially heavy elements like arsenic, ICP-MS is recommended due to its superior sensitivity based on the literature review, it was shown that ICP-Ms is reported for measuring heavy metals in 20% of articles [81]. In contrast, XRF was mentioned in only 1% of articles (Appendix 1). XRF is a fast, affordable, and non-destructive analytical method for detecting a variety of hazardous materials, capable of simultaneously identifying up to 30 elements [71] through the interaction of X-ray radiation with atoms [82].
The ICP-OES technique offers superior sensitivity, broader elemental coverage, and lower detection limits than the XRF method. This condition makes ICP-OES an ideal technique for trace-level analysis of trace heavy metals. On the other hand, XRF is a versatile technique suited for qualitative and semi-quantitative analysis, especially when analyzing bulk samples with minimal preparation [83]. Overall, selecting a technique should consider factors such as detection limit, accuracy, sensitivity, expected concentration levels of heavy metals, the number of elements, the frequency of sample observation, and the presence of interfering components in sample matrices.
Concentration of PTEs in Fish
Fish is an exceptional source of high-quality protein, micronutrients, vitamins, and n-3 fatty acids, in specific unique fatty acids such as eicosapentaenoic (EPA 20:5) and docosahexaenoic (DHA 22:6) acid. The scientific report shows the association of fish consumption with various health benefits [84]. However, fish can be a source of potentially toxic elements if exposed to contamination in water. The results indicate a significant disparity in heavy metal in common carp (Cyprinus carpio) fish and sampling areas.
The bioaccumulation of toxic trace elements in fish depends on non-biotic criteria such as water pH and the chemical form of the element. In contrast, some intrinsic factors of the fish, such as age and physiologic conditions, are vital in accumulating PTEs in the body of fish [85]. Scientific reports on the levels of heavy metals in fish tissues are vital for human consumption. Significant differences have been observed worldwide in the levels of PTEs in fish, which may be attributed to this fish species’ metabolism and feeding patterns [86].
The rank order of PTEs in the fillet of common carp (Cyprinus carpio) based on pooled concentration was Cu (0.4550 mg/kg-ww) > MeHg (0.2000 mg/kg-ww) > Ni (0.1540 mg/kg-ww) > iAs (0.0260 mg/kg-ww) > Pb (0.0036 mg/kg-ww) > Cd (0.0030 mg/kg-ww) (Tables 1, 2, 3, 4, 5, and 6). Statistical comparisons revealed that metals in varying quantities could be attributed to the examined tissues (gill, gut, liver, muscle, kidney, skin) in common carp (Cyprinus carpio). This suggests the physiological potential of different organs in the accumulating heavy metals [87]. In aquatic environments, the health status of fish and the different organs of this animal serve as indicators of water pollution and quality [88]. The consumption of fish muscle is considered an important part of the routine metal contamination [89]. Different amounts of heavy metals are mentioned in fish tissue, which might result from their capacity to induce metal-binding proteins such as metallothioneins [90, 91].
The gill plays a main role in fish exposure at the interface and direct contact with the marine environment. This organ regulates metal ions and nitrogenous waste excretion [92]. Heavy metals have been detected in fish lungs, likely due to their thin epithelium and susceptibility to metal penetration [86]. In some reports, the liver was mentioned as the tissue with the highest concentration of heavy metals. The liver is an active central site for uptaking and storing the metals and their detoxification excretion [93, 94]. The high concentration of heavy metals mentioned in the liver of carp in a study in Serbia was attributed to the high level in the gut and transferred to the liver [90].
