Abstract
The present study was conducted to evaluate the effect of feeding conch shell (Turbinella pyrum) powder (either fresh or calcined) as a marine organic source of calcium (Ca) supplemented in the diet of crossbred calves on voluntary intake, growth performance, and blood biochemistry in growing crossbred Jersey calves. A growth trial of 90 days was conducted on 15 Jersey crossbred female calves (Av. weight, 70.68 ± 2.90 kg; Av. age, 197.73 ± 12.40 days), equally divided into three groups of 5 animals each, i.e., control (T0), treatment 1 (T1), and treatment 2 (T2). All animals were fed total mixed ration (TMR) prepared with a concentrate mixture, chaffed paddy straw, and green fodder at the ratio of 40:30:30 on DM basis. Calves under the control group were fed with TMR containing a standard mineral mixture having dicalcium phosphate (DCP) as a Ca source. Calves under T1 group were supplemented with TMR containing fresh conch shell powder (FCSP), and T2 calves were fed with TMR containing conch shell calcined powder (CSCP) as Ca source. We observed 11.66% increase (p < 0.01) in Ca concentration in CSCP compared to FCSP. The concentration of minerals like Mg, Co, Mn, and Fe was enhanced in CSCP compared to the FCSP. However, the calcination process of fresh conch shell powder (FCSP) reduced the concentration of Cu, and Zn. The Ca/P ratio was estimated as 2.11, 2.06, and 2.10 in T0, T1, and T2 diets, which could be considered ideal for calf ration. Calves under T1, and T2 groups consumed significantly (p < 0.001) greater amounts (g/kg W0.75) of DM and CP compared to T0. However, increased voluntary intake did not translate into increased body weight gain (kg), and feed conversion ratio (kg DMI/kg gain) in T1 and T2 groups in comparison to T0. We observed similar blood glucose, urea, alkaline phosphatase (ALP), aspartate aminotransferase (AST), and alanine transaminase (ALT) concentration among the three treatments. Ca, and P levels in blood plasma were also identical among the three groups. The digestibility of Ca was increased significantly (p = 0.01) in FCSP (T1)- and CSCP (T2)-treated calves compared to control (T0) calves. Similarly, T1 and T2 enhanced P digestibility compared to T0. This first report with short-term experimentation depicted some promising scope for the use of locally available conch shell powder (fresh or calcined form) as a potential source of Ca for feeding to livestock, because these new sources of Ca did not affect intake, digestibility of Ca and P, growth performance, blood chemistry, and liver enzymes negatively in weaned crossbred calves.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The total coastal length in India (including Andaman and Nicobar Islands and Lakshadweep) is 7516.6 km [1] which has plenty of sea/marine shells [2]. Bay of Bengal, Arabian Sea, and Indian Ocean, surrounding Indian subcontinent, are wealthier in biological resources of calcium (Ca) and other important minerals. The major sources of Ca include crustacean shells, fish bones, coral, shell fish, and seaweed. Sometimes, marine processing leftovers are considered unusable; however, it is a copious and cheaper source of Ca [3]. “Turbinella pyrum,” a marine animal of “mollusc” group under the family Turbinellidae [4], is used as conch shells or Shankha (Chart 1) in India. Molluscs are a class of animals that have no bones and have a hard outer shell [5]. Hindu religious groups in India use Shankha and ladies’ Bangles, commercially produced from conch shells (Chart 1) which are fished in southern coastal lines in India and Sri Lanka. Fished conch shells are processed in several small-scale industries located in the state of West Bengal, India. The conch shell powder is the by-product produced during the process of hand-crafting of Shankha, Bangles, and other valued products (Chart 1). The calcined product of conch shell powder is known as Shankha Bhasma which is traditionally used in Ayurvedic medicines to treat many ailments in human beings [6] like peptic ulcers, piles, cough, and certain gastrointestinal disorders [7]. Minerals accomplish various basic physiological functions within the tissues and organs; so, insufficiency or surplus of one or more of them may result into metabolic dysfunctions that may cause low productive and reproductive efficiency [8]. Ca is an essential macro-mineral required for appropriate bone health, integrity, and metabolism in livestock and human beings. Ca enters the circulation through the feed/food chain or Ca supplemented through the mineral mixture, and a dynamic homeostatic balance is maintained between blood and bone Ca [9]. Growing calves need to be supplied with adequate amounts of Ca in order to accomplish acceptable performance, as well as sufficient bone mineralization. Lack of information about Ca availability in different feed resources may impair the accuracy of ruminant’s ration formulation [10]. In recent years, Ca supplements of marine origins have gained considerable attention due to their copious reserves, higher biological safety, and activity [11, 12].
The production of meat (chicken, pork, chevon, mutton, and beef) and milk will accelerate to meet the increasing demand of human population. Hence, the requirements for different nutrients, including minerals, will also increase in the future. Animals can never fully utilize their dietary Ca and P, as some of them are inaccessible or lost during normal digestion and metabolic processes or have an unfavorable impact on their absorption due to a number of dietary or non-dietary variables [13]. The utilization of Ca and P in animals depends upon their bioavailability in feed resources [14]. However, an inappropriate diet of livestock and poultry birds may limit the bioavailability of Ca and P. For example, the presence of phytic acid in cereal grains and oxalic acid in some green fodder can cause Ca to precipitate as calcium phytate and calcium oxalate, which are insoluble compounds [15, 16]. An adequate supply of Ca in the diet could entirely be provided by animal-based and inorganic mineral salts, the most common sources of which include ground limestone, calcium chloride, mono- and dibasic calcium phosphate, and oyster shells [17]. However, the cost of mineral mixtures is a concern these days due to the increased cost of various mineral salts. Instead of purchasing expensive mineral salt, we may use locally available mineral resources of natural origin. For example, Ca from CaCO2 can be obtained from marine shells or wastes such as crustaceans and bivalve shells. The seashells are composed of more than 95% inorganic minerals and a small quantity of organic matrix [18, 19]. Xu et al. [3] reported that the marine shell, an organic source of Ca, contained about 95% CaCO2. CaCO3 in marine shells is commonly found as calcite and aragonite in adult individuals and often amorphous CaCO3 (ACC) in young animals [20].
