Abstract
Aluminum chloride is an inorganic polymeric coagulant commonly found in daily life and various materials. Although male reproductive toxicity has been associated with AlCl3 exposure, the underlying mechanism remains unclear. This study aimed to examine the impact of AlCl3 exposure on mitophagy and mitochondrial apoptosis in testicular tissue and mouse spermatocytes. Reactive oxygen species (ROS) and ATP levels were measured in GC-2spd after AlCl3 exposure using a multifunctional enzyme labeler. The changes in mitochondrial membrane potential (MMP) and TUNEL were observed through confocal laser microscopy, and the expression of proteins associated with mitophagy and apoptosis was analyzed using Western blot. Our results demonstrated that AlCl3 exposure disrupted mitophagy and increased apoptosis-related protein expression in testicular tissues. In the in vitro experiments, AlCl3 exposure induced ROS production, suppressed cell viability and ATP production, and caused a decrease in MMP, leading to mitophagy and cell apoptosis in GC-2spd cells. Intervention with N-acetylcysteine (NAC) reduced ROS production and partially restored mitochondrial function, thereby reversing the resulting mitophagy and cell apoptosis. Our findings provide evidence that ROS-mediated mitophagy and cell apoptosis play a crucial role in the toxicity of AlCl3 exposure in GC-2spd. These results contribute to the understanding of male reproductive toxicity caused by AlCl3 exposure and offer a foundation for future research in this area.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
According to reports, there is a global increase in infertility cases, with half of them being attributed to male factors. Male infertility can be caused by various factors including genetic and environmental factors [1, 2]. Aluminum (Al), consisting of 8.8% of the earth’s crust [3, 4], is the most abundant metal element. According to the World Health Organization, too much aluminum can harm human health. However, AlCl3 is prevalent in daily life, present in food additives, raw materials for processing, pharmaceuticals, and cosmetics [5, 6]. Consequently, humans can come into contact with and consume AlCl3-containing items and foods. AlCl3 contamination poses a significant threat to human and animal health. Recent studies have repeatedly reported the toxicity of AlCl3 to cells, demonstrating its ability to damage neuronal cells through various pathways, including ROS-mediated mitophagy, DDX3X-NLRP3, and others [7, 8]. Moreover, AlCl3 has been shown to induce intestinal disorders via biochemical and immune pathways [9]. As research has advanced, the detrimental effects of AlCl3 on the reproductive system have also emerged. Excessive AlCl3 intake has been documented to impact the male mammalian reproductive system [10]. Our research group has previously discovered that exposure to AlCl3 can cause proteomic changes in the testicles of rats, resulting in testicular injury and toxicity. Additionally, we found that AlCl3 exposure induces the production of ROS and transcriptomic changes in mouse spermatocytes, leading to toxicity in these cells [11, 12]. Furthermore, Studies have shown that aluminum can also induce the production of ROS, which can in turn lead to an increase in immobile and malformed sperm, thereby affecting male sperm motility [13]. Additionally, some scholars have demonstrated the accumulation of Al in germ cells and associated mitochondrial damage by injecting an AlCl3 solution into mice [14].
Oxidative stress is a significant cellular and in vivo pathogenesis resulting from the excessive production of ROS within cells or organisms. Mitochondria, as the primary organelles responsible for ROS production and being targeted, play a crucial role in this process [15, 16]. Current research suggests that Al-induced cellular toxicity is caused by enhanced production of ROS in the mitochondria due to extrinsic stimuli, resulting in oxidative stress and eventual mitochondrial damage [17]. In response to mitochondrial damage, a selective process of eliminating damaged mitochondria known as mitophagy is activated. A previous study has highlighted that exposure to the heavy metal Cr(VI) impairs mitochondrial function in chicken kidneys, triggering mitophagy [18]. Notably, mitophagy are vital for maintaining spermatogenic cell homeostasis and maturation [19]. When mitochondria are damaged, the outer mitochondrial membrane protein PINK1 recruits Parkin, leading to mitochondrial phosphorylation and subsequent LC3-mediated engulfment of damaged mitochondria [20, 21]. Additionally, p62 recognizes phosphorylated polyubiquitin chains on mitochondrial proteins and promotes autophagosome formation by binding to LC3. Furthermore, PINK1 phosphorylates ubiquitin molecules, resulting in the formation of a polyubiquitin chain receptor protein on the surface of damaged mitochondria [22]. AlCl3 exposure can also induce mitochondrial apoptosis. The mitochondrial pathway plays a crucial role in apoptosis. When the permeability of the mitochondrial membrane increases, Cytochrome C (Cyt-C) is released, altering the ratio of Bax/Bcl2. Cyt-C then binds to apoptotic protease activator 1 (Apaf-1) to form a regulatory death complex, which subsequently activates caspased-9. The activation of caspased-9, along with the activation of effector caspases such as caspased-3, leads to cell apoptosis [23, 24]. NAC is a powerful antioxidant and an effective chelator of heavy metals. Research has indicated that NAC has the ability to decrease ROS levels and reverse cell apoptosis and mitophagy. NAC treatment has shown promising results in alleviating testicular injury caused by metal exposure [25,26,27]. However, the specific mechanism by which NAC reverses mitophagy and apoptosis to ameliorate germ cell toxicity induced by AlCl3 exposure has been rarely investigated.
AlCl3 is extensively utilized in numerous industries, resulting in a rise in human exposure to this metal. This study aimed to investigate the mechanism of mitophagy and apoptosis in rat testicular tissue and in the GC-2spd model exposed to AlCl3. The study examined the impact of AlCl3 exposure on cell viability, intracellular ROS and ATP levels, and mitochondrial membrane potential. Additionally, the expression of mitophagy and apoptosis-related proteins was analyzed to gain further insights into the toxic mechanism of ROS-mediated mitophagy and apoptosis in male germ cells induced by AlCl3 exposure. Moreover, it offers a theoretical basis and new ideas for further research on the mechanisms and preventive strategies against AlCl3-induced damage to the male reproductive system.