The concentration of elements in the gut is valuable because this part can indicate the levels of elements in sediment and natural food sources. The analysis of the gut of fish provides evidence of an accessible pool of elements and the potential for transfer through the gastrointestinal tissue and accumulation in different tissues [90]. The three countries with the highest concentration of iAs observed were Iraq (3.3420 mg/kg-ww), Cyprus (0.4130 mg/kg-ww), and Pakistan (0.2930 mg/kg-ww) (Table 1); Cd, in India and Pakistan (11.600 mg/kg-ww), the Philippines (1.670 mg/kg-ww), and Pakistan (1.131 mg/kg-ww) (Table 2); Pb, in Iraq (0.770 mg/kg-ww), Turkey (0.754 mg/kg-ww), and Cyprus (0.362 mg/kg-ww) (Table 3); Ni, in the Philippines (2.170 mg/kg-ww), Iraq (1.615 mg/kg-ww), and Cyprus (1.470 mg/kg-ww) (Table 4); MeHg, in Turkey (3.074 mg/kg-ww), Pakistan (0.455 mg/kg-ww), and Iraq (0.321 mg/kg-ww) (Table 5); and Cu, in Iraq (3.985 mg/kg-ww), Vietnam (3.499 mg/kg-ww), and Spain (1.517 mg/kg-ww) (Table 6). Many influential factors contribute to differences in heavy metal levels in fish, including the bioavailable metal concentration in the abiotic components of their surroundings, their feeding habits, ecological requirements, metabolism, age, and size of the fish in different countries [86, 95]. Each of these criteria can significantly impact the metals present in fish.
Health Risk Assessment
Non-Carcinogenic Risk
Iraq, Cyprus, and South Korea were the three countries with the highest THQ of iAs (Fig. 3). However, excluding children in Iraq (THQ = 1.5E + 00), THQ for both adults and children in all countries was less than 1. Hence, the non-carcinogenic risk due to iAs is acceptable. For Cd, the countries with the highest THQ were the Philippines, Pakistan, and Iraq. Except for children in the Philippines (THQ = 1.0E + 00), THQ for both adults and children in all countries was less than 1. Hence, the non-carcinogenic risk due to Cd is acceptable (Fig. 4). Cyprus, Vietnam, and the Philippines are the three countries with the highest THQ of Pb. THQ for both adults and children in all countries was less than 1 value. Hence, the non-carcinogenic risk due to Pb is acceptable (Fig. 5). Turkey, South Korea, and China were the three countries with the highest THQ of MeHg. Except turkey (adult = 2.2E + 00 and children = 1.06E + 01) and South Korea (adult = 1.32E + 00 and children = 6.18E + 00). THQ for adults and children in all countries was less than 1; hence, the non-carcinogenic risk due to MeHg is acceptable (Fig. 6). The highest THQ for Ni was found in the Philippines, Cyprus, and Iraq. However, the THQ for both adults and children in all countries was less than 1 value; hence, the non-carcinogenic risk due to Ni is acceptable (Fig. 7). Vietnam, Cyprus and Iraq were the three countries with the highest THQ of Cu. THQ for both adults and children in tall countries was less than 1 value. Hence, the non-carcinogenic risk due to Cu is acceptable (Fig. 8). In a study conducted by Bagheri et al. in Iran on edible fishes of Gorgan Bay, Capsian Sea, the results of THQ value were less than one [89]. Similarly, in a study conducted on the farmed common carp (CYPRINUS CARPIO) in Poland, the THQ index for Cd, As, Pb, Cr, Ni, and Cu were less than 1, indicating the intake of a single metal does not pose health risk [96]. This situation demonstrated for provisional intakes and THQ estimated for a common carp. This means that the consumption of any of the fish above did not appear to be potentially hazardous for the health of Tunisian consumers as they were far below threshold values [97]. Furthermore, in different studies in China, THQ of heavy metals was lower than 1 in examined common carp fish [98, 99]. Totally, based on the results of the current meta-analysis, THQ was less than 1 for all examined heavy metals (iAs, Cd, Pb, MeHg, Cu, and Ni), revealing no serious non-carcinogenic risk for each heavy metal, and it does not have a side effect on consumers. However, continuing exposure to more than one pollutant can synergistically affect consumers.
Carcinogenic Risk
Iraq, Cyprus, and South Korea were the countries with the highest CR of iAs. Except for adult consumers in Iraq, the cancer risk in the other countries was ignorable and/or acceptable due to iAs (Fig. 9). It has been reported that both forms of As (organic and inorganic: Total As) are present in fish [100]. The latter form (iAs) is the most toxic to humans. Based on the current examination, CR for adults and children due to consumption of common carp was not considerable.
