Abstract
Aluminum (Al) exposure can lead to different degrees of damage to various organ systems of the body. It has been previously revealed that Al exposure can damage the liver, causing liver dysfunction. However, the specific mechanism remains unclear. This research aims to uncover the damaging effect of Al exposure on rat liver and to demonstrate the role of autophagy and apoptosis in this effect. Thirty-two Wistar rats were randomly divided into the control group (C group), low-dose Al exposure group (L group), middle-dose Al exposure group (M group), and high-dose Al exposure group (H group) (n = 8). The rats, respectively, received intraperitoneal injections of 0, 5, 10, and 20 mg/kg·day AlCl3 solution for 4 weeks (5 times/week). After the experiment, changes in the ultrastructure and autolysosome in rat liver were observed; the liver function, apoptosis rate, as well as levels of apoptosis-associated proteins and autophagy-associated proteins were detected. The results indicated that Al exposure damaged rat liver function and structure and resulted in an increase in autolysosomes. TUNEL staining revealed an elevated number of apoptotic hepatocytes after Al exposure. Moreover, we found from Western blotting that the levels of autophagy-associated proteins Beclin1 and LC3-II were increased; apoptotic protein Caspase-3 level was elevated and the Bcl-2/Bax ratio was reduced. Our research suggested that Al exposure can lead to high autophagy and apoptosis levels of rat hepatocytes, accompanied by hepatocyte injury and impaired liver function. This study shows that autophagy and apoptosis pathways participate in Al toxication-induced hepatocyte injury.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aluminum (Al) is an abundant metal element in nature and is widely used in our life, industrial production, and healthcare industry due to its excellent characteristics. It can enter the human body through food, drinking water, air, medicine, and other ways [1]. The liver is the largest gland in the body and the most important organ for the metabolism and biotransformation of foreign chemicals, making it the most vulnerable organ [2]. The main absorption site of Al is the duodenum. The absorbed Al combines with proteins and enters the blood; in this way, it was transported to various organs. Al accumulation in the liver can lead to pathological changes and liver dysfunction [3, 4]. It has been reported that Al exposure can cause cristae rupture, dissolution, and collapse in the mitochondria of hepatocytes. Moreover, it can lead to hepatic interstitial fibrosis and inflammatory cell infiltration, and also can result in abnormal synthetic and metabolic functions [5, 6]. Patients taking Al nutrient solution for a long time may develop cholestatic liver disease [7]. Al-induced liver injury has been confirmed in vivo and in vitro, while its mechanism has not been fully elucidated yet [8, 9].
Autophagy and apoptosis are crucial catabolic processes that maintain cell and tissue homeostasis. Apoptosis is the first recognized programmed cell death (PCD) procedure, and it’s role and regulatory network have become gradually clear. However, apoptosis is not the only factor determining the fate of cell death. In recent years, autophagy, known as type II PCD, has been confirmed to regulate cell death together with apoptosis [10, 11]. There is a close and complex relationship between autophagy and apoptosis in determining cell fate [12]. Currently, little is known about the effects of Al exposure on autophagy and apoptosis of hepatocytes, and its role in the process of aluminum-induced liver injury in rats remains unknown.
Our previous studies have also implied that Al exposure can damage the rat’s liver structure and function. This research focuses on the possible role of autophagy and apoptosis in the occurrence and development of Al-induced liver injury to further provide clues for the prevention and treatment of Al-induced liver injury.
Materials and Methods
Reagent
Al chloride (Howei Chemical, Guangzhou, China) was diluted with distilled water before administration.
Animals and Experimental Design
Thirty-two male Wistar rats (5–6 weeks old, 150–180 g weight) (Changsha Tianqin Biotechnology Co., Ltd.) were fed under standard conditions (4 rats in each cage, 24–27 °C, relative humidity of 45–65%, and 12-h day/night cycle). The rats were provided with abundant food and water throughout the study process. The standard rat feed provided to rats was supplied by Beijing Keao Xieli Feed Co., Ltd. (Beijing, China) (http://www.keaoxieli.com/product/137.html). This research was conducted strictly following the Guide to the Management and Use of Laboratory Animals and was approved by the Ethics Committee of Youjiang Medical University for Nationalities (approval date, 20 July 2020). All surgery was performed under pentobarbital anesthesia with minimum suffering in the animals.
After acclimatization for 7 days, 32 Wistar rats were randomly divided into the control group (C group), low-dose Al exposure group (L group), middle-dose Al exposure group (M group), and high-dose Al exposure group (H group) (n = 8 for each group). The rats, respectively, received intraperitoneal injections of 0, 5, 10, and 20 mg/kg·day AlCl3 solution for 4 weeks (5 times/week) [13, 14]. At the end of the 4-week treatment, the blood was collected and allowed to clot to collect serum. Rats were killed, and their liver was removed and then soaked between filter papers to remove excess fluid.