Conch shell powder, along with another P source, can replace the commonly used dicalcium phosphate (DCP) in the mineral mixture for feeding to livestock. Since no published reports are available on the use of conch shell (Turbinella pyrum) powder (either fresh or calcined) in the mineral mixture supplemented in the diet of livestock, the present study was conducted to assess the effect of replacing DCP with fresh conch shell powder or conch shell calcined powder (a marine organic source of Ca) on voluntary intake, growth performance, blood chemistry, and liver enzymes in growing crossbred Jersey calves.
Materials and Methods
Site of the Experiment
The experiment was conducted in the Animal Nutrition Laboratory and Cattle Yard Complex of ICAR-National Dairy Research Institute, Eastern Regional Station, Kalyani, West Bengal (located in the lower Gangetic region of India) as per the approval of the University (20-M-AN-01 of 2020). The latitude and longitude position of the study site is 22°56′30″N and 88°32′04″E, respectively. The experiment was divided into two phases, i.e., I and II.
Phase-I: Physico-chemical Evaluation of Fresh Conch Shell Powder and Conch Shell Calcined Powder
The sufficient quantity of fresh conch shell powder (FCSP) was procured from Rupam Sankha Silpalaya (registered Industry), Kolmijore, Paschim Midnapur, West Bengal, India, for conducting the experiment. Conch shell calcined powder (CSCP) was prepared by ashing part of FCSP in the muffle furnace at 650–700 °C for 7–8 h. FCSP and CSCP were subjected to solubility test using different inorganic acids and water.
Solubility Test
The weighed samples (5 g) of FCSP and CSCP were allowed to dissolve in 25 ml of distilled water, normal tap water (source deep tube well), and concentrated acids like H2SO4, HCl, HNO3, and HClO4. The samples were kept for dissolution for 18 h. The residual samples were filtered through repeated washing with double-distilled water on pre-weighed filter paper no. 1 until the sample present in the beaker was completely filtered. Then, the filter paper, along with undissolved part of the sample, was kept in a hot air oven until complete drying at 80.0 ± 5.0 °C.
Estimation of Minerals
Minerals such as Ca, Mg, Cu, Zn, Mn, Fe, and Co in FCSP, CSCP, feeds, fodders, fecal samples, and water samples were analyzed by using Atomic Absorption Spectrophotometer (Agilent 240AA model). P content in these feeds and mineral resources was evaluated as per the method described by O’Dell [21].
Evaluation of Density and pH
The density of both FCSP and CSCP was evaluated by using the following formula,
Density of powder (g/cm3) = weight (g)/volume (cm3).
The pH of each powder was estimated by dissolving 10 g of each powdered sample in 100 ml of double distilled water. A digital pH meter (Elico, India) was used for the purpose.
Phase-II: Assessment of Fresh Conch Shell Powder and Conch Shell Calcined Powder in Crossbred Calves
Experimental Animals
The experiment was conducted in 2022 on 15 healthy female Jersey crossbred calves (av. weight, 70.68 ± 2.90 kg; av. age, 197.73 ± 12.40 days) divided into 3 equal treatments (5 calves in each group), i.e., T0 as control, T1, and T2. The average initial body weight and age of calves were statistically similar among the three treatments. All calves were housed individually in the well-ventilated experimental shed under similar management conditions. Ad libitum clean and fresh drinking water was offered twice daily to all animals at around 10.00 AM and 4.00 PM. The experimental shed and calves were cleaned regularly throughout the trial period.
Experimental Feeding
The growth trial in crossbred calves continued for 90 days. Total mixed rations (TMRs) (calculated CP 12% and TDN 65%) were prepared separately for each group using concentrate mixture containing separate mineral mixture (Table 1), chaffed paddy straw, and green fodder at the ratio of 40:30:30 on DM basis. We used seasonal oat fodder during the initial 2 months of the experiment, followed by maize fodder, to prepare TMRs for the last month of the experiment.
Control group (T0): Calves under the control group were fed ad libitum TMR (CP 12% and TDN 65%) with a mineral mixture containing dicalcium phosphate (DCP) as a source of Ca and P.
Treatment group (T1): Ad libitum TMR (CP 12% and TDN 65%) with a mineral mixture containing fresh conch shell powder (FCSP) as a source of Ca and diammonium phosphate as a source of P.
Treatment group (T2): Ad libitum TMR (CP 12% and TDN 65%) with a mineral mixture containing conch shell calcined powder (CSCP) as a source of Ca and diammonium phosphate as a source of P.
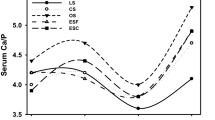
Three experimental mineral mixtures were prepared using the ingredients mentioned in Table 1. The percentage of calcium and phosphorus was kept similar among all mineral mixtures (Fig. 1).
Observations
Chemical Composition of Feeds
Throughout the experimental period, representative samples of feeds and residues were taken and analyzed in triplicate in the Animal Nutrition Laboratory for Proximate Principles [22] and cell wall fractionations [23].
Voluntary Intake of Nutrients, Body Weight, and Feed Conversion Efficiency
The body weight of experimental calves was recorded before the start of the experiment and during different phases (fortnightly) of the trial period by using the ISI-branded digital platform balance (Fig. 2). Fortnightly dry matter intake (DMI) by individual calf was measured by deducting the DM of the residue from the DM of the total feed offered. Similarly, organic matter intake (OMI) and crude protein intake (CPI) patterns were recorded in all experimental animals on fortnightly basis. Average daily body weight gain (ADG), feed conversion efficiency (FCE), and feed conversion ratio (FCR) were calculated based on the following formulae.
Similarly, FCE and FCR were also calculated based on the total crude protein intake (CPI) by the calves under different treatment groups.