Conclusion
The highest concentrations of Cu, methyl-Hg, and Ni were observed in the fillet of common carp fish. Therefore, effective monitoring of sources emitting these elements should receive more attention. Additionally, the concentration of PTEs in the fillet of common carp fish was higher in Iraq, India, Pakistan, the Philippines, and Turkey than in other countries. However, the non-carcinogenic risk was less than 1 value for both adult and child consumers in all countries; hence, the consumption of common carp fish cannot have a non-carcinogenic risk for consumers. Adult consumers in Iraq were exposed to an unacceptable carcinogenic due to iAs in common carp fish. Therefore, it is recommended that plans be implemented to reduce the concentration of PTEs in common carp fish in Iraq. Therefore, the control of iAs in water and food resources of common carp should be given more attention.
Data Availability
No datasets were generated or analysed during the current study.
References
Chen X et al (2023) Near-infrared spectroscopy of Chinese soy sauce for quality evaluation. Qual Assur Saf Crops Foods 15(1):139–151
Wang L et al (2024) Quality assessment of black ginseng materials utilizing chemometrics and modeling inflammation in Zebrafish. Qual Assur Saf Crops Foods 16(1):23–37
Xu M et al (2024) Ultra-high pressure improved gelation and digestive properties of Tai Lake whitebait myofibrillar protein. Food Chemistry: X 21:101061
Lan T et al (2022) Floods and diarrheal morbidity: evidence on the relationship, effect modifiers, and attributable risk from Sichuan Province, China. J Global Health 12:1107
Zhu J et al (2024) p-Phenylenediamine derivatives in tap water: implications for human exposure. Water 16(8):1128
Zamanpour S et al (2023) A systematic review to introduce the most effective postbiotics derived from probiotics for aflatoxin detoxification in vitro. Ital J Food Saf. 35(4):31–49
Basso ABG et al (2023) Individual and combined decontamination effect of fermentation and ultrasound on aflatoxin B1 in wheat-based doughs: a preliminary study. Qual Assur Saf Crops Foods 15(3):96–103
Thakaew R, Chaiklangmuang S (2023) Aflatoxin B1 elimination in low-grade maize by co-influence of heat and chemical treatment. Qual Assur Saf Crops Foods 15(3):55–67
Pires RC et al (2022) Evaluation of anti-aflatoxin M1 effects of heat-killed cells of Saccharomyces cerevisiae in Brazilian commercial yogurts. Qual Assur Saf Crops Foods 14(1):75–81
Pieralisi S et al (2023) Effectiveness of Bdellovibrio bacteriovorus to contain Escherichia coli on milk and temperature impact on predation dynamics. Ital J Food Saf 35(2):80–87
Toplu MS, Tuncer BÖ (2023) Evaluation of the functional properties and safety of enterocin-producing Enterococcus faecium BT29. 11 isolated from Turkish Beyaz cheese and its inhibitory activity against Listeria monocytogenes in UHT whole milk: English. Ital J Food Saf 35(2):54–70
Liang A et al (2024) Dynamic simulation and experimental studies of molecularly imprinted label-free sensor for determination of milk quality marker. Food Chem 449:139238
Munir N et al (2021) Heavy metal contamination of natural foods is a serious health issue: a review. Sustainability 14(1):161
Nie G et al (2023) Accumulation characteristics and evaluation of heavy metals in soils and vegetables of plastic-covered sheds in typical red soil areas of China. Qual Assur Saf Crops Foods 15(3):22–35
Luo C et al (2022) Comparison of the health risks associated with exposure to toxic metals and metalloids following consumption of freshwater catches in China. Qual Assur Saf Crops Foods 14(4):1–12
Bahardoust M et al (2023) Effect of ABO blood group on postoperative overall survival and recurrence-free survival rate in patients with hepatocellular carcinoma after hepatectomy: a multi-center retrospective cohort study. BMC Surg 23(1):324