Materials and Methods
Experimental Materials
The GC-2spd cell line was obtained from Chinese Academy of Sciences (SCSP-5055); aluminum trichloride hexahydrate (AlCl3-6H2O) was purchased from Shanghai Aladdin Biochemical Technology Company (L1706080); ATP assay kit (S0026), reactive oxygen species kit (S0033S), mitochondrial membrane potential as say kit (C2006)were purchased from China Biyuntian Biotechnology Co., Ltd; TUNEL kit(E-CK-A322)was purchased from Elabscience; BAX (60267-1-Ig,1:5000), BCL-2 (2653-1-AP,1:1000), Cleaved caspased-9 (66169-1-Ig,1:1000),, Cyt-c (66264-1-Ig,1:1000), LC3B (14600-1-AP, 1:1000)were purchased from Wuhan Sanying; Cleaved-caspased-9 (#9664,1:1000),P62 (#5114,1:1000), Parkin (#2132,1:1000)were purchased from CST. Pink1 (PA5-18770,1:1000) were purchased from Thermo Fisher. BAX, Cleaved-caspased-9, Cyt-c were murine antibodies, BCL-2, LC3B, Pink1, Cleaved-caspased-3, Parkin, P62 were rabbit antibodies.
Animal Experiments and Grouping
Prior to the experiment, a total of 24 male Wistar rats weighing 180–200 g and aged 8 weeks were given one week to acclimate. Following the experimental methods outlined by Xu et al. [28], the rats were randomly assigned to four groups (six rats per group), including a control group and three groups exposed to different doses (64.18, 128.36, and 256.72 mg/kg) of AlCl3(This dose was based on the median lethal dose of rats with a rat mortality rate of 5%, 10% and 20%). The room was set at 25 ℃ ± 2 ℃ with a 12-h light/dark cycle for 16 weeks, which is roughly equivalent to two spermatogenic cycles of rats. At the end of the feeding period, rats were sacrificed by spinal dislocation and testicular tissue was obtained, A portion of the testicular tissue was fixed in a 4% paraformaldehyde (PFA) solution for histopathological evaluation, while another part of the testicular tissue was frozen in liquid nitrogen and stored at -80℃ for subsequent Western blot analysis. The remaining tissue was left unchanged. Al-containing water for daily consumption of rats was prepared by dissolving a predetermined amount of AlCl3 in drinking water and stored at ambient temperature after shaking for a complete dissolution. The feeding diet was standard rat feed [12]. Animal experiments in this study received approval from the Laboratory Animal Care and Use Ethics Committee of the You-jiang Medical University for Nationalities.
Detection of Aluminum Content in Testicular Tissue by ICP-AES Spectrometer
Testicular tissue was collected and placed into a container. Then, 5.0 ml of nitric acid was added, and the mixture was dried at low temperature. Next, 1.0 ml of perchloric acid was added until white smoke appeared, resulting in a colorless solution. A 10% (v/v) hydrochloric acid solution was transferred into a 10 ml measuring bottle with a fixed volume. Three blank tests were conducted alongside the sample treatment, and the aluminum content was determined using Inductively Coupled Plasma-Atomic Emission Spectroscopy (ICP-AES) under the specified working conditions.
Cell Culture and Grouping
Exponentially growing cells were seeded in 6-well plates and incubated until 75% confluency. The cells were then treated with media containing different concentrations of AlCl3, including 0 mM (control), 1 mM, 2 mM, and 4 mM. The selection of cell culture cycle and cell aluminum concentration was determined according to the previous study [11]. The dose of NAC was determined based on a previous study by Liu et al. [29]. The GC-2spd cells treatments were divided into four groups: 0 mM AlCl3 (0 group), 5 mM NAC (NAC group), 4 mM AlCl3 (AL group), and 4 mM AlCl3 + 5 mM NAC (NAL group). These groups were cultured until they reached 75% confluence and subjected to subsequent experiments 24 h after treatment.
Intracellular ROS Detection
For intracellular ROS detection, 6-well plates were seeded with 3 × 105 cells per well and incubated at 37℃ in a 5% CO2 incubator for 24 h. The cells were then treated with the pre-prepared AlCl3 and NAC solutions according to the pre-defined groups mentioned earlier. After incubating for 24 h, the cells were collected and incubated in a diluted solution of 2',7'-dichlorodihydrofluorescein diacetate (DCFH-DA) for 20 min at 37 °C. The cells were gently inverted every 3–5 min to promote interaction between the probe and the cells. The cells were then washed three times using serum-free culture medium to remove any excess DCFH-DA outside the cells [30]. Finally, the measurements were taken using a (VICTOR NIVO 3F) multimode microplate reader.
Cell Viability Assay
Cell viability was assessed using the cell counting kit-8 (CCK-8) solution, a commonly used reagent for cell viability assay. Cells were plated at a density of 5 × 103 cells/well in a 96-well plate and incubated at 37℃ with 5% CO2 for 24 h. The cells were then treated with the pre-prepared AlCl3 and NAC solutions based on the pre-defined groups mentioned earlier. After 24 h of incubation, CCK-8 solution (10 μL) was added to each well and incubated at 37℃ for 2 hours [31]. The absorbance at 560 nm was measured using a multimode microplate reader (VICTOR NIVO 3F).
Detection of Adenosine Triphosphate (ATP) Production in Cells
Cell seeding plate and intervention methods were the same as (Intracellular ROS detection).After 24 h, the medium was aspirated and discarded. To fully lyse the cells, 200 μL of lysis buffer was added to each well. The cells were harvested and centrifuged at 12,000 × g for 5 min to collect the supernatant [32]. The relative light unit (RLU) value was determined using a multimode microplate reader (VICTOR NIVO 3F).
Assessment of Cellular Mitochondrial Membrane Potential (MMP)
Cells were cultured following the procedures described in the ROS detection method. The cells were then treated with the pre-prepared AlCl3 and NAC solutions based on the pre-defined groups mentioned earlier. After 24 h of incubation, the cells were washed once with PBS and incubated with 1 mL of JC-1 staining solution for 30 min in a 37℃ incubator [33]. After two washes with JC-1 buffer, the cells were cultured in a serum-free medium and imaged under a laser scanning confocal microscope (Stellaris 5, Leica, Wetzlar, Germany). The fluorescence intensity was analyzed using ImageJ v 1.8.0.