Blood Metabolites and Enzymes
Blood samples were collected from the jugular vein of all experimental calves at 0, 45, and 90 days of feeding under aseptic conditions. Some relevant blood metabolites (urea, glucose), minerals (Ca, P), and liver enzymes (alkaline phosphatase (ALP), aspartate aminotransferase (AST), alanine transaminase (ALT)) were analyzed. Blood glucose was estimated using the commercially available glucose kit (GOD-POD) method (Span Diagnostic Ltd., India). Blood urea was estimated using the commercially available Urea Test Kit (urease Berthelot, endpoint assay) method (Span Arkray Healthcare Pvt. Ltd., India). Blood Ca and P were estimated using the commercially available Liquixx Calcium and Phosphorus test kit of Trans Asia Bio Medicals Ltd., India. Blood ALT was evaluated using the commercially available ALT Test Kit of Coral Clinical Systems (P) Ltd., India. We evaluated Blood AST using the commercially available AST Test Kit of Coral Clinical Systems (P) Ltd., India. Blood ALP activity was also measured by the method of pNPP Kinetic method using Diagnostic Kit manufactured by Arkray Healthcare Pvt. India.
Digestibility Trial
A digestion trial of 6-day collection period was conducted on 15 animals (5 experimental calves under each group) by total collection method after the completion of 90 days of the growth trial to study Ca and P intake, outgo through feces, and digestibility. The digestion trial was conducted following the detailed method described by Anil et al. [24]. Ca and P content of feeds offered, residues left, and fecal samples were evaluated as per the methods described in the earlier section.
Statistical Analysis
Data related to the chemical and mineral composition of different samples were statistically analyzed by using one-way analysis of variance (ANOVA) [25]. The intake of nutrients, growth performance, feed conversion efficiency, and blood parameters were analyzed by a mixed ANOVA with repeated measures, where treatment was a fixed factor (between subject effect), time period (within-subject) was a random factor having subject (calf) nested within it, and their (period × treatment) interaction was evaluated. A computerized IBM SPSS 26.0 software program was used for ANOVA. Tukey’s HSD test was used to measure the differences of means. The levels of significance were considered at p < 0.05, and p < 0.01.
Results
Evaluation of Physicochemical Properties of FCSP and CSCP
The FCSP is white colored, while the CSCP is slightly grayish (Chart 1). The mean organic matter, total ash, and acid insoluble ash (AIA) of FCSP were estimated 10.62 ± 16.16, 89.17 ± 1.79, and 0.96 ± 0.01, respectively. The density (g/cm3) of FCSP and CSCP was measured as 0.25 ± 0.0004 and 0.40 ± 0.0004, respectively. The pH of FCSP and CSCP, suspended in doubled distilled water, was 9.80 and 12.02, respectively. The solubility of Ca-rich powders (FCSP, and CSCP) was found to be highest (p < 0.001) in concentrated HCl followed by HNO3, HClO4, H2SO4, tap water, and distilled water (Table 2). However, the overall solubility of FCSP was higher (p < 0.001) than CSCP. The interaction of treatments × solvents was also significant (p < 0.001).
The concentration (%) of Ca was found to be significantly greater (p = 0.005) in CSCP compared to FCSP, wherein the P content was depressed (p < 0.001) in CSCP due to the calcination of FCSP (Table 3). We observed an 11.66% increase in Ca concentration in CSCP compared to FCSP. The concentration of minerals like Mg, Co, Mn, and Fe was enhanced in the CSCP compared to the FCSP (Table 3). However, the calcination process of FCSP reduced the concentration of Cu and Zn, which could be due to the partial vaporization of these essential minerals during the calcination process at high temperatures (> 650 °C).
Evaluation of Chemical and Mineral Composition in Different Feeds
All feed ingredients (oat fodder, paddy straw, maize grain, wheat bran, groundnut cake (GNC), and mustard oil cake (MOC) used for the preparation of total mixed rations (TMRs) were analyzed for chemical composition, such as dry matter (DM), organic matter (OM), crude protein (CP), ether extract (EE), total carbohydrates (TCHO), total ash (TA), neutral detergent fiber (NDF), acid detergent fiber (ADF), hemicellulose, cellulose, and acid detergent lignin (ADL). Table 4 displays the estimated values of different feed ingredients. Oil cakes (GNC, and MOC) contained greater amounts of CP compared to other feed resources, whereas fodder resources (paddy straw, and oats fodder) had higher amounts of NDF and ADF. Ca concentration in oats fodder, and MOC was estimated to be higher than other feed ingredients (Table 4). However, P content was greater in MOC, GNC, and wheat bran than in other feed resources. Table 4 presents the detailed values of micro-minerals (Cu, Zn, Mn, Fe, and Co) of these feed resources.
The TMRs prepared as per the composition mentioned in Table 1 were analyzed for chemical and mineral composition (Table 5). We estimated similar DM, OM, CP, EE, TCHO, total ash, ADF, hemicellulose, and lignin among three TMRs, wherein NDF and cellulose concentration were greater in TMRs under T1 and T2 than T0. Three TMRs had a similar concentration of Ca, and P. TMR T1 and TMR T2 contained greater amounts of Mg, Cu, Zn, Mn, and Fe compared to TMR T0. Cobalt concentration was found identical among the three TMRs.