Nutrition WECoTEiH (1973) Trace elements in human nutrition: report of a WHO expert committee. WHO
Cai S, Zeng B, Li C (2023) Potential health risk assessment of metals in the muscle of seven wild fish species from the Wujiangdu reservoir, China. Qual Assur Saf Crops Foods 15(1):73–83
Bai B et al (2020) Temperature-driven migration of heavy metal Pb2+ along with moisture movement in unsaturated soils. Int J Heat Mass Trans 153:119573
Bai B et al (2022) The remediation efficiency of heavy metal pollutants in water by industrial red mud particle waste. Environ Technol Innov 28:102944
Chasapis CT et al (2020) Recent aspects of the effects of zinc on human health. Arch Toxicol 94:1443–1460
Bhattacharya PT, Misra SR, Hussain M (2016) Nutritional aspects of essential trace elements in oral health and disease: an extensive review. Scientifica 2016:5464373
Bolan S et al (2022) Differential toxicity of potentially toxic elements to human gut microbes. Chemosphere 303:134958
Talema A (2023) Causes, negative effects, and preventive methods of water pollution in Ethiopia. Qual Assur Saf Crops Foods 15(2):29–139
Bai B et al (2024) Corrosion effect of acid/alkali on cementitious red mud-fly ash materials containing heavy metal residues. Environ Technol Innov 33:103485
Ghajarbeygi P et al (2022) The concentration of radioisotopes (Potassium-40, Polonium-210, Radium-226, and Thorium-230) in fillet tissue carp fishes: a systematic review and probabilistic exposure assessment. Int J Environ Health Research 1-22
Fakhri Y et al (2023) The concentration of radionuclides (Lead-210, Polonium-210, and Cesium-137) in the muscle of sardine fish: a global systematic review, meta-analysis, and exposure assessment. Biol Trace Element Res 201(4):2011–2021
Hui X et al (2023) Steroid hormones in surface water resources in China: systematic review and meta-analysis and probabilistic ecological risk assessment. Int J Environ Health Res 1-17
Ghaffari HR et al (2022) Corrigendum to “The concentration of potentially hazardous elements (PHEs) in drinking water and non-carcinogenic risk assessment: A case study in Bandar Abbas, Iran” [Environ. Res. 201 (2021) 111567]. Environ Res 207:112185
Dhaliwal SS et al (2020) Remediation techniques for removal of heavy metals from the soil contaminated through different sources: a review. Environ Sci Pollu Res 27:1319–1333
Aprile A, De Bellis L (2020) Editorial for special issue “Heavy metals accumulation, toxicity, and detoxification in plants”. MDPI. 4103
Ding B et al (2024) Water security assessment for effective water resource management based on multi-temporal blue and green water footprints. J Hydrol 632:130761
Guo B et al (2024) Noble metal phosphides supported on CoNi metaphosphate for efficient overall water splitting. ACS Appl Mater Interfaces 16:8939–8948
Jing et al (2023) Operation analysis and its performance optimizations of the spray dispersion desulfurization tower for the industrial coal-fired boiler. Case Studies in Thermal Engineering 49:103210. https://doi.org/10.1016/j.csite.2023.103210
Zhou et al (2024) Detoxification of phoxim by a gut bacterium of Delia antiqua. Sci Total Environ 943:173866. https://doi.org/10.1016/j.scitotenv.2024.173866
Fenga C et al (2017) Immunological effects of occupational exposure to lead. Mol Me Rep 15(5):3355–3360
Yu D et al (2020) Association of liver and kidney functions with Klotho gene methylation in a population environment exposed to cadmium in China. Int J Environ Health Res 30(1):38–48
del Rosal JJL et al (2021) Mercury deposits as incidental CT findings on a coronary calcium score. Arch Cardiol Mex (Eng) 91(2):208–209