Cell Apoptosis was Detected by TUNEL Assay
A total of 5 × 104 cells were seeded in confocal dishes. The cells were then treated with the pre-prepared AlCl3 and NAC solutions according to the pre-defined groups mentioned earlier. Cells were incubated for 24 h, rinsed with PBS twice, fixed in 4% PFA for 20 min, then washed in PBS three more times before being permeabilized for 10 min. Following three PBS washes, the cells were treated with TdT equilibration buffer (100 μL per dish) at 37℃ for 30 min. The cells were washed three times with PBS, and a fluorescent quenching agent, 4',6-diamidino-2-phenylindole (DAPI), was added [34]. Cell apoptosis was imaged and analyzed using a laser confocal microscope (Stellaris 5, Leica, Wetzlar, Germany) after 10 min incubation.
Western Blot Detection of Mitophagy and Cell Apoptosis-Related Proteins
Rat testes were ground using a tissue grinder and mixed with the appropriate proportion of protein lysis buffer. For cells treated in the six-well plates described above, 200ul of protein lysate was added to each well to extract total protein. The samples were subjected to gel electrophoresis (30 µg per well) and then transferred to membranes. The membranes were blocked with 5% skimmed milk powder for 2 h, followed by overnight incubation with various primary antibodies. After washing the membranes, a secondary antibody was added and incubated at room temperature for 1 h before another round of washing. Finally, the protein bands were visualized using an (Amersham Imager 600) chemiluminescent imaging system and their grayscale values were analyzed using ImageJ v1.8.0.
Statistical Analysis
Data analysis was performed using SPSS 25. For comparison among multiple groups, one-way ANOVA was used. Prior to analysis, normality test and homogeneity analysis of variance were conducted. If the variance was homogeneous, the SNK-q test was used. In cases where the variance was not uniform, the Games-Howell test was employed. The test level was set at a = 0.05. Graphpad Prism (version 9.0) was utilized for plotting the data. Fluorescence intensity was analyzed using Image Jv1.8.0.
Results
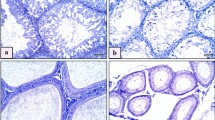
Aluminum Content in Rat Testicular Tissue after Exposure to AlCl3
Results showed that aluminum deposition increased in testicular tissue with increasing AlCl3 exposure concentration. Moreover, there was a statistically significant difference between the medium-dose group and the high-dose group (P = 0.0016, P < 0.001) (Fig. 1).
Effect of AlCl3 Exposure on Mitophagy and Cell Apoptosis in Rat Testicular Tissues
We investigated the impact of AlCl3 exposure on mitophagy in rat testicular tissues through Western blot analysis. It was observed that as the AlCl3 exposure concentration increased, all indexes in the high-dose exposure group showed statistical significance when compared to the control group. The expressions of PARKIN (P < 0.001), PINK1 (P = 0.018), and LCB (P = 0.019) were upregulated, while the levels of autophagy substrate P62 (P = 0.007) were decreased. We also investigated the changes in apoptotic protein levels in rat testicular tissue and observed that the expression of apoptotic proteins increased with higher concentrations of AlCl3 exposure, as shown in Fig. 2A. In comparison to the control group, the high-dose group exhibited upregulation of apoptosis-related proteins BAX (P = 0.023), Cleaved-caspased-3 (P < 0.001), cleaved-Casasded-9 (P = 0.004), and Cyt-c (P = 0.041). Additionally, the anti-apoptotic protein BCL-2 (P = 0.048) showed downregulation (Fig. 2A–J).
Western blot analysis of mitophagy and cell apoptosis-related proteins in testicular tissues of rats exposed to different concentrations of AlCl3. A Western blots of the mitophagy and cell apoptosis-related proteins in testicular tissues of AlCl3-exposed rats. B–J Quantitative analysis of protein levels of mitophagy and cell apoptosis-related proteins in testicular tissues of AlCl3-exposed rats. (C: Control Group. L: Low Dose AlCl3 Exposure Group. M: Medium Dose AlCl3 Exposure Group. H: High Dose AlCl3 Exposure Group. * P < 0.05, ** P < 0.01, *** P < 0.001)
Effect of AlCl3 Exposure and NAC Intervention on ROS Accumulation in GC-2spd Cells
After the intervention of GC-2spd with different concentrations of AlCl3, it was observed in Fig. 3A that the production of ROS changed. The production of ROS was higher in each AlCl3-exposed group compared to the control group, and it increased with increasing AlCl3 concentration. The highest ROS production was observed at 4 mM (P < 0.001). NAC, an oxidative stress inhibitor, effectively suppressed the production of ROS. In Fig. 4A, it was found that NAC successfully inhibited the ROS production induced by AlCl3 in spermatocytes, and the ROS production in the NAC group was significantly lower than in the AlCl3 group (P = 0.03).
Impact of AlCl3 exposure on various parameters in GC-2spd cells. A Changes in ROS production in GC-2spd after AlCl3 exposure. B Changes in cell viability in GC-2spd after AlCl3 exposure. C Changes in ATP production in GC-2spd after AlCl3 exposure. D Quantitative analysis of MMP in GC-2spd after AlCl3 exposure. E Representative image showing changes in MMP in GC-2spd after AlCl3 exposure. F TUNEL-positive apoptotic cells in GC-2spd after AlCl3 exposure. (* P < 0.05, ** P < 0.01, *** P < 0.001)
Impact of AlCl3 exposure and NAC intervention on various parameters in GC-2spd cells. A Changes in ROS production in response to NAC intervention in AlCl3-exposed GC-2spd. B Changes in cell viability in response to NAC intervention in AlCl3-exposed GC-2spd. C Changes in ATP production in response to NAC intervention in AlCl3-exposed GC-2spd. D Quantitative analysis of MMP in response to NAC intervention in AlCl3-exposed GC-2spd. E Image of MMP changes in response to NAC intervention in AlCl3-exposed GC-2spd. F TUNEL-positive apoptotic cells in response to NAC intervention in AlCl3-exposed GC-2spd. (* P < 0.05, ** P < 0.01, *** P < 0.001)
Effect of AlCl3 Exposure and NAC Intervention on Proliferation and ATP Production in GC-2spd Cells
We conducted an initial evaluation of the harmful effects of AlCl3 on GC-2spd cells using CCK-8 assay, and discovered that AlCl3 exposure led to a reduction in the viability of GC-2spd cells (Fig. 3B). In comparison to the control group, the viability of cells in the 1 mM, 2 mM, and 4 mM groups decreased substantially (P = 0.015, P = 0.014, P < 0.001), and this reduction was attributed to the ability of AlCl3 to hinder the proliferation of GC-2spd cells, in accordance with the nature criteria. Additionally, the production of ATP also decreased in a dose-dependent manner with the most significant reduction observed at a concentration of 4 mM (P < 0.001) (Fig. 3C). Following the intervention with NAC, cell viability was restored and cell ATP production was found to be increased compared to the AlCl3 exposure group. In Fig. 4B, it was observed that cell viability was improved in the NAC group compared to the AlCl3 group (P = 0.014), while Fig. 4C showed an increase in ATP production (P = 0.037).