Voluntary Intake of Nutrients and Utilization of Ca and P
Throughout six fortnights, the total dry matter intake (kg/day/calf) varied from 2.21 to 3.20 in the control (T0) group, 2.36 to 3.56 in T1, and 2.33 to 2.97 in T2, respectively. The average DM intake was significantly higher (p < 0.05) in T1 and T2 compared to T0 (Table 6). There was a significant (p < 0.001) difference in total DMI (kg/d/calf) among different fortnights indicating the periodic effect, which was mainly due to gain in body weight (BW) by growing calves over different fortnights. DMI (kg/100 kg body weight and g/kg W0.75) was significantly (p < 0.05) greater in T1 and T2 groups compared to T0 group, with no significant difference between T1 and T2 (Table 6). Similarly, the period effect (Fortnights) was also significant for DMI per unit of body weight (kg/100 kg BW and g/kg W0.75), whereas the interaction between treatment, and period (T × P) showed (p > 0.05) non-significant effect for DMI. Calves under T1 (containing FCSP), and T2 (containing CSCP) consumed a greater (p = 0.012) amount of total OM compared to the control (T0) (Table 6). The OMI (kg)/100 kg BW and OMI (g)/kg W0.75 ranged from 2.59 (T0) to 2.92 (T2) and 79.77 (T0) to 89.78 (T2). The values were statistically greater in T1 and T2 than in T0. However, similar to DMI, the periodic effect on OM intake (kg/d/calf) was also statistically significant (p < 0.001), whereas OM intake per unit BW increased significantly (p < 0.001) as the experiment progressed, indicating a periodic effect. However, the T × P effect was found to be non-significant (p > 0.05) for these parameters. We estimated significantly greater (p < 0.001) crude protein intake per unit body weight (CPI, g/100 kg BW and g/W0.75) in the treatments supplemented with FCSP (T1) and CSCP (T2) compared to the control (T0) treatment, wherein calves under T2 consumed a higher (p = 0.016) amount of total CP than control T0. Similar to DM intake, the periodic effect showed statistical significance (p < 0.001) only for total CPI, and CPI/kg W0.75, whereas the T × P effect was non-significant.
Calves under different treatments consumed (g/day) similar total Ca through TMR diets (Fig. 3) offered during the digestion trial conducted at the last phage of the growth trial. However, Ca intake (g)/kg W0.75 was greater (p = 0.021) in CSCP-treated calves (T2) compared to DCP-treated control calves (T0). Calves under T1 had similar values (Ca intake (g)/kg W0.75) with T0 and T2. Ca outgo (g/day) through feces was identical among three treatments, whereas Ca digestibility increased significantly (p = 0.01) in FCSP (T1) and CSCP (T2) treated calves compared to control T0. During the same digestion trial, we observed similar total P intake among different treatments (Fig. 4). However, P intake (g)/kg W0.75 enhanced (p < 0.001) in CSCP-treated calves (T2) compared to FCSP (T1) and control calves (T0). Fecal outgo of P reduced significantly in T2 in comparison to T0; however, the value of T1 was similar to T0 and T2. The addition of fresh and calcined powder of conch shell increased (p < 0.001) P digestibility in T1 and T2 compared to T0 in the present study (Fig. 4).
Growth Performance and Feed Conversion Efficiency
The initial body weight (kg) was similar among T0, T1, and T2 groups (Table 6). At the end of the 90-day trial, the corresponding final body weight (kg) was 106.03, 106.31, and 106.68 in the T0, T1, and T2 groups, respectively. The statistical difference among the groups was non-significant (p > 0.05). Calves under T1 and T2 treatments gained 3.03, and 5.78% higher compared to T0 during the feeding period; however, the difference was non-significant. The pattern of fortnightly body weight of calves is expressed graphically in Fig. 2, which indicated no difference among different treatments. The average daily body weight gain (g/d/animal) ranged from 366.60 (T0) to 392.24 (T2); the difference was observed similar among the treatment groups. Furthermore, periodic (P), and interaction (P × T) effects were also non-significant (p > 0.05). Similarly, fortnightly body weight gain was also statistically similar among the three treatments.
Different sources of Ca in the present study did not alter the feed conversion efficiency (ADG as percent of DM intake), and feed conversion ratio (kg DMI/kg weight gain) in calves under three treatment groups (Table 6). Similarly, FCE (ADG as percentage of CPI) and FCR (kg CPI/kg weight gain) were also statistically identical among different treatments. However, the periodic effect was found significant (p < 0.05) for FCE (based on DMI and CPI), indicating calves fed at younger age had greater FCE compared to older age. However, the interaction between treatment × period (T × P) was similar for FCE, and FCR.
Blood Metabolites and Enzymes
The blood glucose concentration (mg/dl of plasma) ranged from 49.06 to 50.81 in T0, 47.88 to 51.88 in T1, and 51.30 to 52.14 in T2 groups, respectively (Table 7). Ca from FCSP and CSCP did not alter the blood glucose concentration. Additionally, no statistically significant difference was recorded among different periods of blood sampling. Similarly, the interaction effect between treatment, and period (T × P) was also observed to be non-significant. The average blood urea concentration was 15.65, 15.52, and 15.67 (mg/dl) in the T0, T1, and T2 groups, indicating no significant difference among different treatments. However, the periodic effect was significant (p < 0.001). Furthermore, the interaction effect (T × P) was non-significant.
The Ca concentration (mg/dl of plasma) at the start of the experiment was estimated 10.28, 10.16, and 10.38 (mg/dl) in T0, T1, and T2 groups, and it was 11.41, 11.18, and 11.14 (mg/dl of plasma) at the end of the experiment (Table 7). The average plasma Ca concentration (mg/dl of plasma) in calves of the present study ranged from 10.88 (T0) to 10.97 (T2). However, the difference (p > 0.05) was non-significant among the treated groups. A significant rise (p < 0.001) in blood Ca concentration was observed as the experiment progressed due to the feeding of dicalcium phosphate in T0 and conch shell powders (fresh and calcined) in T1 and T2 as a source of Ca. However, the interaction effect (T × P) was similar for plasma Ca concentration. We observed average plasma P concentration (mg/dl plasma) ranging from 5.45 in T0 to 5.65 in T2. The treatment effect (T), periodic effect (P), and interaction effect (T × P) were indistinguishable among the three treatments.
The average concentration (IU/l of plasma) of alkaline phosphatase (ALP) ranged from 275.76 (T0) to 279.66 (T2) in the study (Table 7). We observed no difference among the three treatments. The values for alkaline phosphatase level in blood were within the normal physiological range which indicated no adverse effect of new Ca source on the liver. The treatment effect (T), periodic effect (P), and interaction (T × P) effect were comparable among the three treatments. The average concentration (IU/l plasma) of aspartate aminotransferase (AST) was observed to be non-significant (p > 0.05) among the different groups (T0 98.77 IU/l, T1 98.77 IU/l, and T2 97.95 IU/l). In addition, the periodic effect, and interaction (T × P) effect were also statistically non-significant. We observed similar average concentrations (IU/l plasma) of alanine transaminase (ALT) in different treatment groups; the values were within the normal physiological range. Moreover, the periodic effect and interaction effect (T × P) were also identical.