Wang S et al (2022) Trends and health risk of trace metals in fishes in Liaodong Bay, China, from 2015 to 2020. Front Mar Sci 2022(8):789572
Ali H, Khan E, Ilahi I (2019) Environmental chemistry and ecotoxicology of hazardous heavy metals: environmental persistence, toxicity, and bioaccumulation. J Chem
Zaynab M et al (2022) Health and environmental effects of heavy metals. J King Saud Univ-Sci 34(1):101653
Mehar S et al (2023) Bioaccumulation of heavy metals in the different tissues of Mackerel scad, Decapterus macarellus (Cuvier, 1833) collected from Karachi and Gwadar Coasts of Pakistan. Saudi J Biol Sci 30(2):103540
Velichka J et al (2023) Elements and omega-3 fatty acids in fishes along a large, dammed river. Environ Pollut 2023:122375
Hosseini S et al (2021) Effect of Zataria multiflora Boiss. essential oil, NaCl, acid, time, and temperature on the growth of Listeria monocytogenes strains in broth and minced rainbow trout. Food Sci Nutr 9(4):2290–2298
Byrd KA, Thilsted SH, Fiorella KJ (2021) Fish nutrient composition: a review of global data from poorly assessed inland and marine species. Public Health Nutr 24(3):476–486
Hanachi P et al (2019) Abundance and properties of microplastics found in commercial fish meal and cultured common carp (Cyprinus carpio). Environ Sci Pollut Res 2019(26):23777–23787
Štrbac S et al (2015) Bioaccumulation of heavy metals and microelements in silver bream (Brama brama L.), northern pike (Esox lucius L.) sterlet (Acipenser ruthenus L.), and common carp (Cyprinus carpio L.) from Tisza River, Serbia. J Toxicol Environ Health Part A 78(11):663–665
Štrbac S et al (2014) Metals in the sediment and liver of four fish species from different trophic levels in Tisza River, Serbia. Chem Ecol 30(2):169–186
Majnoni F et al (2013) Metal concentrations in tissues of common carp, Cyprinus carpio, and silver carp, Hypophthalmichthys molitrix from the Zarivar Wetland in Western Iran. Fish Aquatic Life 21(1):11–18
Pazooki J, Ghaffar HF, and Abtahi B (2011) A comparison of heavy metal concentrations in skin and muscle tissues of wild and cultured carp (Cyprinus carpio) in the southeastern Caspian Sea area of Iran
Alhashemi AH et al (2012) Bioaccumulation of trace elements in water, sediment, and six fish species from a freshwater wetland, Iran. Microchem J 104:1–6
Heshmati A et al (2017) Dietary exposure to toxic and essential trace elements by consumption of wild and farmed carp (Cyprinus carpio) and Caspian kutum (Rutilus frisii kutum) in Iran. Chemosphere 173:207–215
Ariyaee M et al (2015) Comparison of metal concentrations in the organs of two fish species from the Zabol Chahnimeh Reservoirs, Iran. Bull Environ Contam Toxicol 94:715–721
Moher D et al (2015) Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 4(1):1–9
Li X et al (2023) Role of arbuscular mycorrhizal fungi in cadmium tolerance in rice (Oryza sativa L): a meta-analysis. Qual Assur Saf Crops Foods 15(2):59–70
Tian Z et al (2023) Efficacy and safety of Shaoyao Gancao Tang for restless leg syndrome: a systematic review and meta-analysis. Qual Assur Saf Crops Foods 15(1):169–181
Gao P et al (2023) Effects of vitamins A, C, and D and zinc on urinary tract infections: a systematic review and meta-analysis. Qual Assur Saf Crops Foods 15(3):88–95
Aranega JP, Oliveira CA (2022) Occurrence of mycotoxins in pastures: a systematic review. Qual Assur Saf Crops Foods. 14(3):135–144
Qin Y et al (2023) Relative bioavailability of selenium in rice using a rat model and its application to human health risk assessment. Environ Pollut 338:122675
Özlü H (2024) Occurrence, dietary exposure and risk assessment to aflatoxins in red pepper flakes from Southeast of Türkiye. Qual Assur Saf Crops Foods 16(1):69–77