Effect of AlCl3 Exposure and NAC Intervention on MMP in GC-2spd Cells
JC-1 is a fluorescent dye that indicates MMP. When it aggregates in the mitochondrial matrix, it emits red fluorescence, indicating high MMP. Conversely, when MMP decreases, it emits green fluorescence, indicating a reduction in MMP. Compared with the control group (Fig. 3D–E), the AlCl3-exposed GC-2spd cells showed a decrease in the ratio of red fluorescence to green fluorescence, indicating reduced MMP. We observed significant reductions in MMP in response to AlCl3 exposure at concentrations of 1 mM (P > 0.05), 2 mM (P > 0.05), and 4 mM (P = 0.025). The decrease in MMP was concentration-dependent. Following NAC intervention, we observed an increase in MMP in the NAL group compared to the AL group (P = 0.039) (Fig. 4D–E), indicating the restoration of mitochondrial function of GC-2spd cells after NAC intervention.
Effect of AlCl3 Exposure and NAC Intervention on Apoptosis of GC-2spd Cells
The localization of red fluorescence, representing apoptotic bodies, and blue fluorescence, symbolizing the cell nucleus, increased with the rising concentration of AlCl3, indicating a negative impact on cellular survival. Among the tested concentrations, the most significant reduction in cell survival was observed at an AlCl3 concentration of 4 mM, as depicted in Fig. 3F. Subsequent treatment with NAC resulted in a decrease in apoptotic bodies in Fig. 4F, compared to the AL group, suggesting a protective effect of NAC against apoptosis.
The Impact of AlCl3 Exposure and NAC Intervention on Proteins Related to Mitophagy and Cell Apoptosis in GC-2spd Cells
Proteins related to mitophagy and apoptosis were detected in GC-2spd cells following exposure to AlCl3 and intervention with NAC. The results indicated that AlCl3 exposure activated mitophagy and apoptosis in spermatocytes, as compared to the control group (Fig. 5A–J) and the 4 mM AlCl3 exposure group. The expression levels of Parkin (P < 0.001), Pink1 (P < 0.001), LC3B (P < 0.001), BAX (P = 0.0027), Cleaved-caspased-3 (P = 0.031), Cleaved-caspased-9 (P = 0.017), and Cyt-c were upregulated (P < 0.05). P62 (P = 0.027) and BCL-2 (P = 0.026) decreased in a concentration-dependent manner. Treatment of AlCl3-exposed GC-2spd cells with NAC (Fig. 6A–J) reduced AlCl3-induced mitophagy and apoptosis. Compared to the AlCl3-treated group (AL), the NAC-treated group (NAL) showed decreased expression of Parkin (P = 0.047), Pink1 (P = 0.017), LC3B (P = 0.017), BAX (P = 0.038), Cleaved-caspased-3 (P = 0.018), Cleaved-caspased-9 (P = 0.048), and Cyt-c proteins (P < 0.05). P62 (P = 0.0016) and the expression of BCL-2 were upregulated (P = 0.012).
The effects of AlCl3 exposure and NAC intervention on the proteins related to mitophagy and cell apoptosis of GC-2spd cells. A Western blots of the mitophagy and cell apoptosis-related proteins in response to NAC intervention in AlCl3-exposed GC-2spd. B–J Quantitative analysis of protein levels of mitophagy and cell apoptosis-related proteins in response to NAC intervention in AlCl3-exposed GC-2spd. (* P < 0.05, ** P < 0.01, *** P < 0.001)
Discussion
AlCl3 is widely demanded in various fields, but its notable neurotoxicity, cardiotoxicity, and reproductive toxicity have impeded its application. Previous studies have reported that Al exposure can induce apoptosis in astrocytes and rat cardiomyocytes [35]. The present study aims to investigate the potential mechanisms behind male reproductive toxicity caused by exposure to AlCl3. Our findings demonstrate that aluminum accumulates in testis tissue and triggers mitophagy and apoptosis in both testis tissue and spermatocytes of rats. This is evidenced by the upregulation of PARKIN, PINK1, and LC3B expression, as well as the downregulation of P62 expression. Additionally, the expression of apoptosis-related proteins such as BAX, Cleaved-caspased-9, Cyt-c, and Cleaved-caspased-3 was found to increase, while the expression of BCL-2 decreased. Moreover, exposure to AlCl3 was observed to inhibit spermatocyte viability, increase ROS production, reduce ATP production, and decrease mitochondrial membrane potential. In our cell model, treatment with NAC effectively reduced ROS production, enhanced cell viability, ATP production, and mitochondrial membrane potential, and suppressed mitophagy and apoptosis. These results strongly suggest that ROS plays a significant role in AlCl3-induced GC-2spd toxicity.