Discussion
Physico-chemical Properties of FCSP and CSCP
The calcination process of fresh conch shell powder (FCSP) in the present study transformed the most stable polymorphs of CaCO3 of conch shell (shankha) aragonite form into calcite form, along with the formation of portlandite and lime as the majority component [26]. Similar to our findings, properly ground ocean quahog clamp shells (OQCS), and surf clam shells (SCS) contain a higher percentage of Ca (> 36%) [27]. Both FCSP and conch shell calcined powder (CSCP) had greater solubility in HCl, which is advantageous to livestock due to the acidic environment in the stomach (including abomasum in ruminants). Similarly, Rasheed et al. [7] concluded that conch shell powder was soluble in diluted HCl. Similar to our finding, Savita et al. [26] also reported pH values of 12.80 and 9.05 for conch shell calcined powder, and fresh conch shell powder. The acid-insoluble ash (AIA) content of FCSP corroborated the findings of Savita et al. [26] who observed 0.96% AIA in fresh conch shell powder. The calcination process increased the Ca concentration of conch shell powder; whereas it depressed the P concentration in the present study. High temperature (> 650 °C) during the calcination process in muffle furnace could have resulted in the reduction of P, Cu, and Zn in conch shell powder, whereas the level of Mg, Co, Mn, and Fe increased in this powder along with Ca due to such calcination process. The elevated levels of these essential minerals might be advantageous to the livestock to fulfill their nutritional requirements.
Voluntary Intake of Nutrients and Utilization of Ca and P
The incorporation of FCSP and CSCP in the total mixed ration (TMR) of T1 and T2 resulted in similar Ca, and P concentrations with that of control T0 TMR. The Ca/P ratio was estimated at 2.11, 2.06, and 2.10 in T0, T1, and T2, which could be considered ideal for calf ration (Fig. 1). The Ca:P ratio of 2:1 or greater was recommended by NRC [28] to avoid the formation of urinary calculi in sheep. Similarly, it was investigated that the Ca/P ratio in the complete ration plays an imperative role in plummeting the occurrence of mineral calculi in beef cattle, and that a good balanced diet could have an essential protective role in the development of this pathology [29]. The Ca/P ratio should be maintained 2:1 in cattle diet; any Ca/P imbalance may result in higher phosphate elimination through urine which is an imperative factor in the genesis of calculi [30]. Antonio Esteves et al. [16] also explained that the ratio of Ca/P has a great impact on the degree of absorption of both elements in animals. An excess of either causes amplification in fecal voidance by the development of insoluble Ca that is not obtainable for absorption. However, Mg, Cu, Zn, Mn, and Fe increased significantly due to the addition of FCSP and CSCP during the formulation of TMR in T1 and T2 in the present study. Voluntary intake of nutrients (DM, OM, and CP) as expressed per unit of body weight (per 100 kg body weight or kg W0.75) enhanced due to the replacement of DCP with FCSP, and CSCP in the diets of crossbred calves. Calves under three treatments fulfilled their nutritional requirements in terms of DM, and CP intake as recommended by NRC [31]. Anil et al. [24] also reported a similar DM intake pattern per 100 kg body weight of finisher female calf fed with TMR (DCP 7.85%, TDN 57.51%) under an intensive system of management.
We have not found any published reports on the effect of feeding conch shell powder or conch shell calcined powder in ruminants (calves or heifers or dairy cows) and non-ruminants (including poultry birds) to compare our present results. However, some reports are available on the use of different marine shells as a source of Ca. Similar to our findings, Finkelstein et al. [27] reported that the DMI was lowest for cows fed aragonite and greatest for cows fed ocean quahog clam shell (Arctica islandica), and surf clam shell (Spisula solidissima). However, some researchers reported that the oceanic source of oyster shells could be used effectively in poultry birds as a source of Ca. Similar to our findings, Lana [32] discovered that broiler hens fed diets with 0.42% calcium from Charru Mussel Shell meal consumed more feed than the other groups fed different organic calcium sources. Similarly, Oso et al. [33] also reported that broilers fed a diet containing oyster shells as the Ca source had greater feed consumption, and body weight gain than broilers fed a diet having limestone. Daily intake of Ca significantly increased in poultry birds fed with oyster shells, and limestone compared to control [34]. Cambra-Lopez et al. [35] evaluated the P digestibility of the three commercial sources of DCP, and found that the digestibility of P was 80.1% (DCP1), 77.4% (DCP2), and 71.4% (DCP3), respectively. We have also recorded similar digestibility of P in the present study. Omole et al. [36] discovered that replacing dicalcium phosphate with oyster shell did not reduce feed intake but increased growth rate in finishing broiler. Mako et al. [37] observed that the amount of feed consumed by the poultry birds on diet 1 (oyster shells 54% and bone meal 46%) was greater than other treatment combinations. Similarly, the report by Olgun [38] showed that the supplementation of oyster shells up to one-third of the Ca requirement increased eggshell weight; however, it had an antagonistic effect on feed consumption and egg weight. This finding, however, conflicts with a study by Ahmed et al. [39] that claimed 24-week-old Bovan pullets treated with oyster shells had reduced feed intake than those treated with limestone. Badejo et al. [40] observed no significant (p < 0.05) difference between the oyster shell group, and the periwinkle shell group concerning feed consumption in the spent layer. CaCO3 supplementation improved apparent starch digestibility, whereas the digestibility of other nutrients (DM, OM, CP, EE, and ADF) was not affected by CaCO3 [41]. Santana et al. [42] investigated that Ca sources did not affect the digestibility coefficients in pigs.