Zhang X et al (2024) Dissipation kinetics, residue level, and risk assessment of chlorantraniliprole in Rosa roxburghii and its residue removal using household decontamination technique. Qual Assur Saf Crops Foods 16(1):108–120
EPA, (2011) Exposure Factors Handbook . file:///C:/Users/Markazi__PC/Desktop/EFH-CHAPTER08.PDF. 15
Xiong J et al (2022) Occurrence of aflatoxin M1 in three types of milk from Xinjiang, China, and the risk of exposure for milk consumers in different age-sex groups. Foods 11(23):3922
USEPA (2005) U.S. Environmental Protection Agency. Human Health Risk Assessment. https://www.epa.gov/risk/human-health-risk-assessment. USA. 15–19
EPA (2016) USEPA, 2016. Integrated risk information system. https://www.epa.gov/iris/
Bai S et al (2023) Elemental analysis of wild Eriocheir sinensis: determining the geographic origin and human health risk assessment. Qual Assur Saf Crops Foods 15(4):133–142
Gao L et al (2022) Concentrations and health risk assessment of 24 residual heavy metals in Chinese mitten crab (Eriocheir sinensis). Qual Assur Saf Crops Foods 14(1):82–91
Meng T et al (2021) Occurrence of antibiotics in rural drinking water and related human health risk assessment. Environ Technol 42(5):671–681
Fathabad AE et al (2021) The concentration of the potentially toxic elements (PTEs) in the muscle of fishes collected from Caspian Sea: A health risk assessment study. Food Chem Toxicol 154:112349
Płotka-Wasylka J (2018) A new tool for the evaluation of the analytical procedure: Green Analytical Procedure Index. Talanta 181:204–209
Ahmed A (2021) Evaluation of the heavy metal content in the muscle tissue of common carp (Cyprinus carpio L) reared in groundwater in Basrah province, Iraq. Iraqi J Vet Sci 35(1):157–161
Kroukamp E, Wondimu T, Forbes PB (2016) Metal and metalloid speciation in plants: overview, instrumentation, approaches and commonly assessed elements. TrAC, Trends Anal Chem 77:87–99
Hossain M et al (2021) Recent trends in the analysis of trace elements in the field of environmental research: a review. Microchem J 165:106086
Yip YC, Lam JCW, Tong WF (2009) Commonly used methodologies for inorganic analysis in international key comparisons. TrAC Trends Anal Chem 28(2):214–236
Abrham F, Gholap A (2021) Analysis of heavy metal concentration in some vegetables using atomic absorption spectroscopy. Pollution 7(1):205–216
Wu P et al (2009) Flame furnace atomic absorption spectrometry: a review. Appl Spectroscopy Rev 44(5):411–437
Nielsen SS, Miller DD, Rutzke MA (2010) Atomic absorption spectroscopy, atomic emission spectroscopy, and inductively coupled plasma-mass spectrometry. Food Anal 421-442
Butcher DJ (2023) Innovations and developments in graphite furnace atomic absorption spectrometry (GFAAS). Appl Spectroscopy Rev 58(1):65–82
Linge KL, Jarvis KE (2009) Quadrupole ICP-MS: introduction to instrumentation, measurement techniques and analytical capabilities. Geostandards Geoanal Res 33(4):445–467
Sneddon J, Vincent MD (2008) ICP-OES and ICP-MS for the determination of metals: application to oysters. Anal Lett 41(8):1291–1303
Limbeck A, Bonta M, Nischkauer W (2017) Improvements in the direct analysis of advanced materials using ICP-based measurement techniques. J Ana Atomic Spectrometry 32(2):212–232
Klisińska-Kopacz A (2024) X-ray fluorescence spectroscopy, In Non-Destructive Material Characterization Methods. Elsevier. 487–523
Carter S et al (2017) Atomic spectrometry update: review of advances in the analysis of metals, chemicals and materials. J Anal Atomic Spectrometry 32(11):2068–2117
Ahmed M et al (2016) Tissue specific metal characterization of selected fish species in Pakistan. Environ Monit Assess 188:1–9
Fernández-Trujillo S et al (2021) Metals and metalloids in freshwater fish from the floodplain of Tablas de Daimiel National Park, Spain. Ecotoxicol Environ Saf 208:111602