Determination of Aluminum
The Al level in liver tissues was determined using the ICP-OES method. The sample pretreatment methods were as follows: the samples in the polytetrafluoroethylene digestion tube were weighed. The mixture of 0.5 mL hydrofluoric acid and 7 mL nitric acid was added, which was then placed in the digestion tank at 180 °C for 8 h. After complete cooling, the samples were transferred to the constant volume instrument for testing.
Measurement of Alanine Aminotransferase (ALT) and Aspartate Aminotransferase (AST)
The contents of liver function indices ALT and AST were determined using an automatic biochemical analyzer.
Histopathological Examination
For hematoxylin–eosin (HE) staining, the rat liver tissues were fixed in paraformaldehyde for 48 h, embedded in paraffin, sectioned, and stained with HE. Then, the morphological changes of the liver were observed under a light microscope.
Transmission Electron Microscopy (TEM) Observation
Liver tissue blocks were rinsed with normal saline and cut into small cubes, which were placed into the prepared fixation liquid, sectioned, and stained. The ultrathin sections were put onto a single-hole copper mesh box, and a TEM was used for observation and photographing.
Terminal Deoxynucleotidyl Transferase-Mediated Deoxyuridine Triphosphate Nick End-Labeling (TUNEL) Assay
The liver tissue samples were embedded in paraffin and regularly sectioned. The dewaxed sections were treated with proteinase K to detach the membrane proteins and nucleoproteins, which were then incubated with TUNEL reaction solution at 37 °C for 1 h. Incubated with the anti-fluorescein antibody, the sections were developed using diaminobenzidine (DAB), counterstained, dehydrated, permeabilized, and sealed. For the judgment of the results, cells with brownish-yellow-stained nuclei were positively expressed apoptotic cells. Three fields were randomly selected from each section to calculate the apoptosis rate in each of them based on the following formula: apoptosis rate (%) = the number of apoptotic cells / the number of total cells.
Western Blotting
The liver tissues (100 mg) were homogenated with 1 mL lysis solution containing proteinase inhibitor under low temperature. The fully lysed cells were centrifuged at 4 °C and 13,000 r/min for 10 min with the supernatant collected. Bicinchoninic acid (BCA) kit (Beyotime Bio, Shanghai, China) was used to detect the protein concentration and adjust it to be consistent. The samples were added with protein loading buffer with the loading amount balanced, performed with 70 °C water bath for 10 min, and sub-packaged after protein denaturation. Then, gels with different concentrations based on the proteins to be determined were prepared: 5% spacer gel, 15% separation gel for LC3-II (Abcam, Cambridge, UK), and 12% separation gel for Beclin1 (Abcam, Cambridge, UK), B-cell lymphoma-2 gene (Bcl-2), Bax, Caspase-3 (ProteinTech, Wuhan, China), respectively, showing luminescence on different membranes. The proteins were loaded, and the constant voltage was regularly set to 80 V, which was then adjusted to 120 V after the bromophenol blue indicator became linear at the junction of spacer gel and separation gel with the electrophoresis gone on. The electrophoresis was continued until the bromophenol blue reached the bottom of the gel, and the electricity was adjusted to 250 mA constant current. The samples were transferred onto membranes in the prepared and precooled electroporation buffer (45 min for LC3-II and 80 min for Beclin1, Bcl-2, Bax, and Caspase-3). Next, the membranes were blocked with 5% blocking buffer and incubated with the relative primary antibodies at 4 °C overnight. Subsequently, the membranes were washed and incubated with the secondary antibodies at room temperature for 2 h. Finally, a moderate amount of chemiluminescent liquid was prepared at a ratio of A liquid/B liquid = 1:1. After development, the films were scanned and the gray values were analyzed using Image-Pro Plus 6.0 (Media Cybemetics, USA).
Statistical Analysis
All data analyses were conducted using SPSS 22.0 software, and the measurement data conforming to the normal distribution were expressed as mean ± standard deviation. The results were statistically analyzed using a one-way analysis of variance followed by the Student–Newman–Keuls test. The level of significance was set at p < 0.05 for all statistical analyses.
Results
Al Content in the Liver Tissues of Rats
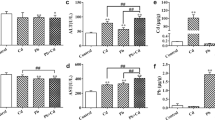
The Al content in rat liver tissues rats was detected after 28 days of Al exposure. It was found that with the increase of Al exposure concentration, the Al content in liver tissues presented an upward trend. The Al content in the liver tissues of rats in the L, M, and H groups was significantly increased in a dose-dependent manner compared with that in the C group (p < 0.001) (Fig. 1).