Growth Performance and Feed Conversion Efficiency
Despite increased voluntary intake, the new marine source of Ca, either in fresh conch shell powder (FCSP) form or conch shell calcined powder (CSCP) form, did not alter the growth pattern in weaned crossbred calves compared with control calves fed with DCP. We did not find any published literature to compare the works on the usage of conch shell powder (fresh or calcined) as a potential source of Ca in ruminants. However, our findings are in close agreement with the finding of Santana et al. [42] who investigated that calcium supplied in the form of calcitic limestone, monodicalcium phosphate, calcinated bone flour, and oyster flour had no difference in growth performance of pigs. Also, similar results were obtained in brown laying hens; different Ca sources (100% fine limestone; 60% fine limestone and 40% large limestone; and 60% fine limestone and 40% oyster shell) had identical effects on body weight gain [43]. The FCE and FCR remained unaltered due to FCSP and CSCP treatments in calves. The results corroborated the findings of Olgun et al. [38], who reported no significant difference between the control (limestone) and the treatments (replaced with 1/3 eggshell, 1/3 oyster shell, 1/3 eggshell + 1/3 oyster shell, 2/3 eggshell, and 2/3 oyster shell) to modify FCR. The study by Badejo et al. [40] revealed that feed consumption and feed conversion ratio in the spent layer did not differ significantly among different Ca sources (bone meal, oyster shell, periwinkle shell, and limestone).
Blood Metabolites and Enzymes
Ca from three sources (DCP, FCSP, and CSCP) did not affect blood glucose, and blood urea concentration in growing calves. Similarly, the concentration of plasma Ca, and P was also similar among the three treatments. The results clearly indicated that marine sources of Ca in different forms (fresh and calcined) had no adverse effects on the concentration of glucose, urea, Ca, and P in blood plasma. The concentration of ALP, AST, and ALT did not differ among the three treatments, indicating no adverse effects on liver enzymes due to the feeding of new Ca sources. All these parameters were within the normal physiological range. Manowari and Singh [44] estimated almost similar blood glucose levels in crossbred heifers and cows. The mean blood urea value in normal healthy control livestock was reported as 26.76 mg/dl [45]. Reece and Hotchkiss [46] measured similar Ca, and P levels in calves fed with different feeding modules. Gading et al. [47] also observed identical blood Ca, and P concentrations in weaner calves. The phosphorus concentration in the present study corroborated the finding of Ramana et al. [48] who revealed that plasma P (mg%) concentrations in stall-fed heifers and cattle calves were from 5.03 to 5.18 mg% and 5.24 to 6.60 mg%, respectively. Brazilian male sheep fed a basal ration supplemented with different sources of Ca, namely limestone (L), alfalfa hay (AH), oyster shell meal (OSM), and citrus pulp (CTP) utilized inorganic sources of Ca in a greater way compared to other sources [10]. The average ALT values reported in this study were within the normal physiological range of cattle [49]. The typical ALT range is 11–40 IU/l of plasma [50]. Similarly, the normal activity of AST in the blood is 78 to 132 IU/l in healthy cattle [50]. Mamun et al. [51] reported a similar pattern of blood ALP, AST, ALT, and P levels in cattle reared in the tropical zone of Bangladesh. However, we estimated greater plasma Ca concentration in the present study compared to the study of Mamun et al. [51].
Conclusions
Both fresh conch shell powder, and conch shell calcined powder are rich in Ca, Zn, Fe, and Co; however, CSCP is richer in Ca, Mg, Mn, and Fe, whereas FCSP contained a greater amount of Cu, and Zn. Despite enhanced voluntary intake of different nutrients, the new marine organic source of Ca, either in fresh or conch shell calcined powder form, did not modify the growth performance in weaned crossbred calves compared to control dicalcium phosphate-fed calves. Blood metabolites and liver enzyme profiles remained unaffected on supplementation of fresh or calcined conch shell powder. Short-term experimentation depicted some promising scope for the use of locally available conch shell powder (fresh/calcined) as a potential source of Ca for feeding to the livestock; however, long-term experimentation can ascertain the positive response of this marine-based organic Ca source in growing, pregnant, and lactating livestock as well as in poultry birds.
Data Availability
The data will be made available from the corresponding author on reasonable request.
References
NCERT (2015) India – size and location. National Council of Education, Research and Training. https://ncert.nic.in/ncerts/l/iess101.pdf (assessed on 08.11.2023)
Silva HT, Mesquita-Guimaraes J, Henriques B, Silva FS, Fredel MC (2019) The potential use of oyster shell waste in new value-added by-product. Resources 8:13. https://doi.org/10.3390/resources8010013
Xu Y, Ye J, Zhou DJ, Su L (2020) Research progress on applications of calcium derived from marine organisms. Scientific Rep 10:18425. https://doi.org/10.1038/s41598-020-75575-8
Wikipedia. Conch. Available online: https://en.wikipedia.org/wiki/Conch (accessed on 11 Nov. 2023)
Babafakruddin DH, Doddamani MS (2017) Marine products and their therapeutic importance. Internat Ayurveda Pub 2:527–533
Gupta LN (2020) Physicochemical evaluation of Shankha Bhasma: a calcium-enriched ayurvedic preparation. World J Pharmacol Res Technol 9:1–9