Aissaoui A et al (2017) Assessment and biomonitoring of aquatic pollution by heavy metals (Cd, Cr, Cu, Pb and Zn) in Hammam Grouz Dam of Mila (Algeria). Int J Environ Studies 74(3):428–442
Hasan GA et al (2023) Distribution of Cr, Cd, Cu, Pb and Zn in organs of three selected local fish species of Turag river, Bangladesh and impact assessment on human health. Emerging Contam 9(1):100197
Orso G et al (2023) A deep survey of fish health for the recognition of useful biomarkers to monitor water pollution. Environments 10(12):219
Bagheri T et al (2023) Health risk assessment of some heavy metals detected in edible fishes of Gorgan Bay, Caspian Sea (Iran), for human. Environ Sci Pollut Res 30(15):44480–44489
Dulić Z et al (2018) Accumulation and seasonal variation of toxic and trace elements in tissues of Cyprinus carpio from semi-intensive aquaculture ponds. In Annales de Limnologie-Int J Limnol. EDP Sciences
Ettefaghdoost M (2019) Study of heavy metal bio-accumulation in the edible muscle tissue of Common carp (Cyprinus carpio linnaeus, 1758) from the Siah Darvishan River, Guilan province, Iran. J Food Sci Technol (Iran) 16(86):251–261
Canli M, Atli G (2003) The relationships between heavy metal (Cd, Cr, Cu, Fe, Pb, Zn) levels and the size of six Mediterranean fish species. Environ Pollut 121(1):129–136
Alam I et al (2020) Heavy metals assessment in water, sediments, algae and two fish species from river swat, Pakistan. Bull Environ Contam Toxicol 105:546–552
Alvarado C et al (2021) Metal bioaccumulation by carp and catfish cultured in lake chapala, and weekly intake assessment. Appl Sci 11(13):6087
Li D et al (2023) Bioaccumulation and health risks of multiple trace metals in fish species from the heavily sediment-laden Yellow River. Mar Pollut Bull 188:114664
Tkachenko H et al (2021) Dietary nutrients and health risks from exposure to some heavy metals through the consumption of the farmed common carp (Cyprinus carpio). J Environ Health Sci Eng 19:793–804
Khemis IB et al (2017) Heavy metals and minerals contents in pikeperch (Sander lucioperca), carp (Cyprinus carpio) and flathead grey mullet (Mugil cephalus) from Sidi Salem Reservoir (Tunisia): health risk assessment related to fish consumption. Environ Sci Pollut Res 24:19494–19507
Xu X et al (2019) Bioaccumulation and health risk assessment of trace metals in fish from freshwater polyculture ponds in Chengdu, China. Environ Sci Pollut Res 26:33466–33477
Zhao L et al (2022) Evaluation of the carbon sink capacity of the proposed Kunlun Mountain National Park. Int J Environ Res Public Health 19(16):9887
Varol M, Sünbül MR (2020) Macroelements and toxic trace elements in muscle and liver of fish species from the largest three reservoirs in Turkey and human risk assessment based on the worst-case scenarios. Environ Res 184:109298
Author information
Authors and Affiliations
Contributions
Search in databases was conducted by Yadolah Fakhri, Zahra Pilevar, Intissar Limam, data collection by Intissar Limam, Zahra Esfandiari, Ali Zare; Data Analysis by Yadolah Fakhri, Zahra Pilevar, and the manuscript and editing by Yadolah Fakhri, Zahra Pilevar, Intissar Limam, Zahra Esfandiari, Behnam Khodadoust.
Corresponding author
Ethics declarations
Consent to Participate
The authors declare their consent to participate in this study.
Consent for Publication
The authors declare their consent to publish this study.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fakhri, Y., Pilevar, Z., Limam, I. et al. The Concentration of Potentially Toxic Elements in Common Carp (Cyprinus carpio) in Fish: Systematic Review and Meta-Analysis and Dietary Health Risk Assessment. Biol Trace Elem Res (2024). https://doi.org/10.1007/s12011-024-04340-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12011-024-04340-z