The disruption of cellular redox balance by increased ROS has been widely recognized as a crucial factor leading to oxidative stress-induced apoptosis [36]. Furthermore, elevated ROS production can also induce mitochondrial dysfunction, resulting in impaired mitochondrial oxidative phosphorylation and reduced ATP production. In our study, exposure to AlCl3 resulted in a decrease in ATP production and cell viability in spermatocytes. This decrease may be attributed to the increase in ROS. However, treatment with the antioxidant NAC successfully mitigated the AlCl3-induced increase in ROS levels, leading to the recovery of cell viability and ATP production. This finding is consistent with a previous study that investigated the effects of polystyrene microparticles and nanoparticles on neuronal cells. In that study, cell viability decreased and ROS production increased, but treatment with NAC effectively restored cell viability and reduced ROS levels [37]. ATP is a source of energy produced by the inner mitochondrial membrane transition of protons that generates a transmembrane potential [38]. ATP serves as the source of proton transfer across the inner mitochondrial membrane, leading to the formation of a transmembrane potential [39]. This potential plays a crucial role in maintaining energy supply and preventing apoptosis. When mitochondrial function is impaired, ATP levels decrease, resulting in a reduction in mitochondrial membrane potential and ultimately leading to cell apoptosis. Additionally, the decrease in mitochondrial membrane potential leads to an upregulation of the pro-apoptotic gene Bax and a downregulation of the anti-apoptotic gene Bcl-2. Our in vitro and in vivo studies revealed that exposure to AlCl3 increased apoptosis in testicular tissue and GC-2spd cells. This exposure also resulted in an increase in the expression of Bax, Cleaved-caspased-3, Cleaved-caspased-9, and Cyt-c, while decreasing the expression of the anti-apoptotic protein BCL-2. TUNEL results further demonstrated an increase in the production of apoptotic bodies in GC-2spd cells exposed to AlCl3. Interestingly, we observed that NAC treatment inhibited AlCl3-induced mitochondrial pathway apoptosis in vitro. This inhibition was accompanied by a downregulation of related protein expression and an increase in the expression of the anti-apoptotic protein BCL-2. The occurrence of apoptosis in both ammonia treated porcine IPEC-J2 intestinal epithelial cells and diisononyl phthalate treated ovarian granulosa cells can be inhibited by NAC treatment [40, 41]. Additionally, the use of NAC can also inhibit the apoptosis induced by organic arsenic in gastric cancer cells [42]. These findings align with and further support the reliability of our results.
The excessive production of ROS can lead to mitochondrial dysfunction. When mitochondria are damaged, they undergo depolarization and activate mitophagy. Mitophagy is an important pathway that eliminates damaged mitochondria, protecting cells from excessive oxidative stress and cell death by selectively degrading dysfunctional mitochondria [43, 44]. The activation of mitophagy has been observed to be involved in the reproductive toxicity caused by different pollutants [45, 46]. Our findings revealed an upregulation of Parkin and PINK1 expression with increasing concentrations of AlCl3 exposure. Furthermore, we observed an increase in the expression of the autophagy marker protein LC3B and a decrease in p62 expression, an autophagy substrate, indicating the activation of autophagy. Molybdenum and cadmium disrupt mitochondrial function in the duck hypothalamus and trigger mitophagy [46, 47]. In hepatocytes, fluoride exposure can also induce mitophagy by damaging mitochondria [48]. Also, DEHP can cause damage to MC3T3-E1 cells by increasing ROS generation and activating Parkin/PINK1-mediated mitophagy [49]. Furthermore, we conducted additional experiments by treating GC-2spd with NAC. We observed a decrease in the expression of PARKIN, PINK1, and LC3B, while the autophagy substrate P62 showed an increase after NAC treatment. Interestingly, we also found that NAC intervention can mitigate the mitophagy and apoptosis induced by fluoride exposure in bone cells [24]. In a separate study, it was discovered that Imidacloprid (IMI), a widely used neonicotinoid compound in agricultural production, can induce mitophagy in hepatocytes. However, the intervention of NAC was able to reverse this effect [50]. Consistent with these previous findings, our results indicate that the toxicity of AlCl3 on GC-2spd is attributed to ROS-mediated mitophagy.
Based on previous studies, we hypothesized that mitophagy could potentially serve as a novel mechanism in mediating AlCl3-induced spermatocyte death. However, there are still several unknown mechanisms that need to be investigated. Mitophagy is a double-edged sword, as moderate levels of mitophagy can be beneficial for cell survival, while excessive levels can trigger apoptosis. For instance, in acrylamide exposed Purkinje cells, activation of mitophagy has been shown to prevent cell apoptosis [51], whereas in DEHP exposed cells, inhibition of mitophagy can reduce cell death [33]. These findings highlight the complexity of the relationship between mitophagy and apoptosis, which may be influenced by factors such as the duration and intensity of mitophagy activation. Further research is needed to fully understand this relationship. In our study, we observed that AlCl3 exposure led to the activation of mitophagy and apoptosis, which could be reversed by using NAC. However, the specific relationship between mitophagy and apoptosis in GC-2spd cells induced by AlCl3 exposure has not been explored in depth. Therefore, we plan to investigate this relationship by utilizing mitophagy activators and inhibitors. Our study aims to contribute to the understanding of the male reproductive toxicity caused by AlCl3 (Fig. 7).
Conclusion
To sum up, our study unveiled that AlCl3 exposure could activate mitophagy and cell apoptosis in rat testes. Moreover, AlCl3 exposure increased ROS production, decreased cell viability and ATP production, as well as mitochondrial dysfunction, autophagy activation, and cell apoptosis. However, intervention with NAC could reverse the cytotoxicity caused by AlCl3 and curb mitophagy and cell apoptosis. However, intervention with NAC effectively reversed the cytotoxic effects on GC-2spd cells induced by AlCl3 and inhibited mitophagy and cell apoptosis. Overall, this research provides a foundational framework for investigating the reproductive toxicity associated with AlCl3 exposure.
Data Availability
All data will be provided upon request.