Rasheed SP, Shivashankar M (2017) Synthesis and characterization of Shanku Bhasma - an anti-ulcer herbomineral formulation. In IOP Conference Series: Materials Sci Eng 263:022026. https://doi.org/10.1088/1757-899X/263/2/022026
Ammerman CB, Goodrich D (1983) Advances in mineral nutrition in ruminants. J Anim Sci 57 (suppl 2):519-533. https://pubmed.ncbi.nlm.nih.gov/6352594 (assessed on 09.11.2023)
Shojaeian Z, Sadeghi R, Roudsari RL (2019) Calcium and vitamin D supplementation effects on metabolic factors, menstrual cycles and follicular responses in women with polycystic ovary syndrome: a systematic review and meta-analysis. PubMed 10:359–369. https://doi.org/10.22088/cjim.10.4.359
Dias RS, Kebreab E, Vitti DMSS, Roque AP (2008) Application and comparison of two models to study effects of calcium sources in sheep. Anim Feed Sci Technol 143:89–103. https://doi.org/10.1016/j.anifeedsci.2007.05.006
Jm L, Lamotte C, Boukandoura B, Cayzeele A, Libersa C, Delannoy CW, Borgies B (2007) Effects of two marine dietary supplements with high calcium content on calcium metabolism and biochemical marker of bone resorption. Eur J Clin Nutr 62:879–884. https://doi.org/10.1038/sj.ejcn.1602797
Flammini L, Martuzzi F, Vivo V, Ghirri A, Salomi E, Bignetti E, Barocelli E (2016) Hake fish bone as a calcium source for efficient bone mineralization. Internat J Food Sci Nutri 67:265–273. https://doi.org/10.3109/09637486.2016.1150434
Kiarie E, Nyachoti CM (2010) Bioavailability of calcium and phosphorus in feedstuffs for farm animals. In: Vitti DMSS, Kebreab E (eds) Phosphorus and calcium utilization and requirements in farm animals, Chapter 6. Publisher CABI, pp 76–93. https://doi.org/10.1079/9781845936266.0076
Coffey RD (1994) The biological availability of phosphorus in various feed ingredients for pigs and chicks. PhD thesis submitted to University of Kentucky, Princeton, Kentucky, USA. http://ci.nii.ac.jp/ncid/BA47748061
Kim S, Ravichandran YD, Kong C (2012) Applications of calcium and its supplement derived from marine organisms. Critical Rev Food Sci Nutr 52:469–474. https://doi.org/10.1080/10408391003753910
Antonio ELM, Roldan STMC, Avendaño RL, Minor PF, González-Reyna A, Javier Trejo Meza F, Cesar CRJ (2023) Importance of minerals in the diet of cattle in the tropical climate of Mexico. In: Book - Landraces - its productive conservation in animals and plants. Editors: González-Reyna. A. and Kaushik P. pp 1–136. IntechOpen. https://doi.org/10.5772/intechopen.110491
NRC (2005) Mineral tolerance of animals, National Research Council, 2nd edn. National Academy of Sciences National Academy Press, Washington, DC
Marin F, Roy NL, Marie B (2012) The formation and mineralization of mollusk shell. Front Biosci S4:1099–1125. https://doi.org/10.2741/321
Checa AG (2018) Physical and biological determinants of the fabrication of molluscan shell microstructures. Front Mar Sci 5:353. https://doi.org/10.3389/fmars.2018.00353
McDougall C, Degnan BM (2018) The evolution of mollusc shells. Wires Dev Biol e313. https://doi.org/10.1002/wdev.313
O’Dell JW (1993) Determination of phosphorus by semi-automated colorimetry. Method 365. 1 Environmental Monitoring Systems Laboratory Office of Research and Development. U.S. Environmental Protection Agency Cincinnati, Ohio 45268. In Elsevier eBooks (pp. 479–495). https://doi.org/10.1016/b978-0-8155-1398-8.50027-6
AOAC (2012) Official methods of analysis of AOAC International, 19th ed. Gaithersburg, Maryland, USA
Van Soest PJ, Robertson JB, Levis BA (1991) Method of dietary fibre, neutral detergent fibre and non-starch polysaccharides in relation to animal nutrition. J Dairy Sci 74:3583–3587
Anil Dutta TK, Chatterjee A, Yadav SK, Mandal DK, Mohammad A (2023) Effect of exogenous fibrolytic enzymes supplementation to improve voluntary intake, availability of nutrients and growth performance in weaned crossbred calves. Indian J Anim Sci 93:896–902. https://doi.org/10.56093/ijans.v93i9.131419
Snedecor GW, Cochran WG (1994) Statistical methods. Oxford and IBH Publishing Co., New Delhi
Savita S, Yadevedra Y, Usha S, Sushma R, Khemchand S (2020) Characterization of conch shell nanoparticles (Shanka Bhasma) synthesized by the classical method. Scholars Inter J Trad Compl Med 3:90–99. https://doi.org/10.36348/sijtcm.2020.v03i05.002
Finkelstein AD, Wohlt JE, Emanuele SM, Tweed SM (1993) Composition and nutritive value of ground sea clam shells as calcium supplements for lactating Holstein cows. J Dairy Sci 76(2):582–589. https://doi.org/10.3168/jds.S0022-0302(93)77378-6
NRC (1985) Nutrient requirements of sheep. National Research Council, 6th Edition, National Academy of Sciences, National Academy Press, Washington, D.C
Gianesella M, Giudice E, Messina V, Cannizzo C, Florian E, Piccione G, Morgante M (2010) Effect of an unbalanced Ca/P diet on blood parameters and urolithiasis in growing calves. VeterinarijaIr Zootechnika 32–36. https://vetzoo.lsmuni.lt/data/vols/2010/49/pdf/gianes (assessed on 13.11.2023)
Rosol TJ, Capen CC (1997) Calcium-regulating hormones and diseases of abnormal mineral (calcium, phosphorus, magnesium) metabolism. In: Elsevier eBooks, 619–702. https://doi.org/10.1016/b978-012396305-5/50024-5
NRC (2001) Nutrient requirements of dairy cattle. 7th, Revised. National Academy Press, National Research Council, Washington, DC
Lana GRQ (2017) Calcium sources in the coastal region of Alagoas in diets for broiler chickens. Doctoral thesis submitted to Federal University of Alagoas, Rio Largo, AL, Brazil
Oso AO, Idowu AA, Niameh OT (2011) Growth response, nutrient and mineral retention, bone mineralisation and walking ability of broiler chickens fed with dietary inclusion of various unconventional mineral sources. J Anim Physiol Anim Nutri 95:461–467. https://doi.org/10.1111/j.1439-0396.2010.01073.x