Aluminum concentrations in the liver. C, L, M, and H refer to 0, 5, 10, and 20 mg/kg·day of AlCl3 solution, respectively. Results are expressed as mean ± SD, Statistical significance was determined by one-way analysis of variance (ANOVA). n = 5; “*” indicates statistically significant difference with the control group *p < 0.05, **p < 0.01, and ***p < 0.001). “#” indicates statistically significant difference between two different dose groups (#p < 0.05, ##p < 0.01, and.###p < 0.001)
Effect of Al Exposure on ALT and AST Levels in Rats
To understand the effect of Al exposure on rat liver function, ALT and AST levels under different concentrations of Al exposure were detected. As shown in Fig. 2, Al exposure caused different degrees of liver dysfunction in a concentration-dependent manner. Namely, a higher Al exposure concentration was associated with a higher ALT level. Compared with those in the C group, serum ALT and AST levels were remarkably increased in rats in the M group and H group (both p < 0.05), and rats in the M group showed the highest serum AST level.
Effects of aluminum exposure on ALT (A) and AST (B). C, L, M, and H refer to 0, 5, 10, and 20 mg/kg·day of AlCl3 solution, respectively. Results are expressed as mean ± SD (n = 8). ALT, alanine aminotransferase; AST, aspartate aminotransferase; “*” indicates statistically significant difference with the C group (*p < 0.05, **p < 0.01, and ***p < 0.001). “#” indicates statistically significant difference between two different dose groups (#p < 0.05, ##p < 0.01, and.###p < 0.001)
Pathological Changes of Rat Liver
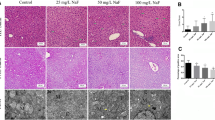
The effect of Al exposure on rat liver histopathological changes was observed using HE staining. As indicated by the histopathological results, hepatocytes in the C group were closely arranged, showing clear boundary, abundant cytoplasm, uniform staining, round nucleus, normal size, and intact and normal venous endothelium; no obvious abnormality was found in the tissues in the C group. However, the Al exposure groups showed loosely arranged and light-stained cytoplasm in hepatocytes (black arrow), some vacuolated cells, necrotic hepatocytes, nucleus condensation, deep staining, and enhanced acidophilia (red arrow); the group H exhibited the highest degree of pathological damage (Fig. 3).
Pathologic changes of hepatic tissue tissues of rats in each group (HE × 400). The scale bar represents 50 μm. C, L, M, and H refer to 0, 5, 10, and 20 mg/kg·day of AlCl3 solution, respectively. Light-stained cytoplasm of hepatocytes (black arrow), Vacuolated cells, necrotic hepatocytes, nuclear condensation, and deep staining (red arrow)
Effect of Al Rxposure on Rat Hepatocyte Ultrastructure
As observed under a TEM, the hepatocytes of rats in the C group showed a good overall structure, with complete membranes, abundant organelles in the cytoplasm, round nuclei, complete nuclear membranes, normal perinuclear space, and large nucleolus; there were abundant mitochondria with complete membrane and cristae; ribosomes attached to the rough endoplasmic reticulum surface and many evenly distributed glycogen particles in the cytoplasm could be observed; no typical autophagosomes and autophagosomes were found. However, the liver tissues of rats in the L, M, and H groups showed different degrees of fibrosis. Detailedly, hepatocytes presented slight edema and high electron density; there were many collagenous fibers around the cells, tortuous dilation of bile canaliculi, many medullary substances and cholestasis in the lumen, and irregular nuclei with vague nuclear membrane structure; there were numerous mitochondria with slight swelling, irregular shape, and ruptured and decreased cristae; diffusely distributed lipid droplets and many autolysosomes (ASS) (8 in the H group) could be observed (Fig. 4).
Effect of Al Exposure on Rat Hepatocyte Apoptosis
TUNEL staining was performed to histologically explore the effect of Al exposure on hepatocyte apoptosis. Cells with brownish-yellow-stained nuclei were apoptotic cells. As shown in Fig. 5, there was no apoptotic hepatocyte in the C group; the other 3 groups with different doses of Al exposure showed a small number of apoptotic hepatocytes. Furthermore, the results suggested that the H group showed a notably higher apoptosis rate than other groups (p < 0.05).
The TUNEL-positive cells (TUNEL staining, × 200) and TUNEL-positive cell count in rat hepatocyte. C, L, M, and H refer to 0, 5, 10, and 20 mg/kg·day of AlCl3 solution, respectively. Apoptotic cells (blue arrow). Results are expressed as mean ± SD (n = 5). “*” indicates statistically significant difference with the C group (*p < 0.05, **p < 0.01, and ***p < 0.001). “#” indicates statistically significant difference between two different dose groups (#p < 0.05, ##p < 0.01, and.###p < 0.001)
Effect of Al Exposure on Expression of Apoptosis-Associated Proteins and Autophagy-Associated Proteins in Rat Liver Tissues
Western blotting was performed to determine the expression of apoptosis-associated proteins and autophagy-associated proteins in rat liver tissues. As indicated by the results in Fig. 6, there was no significant difference in Beclin1 protein expression between the C group and Al-exposed groups; Beclin1 protein expression in the H group was increased relative to that in the L group (p < 0.01); LC3-II expression was remarkably elevated in the L, M, and H groups in comparison to that in the C group in an Al exposure concentration-dependent manner (p < 0.01) (Fig. 6A). These results indicated that Al exposure can increase autophagy level in hepatocytes. Moreover, the M group showed a markedly higher Bcl-2 protein expression than the C group (p < 0.05); compared with those in the C group, Bax and Caspase-3 expression levels were dramatically elevated in the M and H groups with the increase of Al concentration (p < 0.01); Bcl-2/Bax ratio was decreased in the M and H groups relative to that in the C group, and the H group showed more decrease in Bcl-2/Bax ratio (p < 0.05). These results implied that Al exposure induced hepatocyte apoptosis (Fig. 6B).