References
Carson SA, Kallen AN (2021) Diagnosis and management of Infertility: A review. JAMA 326:65–76. https://doi.org/10.1001/jama.2021.4788
Agarwal A, Baskaran S, Parekh N, Cho CL, Henkel R, Vij S, Arafa M, PannerSelvam MK, Shah R (2021) Male infertility. Lancet (London, England) 397:319–333. https://doi.org/10.1016/s0140-6736(20)32667-2
Klotz K, Weistenhöfer W, Neff F, Hartwig A, van Thriel C, Drexler H (2017) The health effects of aluminum exposure. Deutsches Arzteblatt international 114:653–659. https://doi.org/10.3238/arztebl.2017.0653
Nie J (2018) Exposure to aluminum in daily life and Alzheimer’s disease. Adv Exp Med Biol 1091:99–111. https://doi.org/10.1007/978-981-13-1370-7_6
Huston RK, Heisel CF, Vermillion BR, Christensen JM, Minc L (2017) Aluminum content of neonatal parenteral nutrition solutions. Nutr Clin Pract: Off Publ Am Soc Parenteral Enteral Nutr 32:266–270. https://doi.org/10.1177/0884533616668789
Wang YU, Lv H, Lan J, Zhang X, Zhu K, Yang S, Lv S (2022) Detection of sodium formaldehyde sulfoxylate, aluminum, and borate compounds in bread and pasta products consumed by residents in Jilin Province, China. J Food Protect 85:1142–1147. https://doi.org/10.4315/jfp-22-011
Hao W, Hao C, Wu C, Xu Y, Wu S, Lu X, Yang J, Jin C (2021) Aluminum impairs cognitive function by activating DDX3X-NLRP3-mediated pyroptosis signaling pathway. Food Chem Toxicol: Int J Publ Br Ind Biol Res Assoc 157:112591. https://doi.org/10.1016/j.fct.2021.112591
Laabbar W, Abbaoui A, Elgot A, Mokni M, Amri M, Masmoudi-Kouki O, Gamrani H (2021) Aluminum induced oxidative stress, astrogliosis and cell death in rat astrocytes, is prevented by curcumin. J Chem Neuroanat 112:101915. https://doi.org/10.1016/j.jchemneu.2020.101915
Hao W, Hao C, Wu C, Xu Y, Jin C (2022) Aluminum induced intestinal dysfunction via mechanical, immune, chemical and biological barriers. Chemosphere 288:132556. https://doi.org/10.1016/j.chemosphere.2021.132556
Olanrewaju JA, Akinpade TG, Olatunji SY, Owolabi JO, Enya JI, Adelodun ST, Fabiyi SO, Desalu AB (2021) Observable protective activities of quercetin on aluminum chloride-induced testicular toxicity in adult male Wistar rat. J Hum Reprod Sci 14:113–120. https://doi.org/10.4103/jhrs.jhrs_190_20
Huixin P, Guangji W, Yanxin H, Yanfang P, Huixiong Y, Xiong Z, Yu’an X, Wencheng C (2023) Transcriptome-based analysis of the toxic effects of aluminum chloride exposure on spermatocytes. Toxicol In Vitro: Int J Publ Assoc BIBRA 92:105658. https://doi.org/10.1016/j.tiv.2023.105658
Peng H, Huang Y, Wei G, Pang Y, Yuan H, Zou X, Xie Y, Chen W (2023) Testicular toxicity in rats exposed to AlCl(3): a proteomics study. Biol Trace Elem Res. https://doi.org/10.1007/s12011-023-03745-6
Olawuyi TS, Ukwenya VO, Jimoh AGA, Akinola KB (2019) Histomorphometric evaluation of seminiferous tubules and stereological assessment of germ cells in testes following administration of aqueous leaf-extract of Lawsonia inermis on aluminium-induced oxidative stress in adult Wistar rats. JBRA Assist Reprod 23:24–32. https://doi.org/10.5935/1518-0557.20180080
Maghraoui S, Florea A, Ayadi A, Matei H, Tekaya L (2022) Histological and ultrastructural changes observed in testicles, epididymides, seminal vesicles and liver of rat after intraperitoneal administration of aluminum and indium. J Trace Elem Med Biol: Organ Soc Minerals Trace Elem (GMS) 73:126997. https://doi.org/10.1016/j.jtemb.2022.126997
Alizadeh B, Salehzadeh A, Ranji N, Arasteh A (2022) Effects of N-Acetyl cysteine on genes expression of c-myc, and Ask-1, histopathological, oxidative stress, inflammation, and apoptosis in the liver of male rats exposed to cadmium. Biol Trace Elem Res 200:661–668. https://doi.org/10.1007/s12011-021-02670-w
Wang L, Cao J, Chen D, Liu X, Lu H, Liu Z (2009) Role of oxidative stress, apoptosis, and intracellular homeostasis in primary cultures of rat proximal tubular cells exposed to cadmium. Biol Trace Elem Res 127:53–68. https://doi.org/10.1007/s12011-008-8223-7
Jakubczyk K, Dec K, Kałduńska J, Kawczuga D, Kochman J, Janda K (2020) Reactive oxygen species - sources, functions, oxidative damage. Polski merkuriusz lekarski: organ Polskiego Towarzystwa Lekarskiego 48:124–127
Wang Y, Wang X, Wang L, Cheng G, Zhang M, Xing Y, Zhao X, Liu Y, Liu J (2021) Mitophagy induced by mitochondrial function damage in chicken kidney exposed to Cr(VI). Biol Trace Elem Res 199:703–711. https://doi.org/10.1007/s12011-020-02176-x
Yin J, Ni B, Tian ZQ, Yang F, Liao WG, Gao YQ (2017) Regulatory effects of autophagy on spermatogenesis. Biol Reprod 96:525–530. https://doi.org/10.1095/biolreprod.116.144063
Shiba-Fukushima K, Imai Y, Yoshida S, Ishihama Y, Kanao T, Sato S, Hattori N (2012) PINK1-mediated phosphorylation of the Parkin ubiquitin-like domain primes mitochondrial translocation of Parkin and regulates mitophagy. Sci Rep 2:1002. https://doi.org/10.1038/srep01002