Rathnayaka RMDB, Mutucumarana RK, Andrew MS (2020) Free-choice feeding of three different dietary calcium sources and their influence on egg quality parameters of commercial layers. J Agricul Sci 15:50–62. https://doi.org/10.4038/jas.v15i1.8671
Cambra-Lopez M, Moset V, del Carmen LM, Sebastian Mesa J, Carpintero L, Donadeu A, Pascual JJ (2021) Evaluation of phosphorus digestibility from monocalcium and dicalcium phosphate sources and comparison between total tract and prececal digestibility standard methods in broilers. Anim 11:3427. https://doi.org/10.3390/ani11123427
Omole AJ, Ogbosuka GE, Salako RA, Ajayi OO (2005) Effect of replacing oyster shell with gypsum in broiler finisher diet. J Appl Sci Res 1:245–248
Mako AA, Mosur AO, Adedeji BS, Jemiseye FO, Abokede T (2017) Comparative use of oyster shell and limestone as sources of calcium in the diet of laying chickens. Nigerian J Anim Prod 44:275–281
Olgun O, Yildiz AO, Cufadar Y (2015) The effects of eggshell and oyster shell supplemental as calcium sources on performance, eggshell quality and mineral excretion in laying hens. Indian J Anim Res 49:205–209. https://doi.org/10.5958/0976-0555.2015.00056.4
Ahmed NM, Atti AKA, Elamin KM, Dafalla KY, Malik HEE, Dousa BM (2013) Effect of dietary calcium sources on laying hens performance and egg quality. J Anim Prod Adv 3:226–231
Badejo HA, Dilala MA, Potiskum SB, Doma UD (2019) The effect of various calcium and phosphorus sources on productive and egg quality performances of spent layers. IOSR J24:69–75
Clark JH, Plegge AW, Davis CL, McCoy GC (1989) Effects of calcium carbonate on ruminal fermentation, nutrient digestibility, and cow performance. J Dairy Sci 72:493–500. https://doi.org/10.3168/jds.S0022-0302(89)79131-1
Santana ALA, de Oliveira Carvalho PL, Cristofori EC, da Silva Chambo PC, Barbizan M, Nunes RV, Genova JL (2018) Supplementation of pig diets in the growth and termination phases with different calcium sources. Trop Anim Health Prod 50:477–484. https://doi.org/10.1007/s11250-017-1456-8
Safaa H, Serrano MP, Valencia DG, Frikha M, Jiménez-Moreno E, Mateos GG (2008) Productive performance and egg quality of brown egg-laying hens in the late phase of production as influenced by level and source of calcium in the diet. Poultry Sci 87:2043–2051. https://doi.org/10.3382/ps.2008-00110
Manowari, Singh C (2001) Blood glucose concentration in crossbred (Friesian x Hariana) heifers and cows. Indian J Anim Sci 71: 848-849. http://epubs.icar.org.in/ejournal/index.php/IJAnS/arti (assessed on 10.11.2023)
Hagawane SD, Shinde SB Rajguru DN (2009) Haematological and blood biochemical profile in lactating buffaloes in and around Parbhani city. Vet World 2:467-469. http://www.veterinaryworld.org/Vol.2/December/Haem (assessed on 10.11.2023)
Reece WO, Hotchkiss DK (1987) Blood studies and performance among calves reared by different methods. J Dairy Sci 70:1601–1611. https://doi.org/10.3168/jds.S0022-0302(87)80188-1
Gading BMWT, Agus A, Irawan A, Panjono P (2020) Growth performance, hematological and mineral profile of post-weaning calves as influenced by inclusion of pelleted-concentrate supplement containing essential oils and probiotics. Iranian J Applied Anim Sci 10:461–468. http://ijas.iaurasht.ac.ir/article_675343_885d26155ceb8c4190bf285af6c538b6.pdf (assessed 13.11.2023)
Ramana DBV, Nirmala G, Kumar AV (2011) Mineral profile of feeds, fodders and blood plasma of dairy animals in KVK adopted villages of Ranga Reddy district in Andhra Pradesh. Indian J Dryland Agricul Res Dev 26:26-31. http://www.indianjournals.com/ijor.aspx?target=ijor:ijda (assessed on 05.11.2023)
Stojević Z, Piršljin J, Milinković-Tur S, Zdelar-Tuk M, Ljubić BB (2005) Activities of AST, ALT and GGT in clinically healthy dairy cows during lactation and in the dry period. Veterinarski Arhiv 75:67–73 (http://ufrgs.br/lacvet/restrito/pdf/stojevic_liver_enzym)
Ingvartsen KL (2006) Feeding and management-related diseases in the transition cow: physiological adaptations around calving and strategies to reduce feeding-related diseases. Anim Feed Sci and Technol 126:175–213
Mamun MdAA, Hassan MM, Shaikat AH, Islam S, Hoque MdA, Uddin H, Hossain MB (2014) Biochemical analysis of blood of native cattle in the hilly area of Bangladesh. Bangladesh J Vet Med 11:51–56. https://doi.org/10.3329/bjvm.v11i1.16513
Acknowledgements
Authors express sincere gratitude to the Director, ICAR-National Dairy Research Institute (Deemed University), Karnal for providing all facilities for this study.
Funding
The project was funded and supported by Indian Council of Agricultural Research (ICAR)-National Dairy Research Institute, Karnal, Haryana, India.
Author information
Authors and Affiliations
Contributions
TKD, and AC conceived and designed the experiment; JB, TKD, AC, and SR conducted experiment and formal analysis; AM, and SR analyzed the data; JB, TKD, and AC wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethical Approval
The animal feeding experiment was conducted as per committee approval of ICAR-National Dairy Research Institute (NDRI Deemed University), Karnal, Haryana, India (NDRI Reg. No. 20-M-AN-01, 2020), and accordingly, all standard Institutional and Government of India ethical protocols including animal right/welfare issues were followed during the entire animal trial period.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bhagat, J., Dutta, T.K., Chatterjee, A. et al. Conch Shell (Turbinella pyrum) Powder: A Potential Marine Biological Source of Calcium and Some Trace Minerals for Growing Crossbred Calves. Biol Trace Elem Res (2024). https://doi.org/10.1007/s12011-024-04104-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12011-024-04104-9