The effect of aluminum exposure on expression of apoptosis-associated proteins and autophagy-associated proteins. A The protein expression of Beclin1 and LC3-II. B The protein expression Bcl-2, Bax, and Caspase-3. C, L, M, and H refer to 0, 5, 10, and 20 mg/kg·day of AlCl3 solution, respectively. All data were expressed as mean ± SD (n = 5). “*” indicates statistically significant difference with the C group (*p < 0.05, **p < 0.01, and ***p < 0.001). “#” indicates statistically significant difference between two different dose groups (#p < 0.05, ##p < 0.01, and.###p < 0.001)
Discussion
Al is not an essential trace element for the human body, and excessive Al intake has potential toxicity to the human body [15]. Part of Al can be metabolized in the liver after entering the body; therefore, excessive Al intake can cause damage to the liver [16, 17]. Our study showed that the Al concentration in the liver tissues of rats exposed to Al was significantly increased compared with that in the control group, suggesting that Al may accumulate in the liver and result in damage. ALT and AST are liver function enzymes closely related to liver function, which can be used for the diagnosis of hepatocyte injury when too many liver function enzymes were transferred into the blood circulation. Therefore, ALT and AST are biomarkers reflecting the severity of multiple liver diseases [18]. This research revealed that the levels of ALT and AST in rats in the M and H groups were significantly enhanced after Al exposure. As observed under a TEM, there were hepatocyte edema, slight swelling in mitochondria, irregular shape, and ruptured and decreased cristae in rats exposed to Al, which were consistent with the results of a study by Al-Hazmi MA [19]. These data indicated that Al exposure did cause damage to rat liver function and structure.
The mechanism of Al toxicity may be related to the following aspects: oxidative stress, mitochondrial dysfunction, endoplasmic reticulum stress, autophagy disorder, apoptosis, and disturbed homeostasis of other metal ions [20, 21]. However, the accumulation of Al in different tissues is different [22], and the toxicity of Al to different tissues and organs is not the same as well [23]. The specific mechanism of Al toxicity to the liver is not clear and needs to be further explored. Al is a positive trivalent element, which is more active and easy to lose electrons. It can promote the formation of reactive oxygen species (ROS), improve the oxidation level, reduce the function of the antioxidant defense system, and induce the oxidative stress response in cells [24]. Moreover, ROS can also lead to endoplasmic reticulum stress and mitochondrial dysfunction through a series of pathways [25]. It has been reported that oxidative stress, mitochondrial dysfunction, and endoplasmic reticulum stress can eventually induce abnormal autophagy or excessive apoptosis, thus damaging cells and tissues and further affecting their biological activities [26,27,28]. As a key way of affecting tissue function, it is particularly important to explore the significance of autophagy and apoptosis in liver injury. There are few studies on autophagy disorder and apoptosis in Al-induced liver injury. Therefore, this study mainly discussed the mechanism of Al-induced liver injury based on autophagy and apoptosis.
Apoptosis is a process of autonomous cell death controlled by genes. Among many factors regulating apoptosis, the Bcl-2 family and Caspase family play an important role [29]. Bcl-2 is an inhibitor of the apoptosis gene [30], which can inhibit cell apoptosis. Bcl-2 protein is related to the stability of mitochondria, which can protect the stability of cell membranes and prevent the transmission of apoptosis signals; Bax is an apoptosis-promoting protein, and the dimer formed by Bax itself can directly promote cell apoptosis [31]. In addition, the increase of Bax protein can lead to the formation of heterodimers with Bcl-2 protein, thus inactivating Bcl-2. The lower the ratio of Bcl-2 to Bax, the higher the probability of cell apoptosis, which leads to the cascade reaction of Caspase [32]. Apoptotic cell death is ultimately completed by the Caspase family [33]. Caspase-3 is located in the center of the Caspase cascade reaction and is the executor of apoptosis. When Caspase-3 is activated by the upstream signal, it will cleave itself into an active state, hydrolyze the downstream substrate, and cut off DNA, leading to apoptosis [34, 35]. Once Caspase-3 is activated, cell death cannot be reversed [36]. The results of our research showed that Caspase-3 and Bax expression levels were increased and the anti-apoptotic protein Bcl-2 level was also slightly increased under Al exposure; the ratio of Bcl-2 to Bax was decreased, suggesting that Al exposure can promote the occurrence of apoptosis. TUNEL staining showed that the apoptosis of the H group was higher than that of the control group. These results were further verified by histological examination, which confirmed that apoptosis was closely involved in the liver injury effect. However, the number of apoptotic cells was small and the liver has a strong compensatory ability. Therefore, apoptosis may not have a significant impact on liver function damage. Furthermore, there may be other pathways participating in the liver injury effect.