Vives-Bauza C, Zhou C, Huang Y, Cui M, de Vries RL, Kim J, May J, Tocilescu MA, Liu W, Ko HS, Magrané J, Moore DJ, Dawson VL, Grailhe R, Dawson TM, Li C, Tieu K, Przedborski S (2010) PINK1-dependent recruitment of Parkin to mitochondria in mitophagy. Proc Natl Acad Sci USA 107:378–383. https://doi.org/10.1073/pnas.0911187107
Ordureau A, Sarraf SA, Duda DM, Heo JM, Jedrychowski MP, Sviderskiy VO, Olszewski JL, Koerber JT, Xie T, Beausoleil SA, Wells JA, Gygi SP, Schulman BA, Harper JW (2014) Quantitative proteomics reveal a feedforward mechanism for mitochondrial PARKIN translocation and ubiquitin chain synthesis. Mol Cell 56:360–375. https://doi.org/10.1016/j.molcel.2014.09.007
Anand H, Misro MM, Sharma SB, Prakash S (2015) Protective effects of Eugenia jambolana extract versus N-acetyl cysteine against cisplatin-induced damage in rat testis. Andrologia 47:194–208. https://doi.org/10.1111/and.12247
Zhang Y, Dong F, Wang Z, Xu B, Zhang T, Wang Q, Lin Q (2023) Fluoride exposure provokes mitochondria-mediated apoptosis and increases mitophagy in osteocytes via increasing ROS production. Biol Trace Elem Res 201:3994–4007. https://doi.org/10.1007/s12011-022-03450-w
Lin YC, Lin YC, Tsai ML, Liao WT, Hung CH (2022) TSLP regulates mitochondrial ROS-induced mitophagy via histone modification in human monocytes. Cell Biosci 12:32. https://doi.org/10.1186/s13578-022-00767-w
Bai H, Yang F, Jiang W, Hu A, Chang H, Zhang Y, Jiang L, Lin S, Lu Z, Zhang C, Cao H (2021) Molybdenum and cadmium co-induce mitophagy and mitochondrial dysfunction via ROS-mediated PINK1/Parkin pathway in Hepa1–6 cells. Ecotoxicol Environ Saf 224:112618. https://doi.org/10.1016/j.ecoenv.2021.112618
Wang X, Tian X, Yan H, Zhu T, Ren H, Zhou Y, Zhao D, Xu D, Lian X, Fang L, Yu Y, Liao X, Liu Y, Sun J (2023) Exposure to salinomycin dysregulates interplay between mitophagy and oxidative response to damage the porcine jejunal cells. Sci Total Environ: 166441. https://doi.org/10.1016/j.scitotenv.2023.166441
Xu F, Liu Y, Zhao H, Yu K, Song M, Zhu Y, Li Y (2017) Aluminum chloride caused liver dysfunction and mitochondrial energy metabolism disorder in rat. J Inorg Biochem 174:55–62. https://doi.org/10.1016/j.jinorgbio.2017.04.016
Liu M, Wu X, Cui Y, Liu P, Xiao B, Zhang X, Zhang J, Sun Z, Song M, Shao B, Li Y (2021) Mitophagy and apoptosis mediated by ROS participate in AlCl(3)-induced MC3T3-E1 cell dysfunction. Food Chem Toxicol: Int J Publ Br Ind Biol Res Assoc 155:112388. https://doi.org/10.1016/j.fct.2021.112388
Hao H, Cao L, Jiang C, Che Y, Zhang S, Takahashi S, Wang G, Gonzalez FJ (2017) Farnesoid X receptor regulation of the NLRP3 inflammasome underlies cholestasis-associated sepsis. Cell Metab 25:856-867.e855. https://doi.org/10.1016/j.cmet.2017.03.007
Huang P, Zhang W, Ji J, Ma J, Cheng H, Qin M, Wei D, Ren L (2023) LncRNA Miat knockdown protects against pirarubicin-induced cardiotoxicity by targeting miRNA-129-1-3p. Environ Toxicol. https://doi.org/10.1002/tox.23910
Huang M, Ivantsova E, Konig I, Patel N, English C, Souders CL 2nd, Martyniuk CJ (2023) Developmental and mitochondrial toxicity assessment of perfluoroheptanoic acid (PFHpA) in zebrafish (Danio rerio). Environ Toxicol Pharmacol 97:104037. https://doi.org/10.1016/j.etap.2022.104037
Xu J, Wang L, Zhang L, Zheng F, Wang F, Leng J, Wang K, Héroux P, Shen HM, Wu Y, Xia D (2021) Mono-2-ethylhexyl phthalate drives progression of PINK1-parkin-mediated mitophagy via increasing mitochondrial ROS to exacerbate cytotoxicity. Redox Biol 38:101776. https://doi.org/10.1016/j.redox.2020.101776
Mandil R, Prakash A, Rahal A, Koli S, Kumar R, Garg SK (2023) Evaluation of oxidative stress-mediated cytotoxicity and genotoxicity of copper and flubendiamide: amelioration by antioxidants in vivo and in vitro. Toxicol Res 12:232–252. https://doi.org/10.1093/toxres/tfad011
Zhou L, He M, Li X, Lin E, Wang Y, Wei H, Wei X (2022) Molecular mechanism of aluminum-induced oxidative damage and apoptosis in rat cardiomyocytes. Biol Trace Elem Res 200:308–317. https://doi.org/10.1007/s12011-021-02646-w
Marrocco I, Altieri F, Peluso I (2017) Measurement and clinical significance of biomarkers of oxidative stress in humans. Oxid Med Cell Longev 2017:6501046. https://doi.org/10.1155/2017/6501046
Yang D, Zhu J, Zhou X, Pan D, Nan S, Yin R, Lei Q, Ma N, Zhu H, Chen J, Han L, Ding M, Ding Y (2022) Polystyrene micro- and nano-particle coexposure injures fetal thalamus by inducing ROS-mediated cell apoptosis. Environ Int 166:107362. https://doi.org/10.1016/j.envint.2022.107362
de la Fuente-Herreruela D, Gónzalez-Charro V, Almendro-Vedia VG, Morán M, Martín M, Lillo MP, Natale P, López-Montero I (2017) Rhodamine-based sensor for real-time imaging of mitochondrial ATP in living fibroblasts. Biochim Biophys Acta 1858:999–1006. https://doi.org/10.1016/j.bbabio.2017.09.004
Fang H, Wu Y, Guo J, Rong J, Ma L, Zhao Z, Zuo D, Peng S (2012) T-2 toxin induces apoptosis in differentiated murine embryonic stem cells through reactive oxygen species-mediated mitochondrial pathway. Apoptosis: Int J Program Cell Death 17:895–907. https://doi.org/10.1007/s10495-012-0724-3