Autophagy is a lysosome-mediated process of protein and organelle degradation, which is involved in the regulation of cell metabolism and survival. Autophagy dysfunction is related to various diseases, such as tumorigenesis [37], aging [38], and neurodegeneration [39]. Proper autophagy is a self-protection mechanism, while excessive autophagy can lead to cell death [40]. Beclin1, a protein encoded by the BECN1 gene, is an important molecule regulating autophagy, which can mediate the autophagy-related proteins to act on autophagic vesicles and react with various proteins to regulate autophagosome formation and maturation [41]. Microtubule-associated protein LC3 is an autophagy-related marker protein and LC3-II positive quantification can be used to evaluate the number of autophagosomes in cells [42]. In this study, as indicated by the Western blotting results, the Beclin1 level showed no significant difference between the experimental groups and the control group. This can be attributed to the dual effects of Beclin1 on the regulation of autophagy via combining with Bcl-2, Bcl XL, etc. Hence, the Beclin1 level cannot be used to measure autophagy. The expression of the autophagy marker protein LC3-II was found significantly increased. Moreover, it was observed under a TEM that rats under Al exposure showed significantly increased autophagosomes in hepatocytes, obviously damaged mitochondria, and increased undegraded lysosomes. These results suggested that the autophagy pathway was tightly implicated in Al-induced liver injury.
Autophagy and apoptosis are two important catabolic processes maintaining cell and tissue homeostasis and widely exist in the eucaryotic organism. Although the characteristics and mechanisms of apoptosis and autophagy are different, these two pathways are not independent of each other. They share the same stimulating factors and regulatory proteins, and there exist complex dialogues between them [43]. The regulation modes of autophagy and apoptosis can be roughly divided into two types: cooperative relationship and antagonistic relationship [44, 45]. In our research, through detecting the expression of autophagy- and apoptosis-related factors in hepatocytes, the effect of Al exposure on hepatocytes was reflected by the upregulation of autophagy level and apoptosis level, suggesting that autophagy and apoptosis pathways were involved in Al-induced liver injury. However, their role in liver injury and the relationship between them is still unclear. Our next step is to intervene and regulate autophagy and apoptosis. We will extend the time of our research to further observe the liver toxic effects of apoptosis and autophagy under different exposure time and intervention levels, thus further exploring the regulatory mechanism between apoptosis and autophagy.
In conclusion, the experimental results in this study demonstrated that Al exposure could induce apoptosis and autophagy in the liver. This study supplements and enriches the mechanism of Al exposure-induced liver injury. Multifaceted analysis should be conducted when discussing the harm of environmental metal pollution to the body. This study may provide a scientific basis for the development of population health protection. Although Al exposure among the population is mainly low concentration exposure, Al exposure exerts long-term chronic negative effects on the human body due to the accumulation and slow toxicity of aluminum. Therefore, the control and management of Al are still of great value to reduce aluminum-induced health damage to the population.
Data Availability
The data presented in this study are available on request from the corresponding author and the first author.
References
Kim H, Lim KY, Kang J, Park JW, Park SH (2020) Macrophagic myofasciitis and subcutaneous pseudolymphoma caused by aluminium adjuvants. Sci Rep 10(1):11834. https://doi.org/10.1038/s41598-020-68849-8
Eraslan G, Sarıca ZS, Bayram L, Tekeli MY, Kanbur M, Karabacak M (2017) The effects of diosmin on aflatoxin-induced liver and kidney damage. Environ Sci Pollut Res Int 24(36):27931–27941. https://doi.org/10.1007/s11356-017-0232-7
Martinez CS, Piagette JT, Escobar AG, Martin A, Palacios R, Pecanha FM, Vassallo DV, Exley C, Alonso MJ, Miguel M, Salaices M, Wiggers GA (2017) Aluminum exposure at human dietary levels promotes vascular dysfunction and increases blood pressure in rats: a concerted action of NAD(P)H oxidase and COX-2. Toxicol 390:10–21. https://doi.org/10.1016/j.tox.2017.08.004
Xiao B, Cui Y, Li B, Zhang J, Zhang X, Song M, Li Y (2022) ROS antagonizes the protection of Parkin-mediated mitophagy against aluminum-induced liver inflammatory injury in mice. Food Chem Toxicol 165:113126. https://doi.org/10.1016/j.fct.2022.113126
Hassan SA, Kadry MO (2021) Neurodegenerative and hepatorenal disorders induced via aluminum chloride in murine system: impact of β-secretase, MAPK, and KIM. Biol Trace Elem Res 199(1):227–236. https://doi.org/10.1007/s12011-020-02132-9
Sajjad S, Malik H, Saeed L, Hashim I, Farooq U, Manzoor F (2019) Synergistic potential of propolis and vitamin e against sub-acute toxicity of AlCl(3) in albino mice: in vivo study. Physiol Res 68(1):67–74. https://doi.org/10.33549/physiolres.933863
Klein GL (2019) Aluminum toxicity to bone: a multisystem effect? Osteoporosis sarcopenia 5(1):2–5. https://doi.org/10.1016/j.afos.2019.01.001
Sieg H, Ellermann AL, Maria Kunz B, Jalili P, Burel A, Hogeveen K, Böhmert L, Chevance S, Braeuning A, Gauffre F, Fessard V, Lampen A (2019) Aluminum in liver cells—the element species matters. Nanotoxicol 13(7):909–922. https://doi.org/10.1080/17435390.2019.1593542
Yang X, Zhang J, Ji Q, Wang F, Song M, Li Y (2018) Autophagy protects MC3T3-E1 cells upon aluminum-induced apoptosis. Biol Trace Elem Res 185(2):433–439. https://doi.org/10.1007/s12011-018-1264-7
Mejías-Peña Y, Estébanez B, Rodriguez-Miguelez P, Fernandez-Gonzalo R, Almar M, de Paz JA, González-Gallego J, Cuevas MJ (2017) Impact of resistance training on the autophagy-inflammation-apoptosis crosstalk in elderly subjects. Aging 9(2):408–418. https://doi.org/10.18632/aging.101167
Frietze KK, Brown AM, Das D, Franks RG, Cunningham JL, Hayward M, Nickels JT Jr (2022) Lipotoxicity reduces DDX58/Rig-1 expression and activity leading to impaired autophagy and cell death. Autophagy 18(1):142–160. https://doi.org/10.1080/15548627.2021.1920818
Stewart T, Kallash M, Vehaskari VM, Hodgeson SM, Aviles DH (2019) Increased autophagy and apoptosis in the kidneys of intrauterine growth restricted rats. Fetal Pediatr Pathol 38(3):185–194. https://doi.org/10.1080/15513815.2018.1564160
Cheraghi E, Golkar A, Roshanaei K, Alani B (2017) Aluminium-induced oxidative stress, apoptosis and alterations in testicular tissue and sperm quality in Wistar rats: ameliorative effects of curcumin. Int J Fertil Steril 11(3):166–175. https://doi.org/10.22074/ijfs.2017.4859
Li Z, Zhao G, Qian S, Yang Z, Chen X, Chen J, Cai C, Liang X, Guo J (2012) Cerebrovascular protection of β-asarone in Alzheimer’s disease rats: a behavioral, cerebral blood flow, biochemical and genic study. J Ethnopharmacol 144(2):305–312. https://doi.org/10.1016/j.jep.2012.09.013
De A, Ghosh S, Chakrabarti M, Ghosh I, Banerjee R, Mukherjee A (2020) Effect of low-dose exposure of aluminium oxide nanoparticles in Swiss albino mice: Histopathological changes and oxidative damage. Toxicol Ind Health 36(8):567–579. https://doi.org/10.1177/0748233720936828
Salem AM, Mohammaden TF, Ali MAM, Mohamed EA, Hasan HF (2016) Ellagic and ferulic acids alleviate gamma radiation and aluminium chloride-induced oxidative damage. Life Sci 160:2–11. https://doi.org/10.1016/j.lfs.2016.07.006
Abu-Elfotuh K, Hussein FH, Abbas AN, Al-Rekabi MD, Barghash SS, Zaghlool SS, El-Emam SZ (2022) Melatonin and zinc supplements with physical and mental activities subside neurodegeneration and hepatorenal injury induced by aluminum chloride in rats: inclusion of GSK-3β-Wnt/β-catenin signaling pathway. Neurotoxicol 91:69–83. https://doi.org/10.1016/j.neuro.2022.05.002