Chen J, Yang S, Ma B, Wang J, Chen J (2022) Di-isononyl phthalate induces apoptosis and autophagy of mouse ovarian granulosa cells via oxidative stress. Ecotoxicol Environ Saf 242:113898. https://doi.org/10.1016/j.ecoenv.2022.113898
Huang Y, Mo S, Jin Y, Zheng Z, Wang H, Wu S, Ren Z, Wu J (2022) Ammonia-induced excess ROS causes impairment and apoptosis in porcine IPEC-J2 intestinal epithelial cells. Ecotoxicol Environ Saf 243:114006. https://doi.org/10.1016/j.ecoenv.2022.114006
Li Y, She W, Guo T, Huang T, Liu Y, Liu P, Xu X, Wang X, Wang M, Yu C, Liu Y, Wei Y (2023) The organic arsenical-derived thioredoxin and glutathione system inhibitor ACZ2 induces apoptosis and autophagy in gastric cancer via ROS-dependent ER stress. Biochem Pharmacol 208:115404. https://doi.org/10.1016/j.bcp.2022.115404
Kang L, Liu S, Li J, Tian Y, Xue Y, Liu X (2020) Parkin and Nrf2 prevent oxidative stress-induced apoptosis in intervertebral endplate chondrocytes via inducing mitophagy and anti-oxidant defenses. Life Sci 243:117244. https://doi.org/10.1016/j.lfs.2019.117244
Zhang T, Wu P, Budbazar E, Zhu Q, Sun C, Mo J, Peng J, Gospodarev V, Tang J, Shi H, Zhang JH (2019) Mitophagy reduces oxidative stress via Keap1 (Kelch-Like Epichlorohydrin-Associated Protein 1)/Nrf2 (Nuclear Factor-E2-Related Factor 2)/PHB2 (Prohibitin 2) pathway after subarachnoid hemorrhage in rats. Stroke 50:978–988. https://doi.org/10.1161/strokeaha.118.021590
Yi L, Shang XJ, Lv L, Wang Y, Zhang J, Quan C, Shi Y, Liu Y, Zhang L (2022) Cadmium-induced apoptosis of Leydig cells is mediated by excessive mitochondrial fission and inhibition of mitophagy. Cell Death Dis 13:928. https://doi.org/10.1038/s41419-022-05364-w
Liu T, Hou B, Zhang Y, Wang Z (2022) Determination of biological and molecular attributes related to polystyrene microplastic-induced reproductive toxicity and its reversibility in male mice. Int J Environ Res Public Health 19. https://doi.org/10.3390/ijerph192114093
Cui T, Jiang W, Yang F, Luo J, Hu R, Cao H, Hu G, Zhang C (2021) Molybdenum and cadmium co-induce hypothalamus toxicity in ducks via disturbing Nrf2-mediated defense response and triggering mitophagy. Ecotoxicol Environ Saf 228:113022. https://doi.org/10.1016/j.ecoenv.2021.113022
Zhao Y, Wang J, Zhang J, Sun Z, Niu R, Manthari RK, Ommati MM, Wang S, Wang J (2022) Fluoride exposure induces mitochondrial damage and mitophagy via activation of the IL-17A pathway in hepatocytes. Sci Total Environ 804:150184. https://doi.org/10.1016/j.scitotenv.2021.150184
Cui Y, Li B, Du J, Huo S, Song M, Shao B, Wang B, Li Y (2022) Dibutyl phthalate causes MC3T3-E1 cell damage by increasing ROS to promote the PINK1/Parkin-mediated mitophagy. Environ Toxicol 37:2341–2353. https://doi.org/10.1002/tox.23600
Miao Z, Miao Z, Wang S, Wu H, Xu S (2022) Exposure to imidacloprid induce oxidative stress, mitochondrial dysfunction, inflammation, apoptosis and mitophagy via NF-kappaB/JNK pathway in grass carp hepatocytes. Fish Shellfish Immunol 120:674–685. https://doi.org/10.1016/j.fsi.2021.12.017
Huang Z, Wang S, Yang Y, Lou J, Liu Z, Liu Z, Yong H, Shan S, Song F (2023) Mitochondrial dysfunction promotes the necroptosis of Purkinje cells in the cerebellum of acrylamide-exposed rats. Food Chem Toxicol: Int J Publ Br Ind Biol Res Assoc 171:113522. https://doi.org/10.1016/j.fct.2022.113522
Funding
We thank the Guangxi Natural Science Foundation Project (2020GXNSFAA297257), National Natural Science Foundation of China (81960303), Guangxi University Young and Middle-aged Teachers' Basic Ability Improvement Project (2020KY13017), Guangxi Zhuang Autonomous Region Administration of Traditional Chinese Medicine Self-financing Scientific Research Project (GZZC2020248), and Guangxi Zhuang Autonomous Region Health and Health Commission Self-financing Scientific Research Course (Z20201416).
Author information
Authors and Affiliations
Contributions
Hui-xin Peng, fu Chai and Ke-heng Chen were responsible for experiment operation and paper writing; Yan-xin Huang, Yan-fang Pang and Hui-xiong Yuan were responsible for animal feeding and modeling; Guang-ji Wei was responsible for partial data analysis; Wen-cheng Chen, Chun-fang Wang and Shi-hua Luo were responsible for experiment design, paper writing guidance, overall framework construction and project fund preparation.
Corresponding authors
Ethics declarations
Competing Interests
There is no conflict of interest among the authors of this article, which will not affect the reporting of the article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Hui-xin Peng, Fu Chai and Ke-heng Chen share the frst authorship.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Peng, H.x., Chai, F., Chen, Kh. et al. Reactive Oxygen Species-Mediated Mitophagy and Cell Apoptosis are Involved in the Toxicity of Aluminum Chloride Exposure in GC-2spd. Biol Trace Elem Res 202, 2616–2629 (2024). https://doi.org/10.1007/s12011-023-03848-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-023-03848-0