Gao HT, Cheng WZ, Xu Q, Shao LX (2017) Dietary restriction reduces blood lipids and ameliorates liver function of mice with hyperlipidemia. J Huazhong Univ Sci Technolog Med Sci 37(1):79–86. https://doi.org/10.1007/s11596-017-1698-8
Al-Hazmi MA, Rawi SM, Hamza RZ (2021) Biochemical, histological, and neuro-physiological effects of long-term aluminum chloride exposure in rats. Metab Brain Dis 36(3):429–436. https://doi.org/10.1007/s11011-020-00664-6
Salimi A, Shabani M, Aylar EM (2022) Inhibition of mitochondrial permeability transition pore and antioxidant effect of Delta-9-tetrahydrocannabinol reduces aluminium phosphide-induced cytotoxicity and dysfunction of cardiac mitochondria. Pesticide Biochem Physiol 184:105117. https://doi.org/10.1016/j.pestbp.2022.105117
Al-Kahtani M, Abdel-Daim MM, Sayed AA, El-Kott A, Morsy K (2020) Curcumin phytosome modulates aluminum-induced hepatotoxicity via regulation of antioxidant, Bcl-2, and caspase-3 in rats. Environ Sci Pollut Res Int 27(17):21977–22185. https://doi.org/10.1007/s11356-020-08636-0
Weisser K, Göen T (2019) Aluminium in plasma and tissues after intramuscular injection of adjuvanted human vaccines in rats. Arch Toxicol 93(10):2787–2796. https://doi.org/10.1007/s00204-019-02561-z
El-Demerdash FM, Baghdadi HH, Ghanem NF, Mhanna ABA (2020) Nephroprotective role of bromelain against oxidative injury induced by aluminium in rats. Environ Toxicol Pharmacol 80:103509. https://doi.org/10.1016/j.etap.2020.103509
Rizvi SH, Parveen A, Ahmad I, Ahmad I, Verma AK, Arshad M, Mahdi AA (2016) Aluminum activates PERK-EIF2alpha signaling and inflammatory proteins in human neuroblastoma SH-SY5Y cells. Biol Trace Elem Res 172(1):108–119. https://doi.org/10.1007/s12011-015-0553-7
Bañuls C, de Marañón AM, Castro-Vega I, López-Doménech S, Escribano-López I, Salom C, Veses S (2019) Hernández-Mijares A (2019) Role of endoplasmic reticulum and oxidative stress parameters in the pathophysiology of disease-related malnutrition in leukocytes of an outpatient population. Nutrients 11(8):1838. https://doi.org/10.3390/nu11081838
Karna KK, Choi BR, Kim MJ, Kim HK, Park JK (2019) The effect of Schisandra chinensis Baillon on cross-talk between oxidative stress, endoplasmic reticulum stress, and mitochondrial signaling pathway in testes of varicocele-induced SD rat. Int J Mol Sci 20(22):5785. https://doi.org/10.3390/ijms20225785
Zhang Y, Li S, Li J, Han L, He Q, Wang R, Wang X, Liu K (2018) Developmental toxicity induced by PM2.5 through endoplasmic reticulum stress and autophagy pathway in zebrafish embryos. Chemosphere 197:611–621. https://doi.org/10.1016/j.chemosphere.2018.01.092
Zou J, Fei Q, Xiao H, Wang H, Liu K, Liu M, Zhang H, Xiao X, Wang K (2019) VEGF-A promotes angiogenesis after acute myocardial infarction through increasing ROS production and enhancing ER stress-mediated autophagy. J Cell Physiol 234(10):17690–17703. https://doi.org/10.1002/jcp.28395
Kalkavan H, Green DR (2018) MOMP, cell suicide as a BCL-2 family business. Cell Death Differ 25(1):46–55. https://doi.org/10.1038/cdd.2017.179
Mohamed AA, Khater SI, Hamed Arisha A, Metwally MMM, Mostafa-Hedeab G, El-Shetry ES (2021) Chitosan-stabilized selenium nanoparticles alleviate cardio-hepatic damage in type 2 diabetes mellitus model via regulation of caspase, Bax/Bcl-2, and Fas/FasL-pathway. Gene 768:145288. https://doi.org/10.1016/j.gene.2020.145288
Khazaei S, Ramachandran V, Abdul Hamid R, Mohd Esa N, Etemad A, Moradipoor S, Ismail P (2017) Flower extract of Allium atroviolaceum triggered apoptosis, activated caspase-3 and down-regulated antiapoptotic Bcl-2 gene in HeLa cancer cell line. Biomed Pharmacother 89:1216–1226. https://doi.org/10.1016/j.biopha.2017.02.082
Liu LS, Bai XQ, Gao Y, Wu Q, Ren Z, Li Q, Pan LH, He NY, Peng J, Tang ZH (2017) PCSK9 promotes oxLDL-induced PC12 cell apoptosis through the Bcl-2/Bax-Caspase 9/3 signaling pathway. J Alzheimers Dis 57(3):723–734. https://doi.org/10.3233/jad-161136
Adams JM, Cory S (1998) The Bcl-2 protein family: arbiters of cell survival. Sci 281(5381):1322–1326. https://doi.org/10.1126/science.281.5381.1322
Xu YR, Yang WX (2018) Roles of three Es-Caspases during spermatogenesis and Cadmium-induced apoptosis in Eriocheir sinensis. Aging 10(5):1146–1165. https://doi.org/10.18632/aging.101454
Sergio L, Thomé AMC, Trajano L, Mencalha AL, Fonseca ASda, Fde Paoli (2018) Photobiomodulation prevents DNA fragmentation of alveolar epithelial cells and alters the mRNA levels of caspase 3 and Bcl-2 genes in acute lung injury. Photochem Photobiol Sci 17(7):975–983. https://doi.org/10.1039/c8pp00109j
Kei S, Sachiko Y-H, Yasuo T, Kimio A, Makoto U (2022) Golgi stress induces upregulation of the ER-Golgi SNARE Syntaxin-5, altered βAPP processing, and Caspase-3-dependent apoptosis in NG108-15 cells. Mol Cell Neurosci 121:103754. https://doi.org/10.1016/j.mcn.2022.103754
Mommersteeg MC, Simovic I, Yu B, van Nieuwenburg SAV, Bruno IMJ, Doukas M, Kuipers EJ, Spaander MCW, Peppelenbosch MP, Castaño-Rodríguez N, Fuhler GM (2022) Autophagy mediates ER stress and inflammation in Helicobacter pylori-related gastric cancer. Gut Microbes 14(1):2015238. https://doi.org/10.1080/19490976.2021.2015238
Murase D, Kusaka-Kikushima A, Hachiya A, Fullenkamp R, Stepp A, Imai A (2020) Autophagy declines with premature skin aging resulting in dynamic alterations in skin pigmentation and epidermal differentiation. Int J Mol Sci 21(16):5708. https://doi.org/10.3390/ijms21165708
Heckmann BL, Teubner BJW, Tummers B, Boada-Romero E, Harris L, Yang M, Guy CS, Zakharenko SS, Green DR (2019) LC3-associated endocytosis facilitates β-amyloid clearance and mitigates neurodegeneration in murine Alzheimer’s disease. Cell 178(3):536–551. https://doi.org/10.1016/j.cell.2019.05.056
Koenig U, Robenek H, Barresi C, Brandstetter M, Resch GP, Gröger M, Pap T, Hartmann C (2020) Cell death induced autophagy contributes to terminal differentiation of skin and skin appendages. Autophagy 16(5):932–945. https://doi.org/10.1080/15548627.2019.1646552
Fernández ÁF, Sebti S, Wei Y, Zou Z, Shi M, McMillan KL, He C, Ting T, Liu Y, Chiang WC, Marciano DK, Schiattarella GG, Bhagat G, Moe OW, Hu MC, Levine B (2018) Disruption of the beclin 1-BCL2 autophagy regulatory complex promotes longevity in mice. Nature 558(7708):136–140. https://doi.org/10.1038/s41586-018-0162-7
Zhang X, Wang RY (2020) Effects of high-load exercise induced skeletal muscle injury on autophagy ultrastructure and Beclin1 and LC3-II / I in rats. Zhongguo Ying Yong Sheng Li Xue Za Zhi 36(4):296–300. https://doi.org/10.12047/j.cjap.5944.2020.064
Nasiri-Ansari N, Nikolopoulou C, Papoutsi K, Kyrou I, Mantzoros CS, Kyriakopoulos G, Chatzigeorgiou A, Kalotychou V, Randeva MS, Chatha K, Kontzoglou K, Kaltsas G, Papavassiliou AG, Randeva HS, Kassi E (2021) Empagliflozin attenuates Non-Alcoholic Fatty Liver Disease (NAFLD) in high fat diet fed ApoE((-/-)) mice by activating autophagy and reducing ER stress and apoptosis. Int J Mol Sci 22(2):818. https://doi.org/10.3390/ijms22020818
Satyavarapu EM, Das R, Mandal C, Mukhopadhyay A, Mandal C (2018) Autophagy-independent induction of LC3B through oxidative stress reveals its non-canonical role in anoikis of ovarian cancer cells. Cell Death Dis 9(10):934. https://doi.org/10.1038/s41419-018-0989-8
Menk M, Graw JA, Poyraz D, Möbius N, Spies CD, Cvon Haefen (2018) von Haefen C Chronic alcohol consumption inhibits autophagy and promotes apoptosis in the liver. Int J Med Sci 15(7):682–688. https://doi.org/10.7150/ijms.25393
Funding
This work was co-financed by the Guangxi Science Natural Foundation for Young Scientists (No. 2019GXNSFBA245045) and the Young and Middle-Aged Backbone Talents Scientific Research Projects of the Affiliated Hospital of Youjiang Medical University for Nationalities in 2021 (No. Y20212606).
Author information
Authors and Affiliations
Contributions
All authors made contributions to the research design. Xi Wei and Dong Li established the animal model and analyzed the samples. Yueling Luo performed a statistical analysis of the data. Biaoliang Wu directed the design of the research. Xi Wei drafted the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wei, X., Li, D., Luo, Y. et al. Role of Autophagy and Apoptosis in Aluminum Exposure-Induced Liver Injury in Rats. Biol Trace Elem Res 201, 3971–3980 (2023). https://doi.org/10.1007/s12011-022-03497-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-022-03497-9