Abstract
Aluminium (Al) toxicokinetics after intramuscular (IM) injection of Al-adjuvanted vaccines is unknown. Since animal data are required for modeling and extrapolation, a rat study was conducted measuring Al in plasma and tissues after IM injection of either plain Al-hydroxide (pAH) or Al-phosphate (pAP) adjuvant (Al dose 1.25 mg), single human doses of three Al-adjuvanted vaccines (V1, V2, and V3; Al doses 0.5–0.82 mg), or vehicle (saline). A significant increase in Al plasma levels compared to controls was observed after pAP (AUC(0–80 d), mean ± SD: 2424 ± 496 vs. 1744 ± 508 µg/L*d). Percentage of Al dose released from injected muscle until day 80 was higher after pAP (66.9%) and AP-adjuvanted V3 (85.5%) than after pAH and AH-adjuvanted V1 (0 and 22.3%, resp.). Estimated absolute Al release was highest for pAP (836.8 µg per rat). Al concentration in humerus bone was increased in all groups, again strongest in the pAP group [3.35 ± 0.39 vs. 0.05 ± 0.06 µg/g wet weight (ww)]. Extrapolated amounts in whole skeleton corresponded to 5–12% of the released Al dose. Very low brain Al concentrations were observed in all groups (adjuvant group means 0.14–0.29 µg/g ww; control 0.13 ± 0.04 µg/g ww). The results demonstrate systemically available Al from marketed vaccines in rats being mainly detectable in bone. Al release appears to be faster from AP- than AH-adjuvants. Dose scaling to human adults suggests that increase of Al in plasma and tissues after single vaccinations will be indistinguishable from baseline levels.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Aluminium (Al) compounds have been widely used for decades as adjuvants in vaccines. They mainly consist of complex morphologies of crystalline Al-oxyhydroxide or amorphous Al hydroxyphosphate (Hem and HogenEsch 2007) referred to below for the ease of reading as Al-hydroxide (“AH”) and Al-phosphate (“AP”). The poorly soluble adsorbents are commercially available as wet gel suspensions (e.g., Alhydrogel® or Adju-Phos®) or are produced by vaccine manufacturers themselves. Many human vaccines are adsorbed on AH or AP, e.g., the toxoid vaccines against diphtheria and tetanus, acellular pertussis, hepatitis B, pneumococcal and meningococcal vaccines, potentiating the immune response to the poorly immunogenic antigens, thereby enabling successful vaccination. Al content in human vaccines is limited to 1.25 mg per dose by WHO (WHO 2016) and European Pharmacopeia (Ph. Eur. 2018), and is labeled in the product information.
Although to date there is no scientific evidence for a causal relationship between Al containing vaccinations and acute or chronic neurological impairment or diseases (Immunization Safety Review 2001, 2004; WHO 2012), there is still concern about the potential toxicity on the central nervous system or bone deriving from vaccine exposure.
Remaining uncertainty could at best be erased by better knowledge of toxicokinetics after intramuscular (IM) injection of Al-adjuvanted vaccines. While Al bioavailability after parenteral administration is supposed to be 100%, the rate of absorption and thus potential Al increase in plasma and tissues over time in man is unknown. A few investigations in rabbits and monkeys suggest that AP has a higher rate of bioavailability than AH (Flarend et al. 1997; Verdier et al. 2005).
A physiology-based toxicokinetic (PBTK) model is urgently needed for extrapolation of animal data to humans (Krewski et al. 2007). However, relevant animal data on Al absorption and distribution after administration of Al-adjuvanted products to inform such a model are lacking (Weisser et al. 2017; Masson et al. 2018).
We therefore aimed at collecting data on Al bioavailability from adjuvants in vivo by injecting a full human dose of unmodified marketed vaccine products IM into rats. Vaccines should represent both adjuvant types at the highest available Al content per dose. Since most studies investigating Al toxicokinetics from soluble species have been conducted in rats (Weisser et al. 2017, 2019), also with regard to model building this species was considered most appropriate. We monitored Al concentrations in plasma, at the injection site, in bone, and in whole brain hemisphere up to 80 days post-injection.
Materials and methods
Animals
In vivo studies in male Wistar rats (approx. 2 months; body weight 350 g ± 65 g, Charles River Labs, Sulzfeld) were conducted by preclinics GmbH (Potsdam, Germany).
Rats were randomly assigned to treatment groups (no allocation parameter) and were allowed free access to tap water and standard diet [R/M-H, extruded (V1536), Ssniff, Soest, Germany]. The animals were kept under 12 h/12 h light–dark cycle conditions. After 19 days of acclimatization following arrival, animals were anesthetized with 5 vol % isoflurane (IsoFlo 100%; Ecuphar GmbH, Greifswald) and blood was collected from the lateral tail vein to obtain the blank value. Thereafter, treatment preparation or vehicle solution was administered according to the schedule described under treatment.
Rats were housed and handled according to guidelines from the Federation of Laboratory Animal Science Associations (FELASA). The animal study was performed in compliance with the German animal protection law and was registered at the Landesamt für Umwelt, Gesundheit und Verbraucherschutz Brandenburg.
Treatment preparations
Vaccine products (V1, V2, V3) were purchased at a local pharmacy. All three products are marketed in the EU, adjuvanted with either AH (V1), AP (V3) or both AH and AP (V2). A single human dose (0.5 mL) of each vaccine was applied containing 0.5–0.82 mg Al (Table 1). If applicable, fresh preparation was done as indicated in the product information.
Plain adjuvant suspensions (pAH and pAP) were prepared from commercial gels (Alhydrogel® 2% and Adju-Phos®; Brenntag Biosector A/S, Frederikssund, Denmark) by dilution with sterile saline to achieve an Al concentration of 1.25 mg per 0.5 mL. Suspensions were freshly prepared within 24 h and thoroughly vortexed before administration.
Treatment
Each rat received 0.5 mL of either a self-prepared plain adjuvant suspension (pAH or pAP) or a vaccine (V1, V2, or V3; Table 1). A control group receiving 0.5 mL sterile saline (vehicle) was run to monitor the underlying plasma Al steady-state concentration over time (“baseline”) resulting from dietary Al intake. Al contamination of the saline vehicle solution was controlled and found negligibly small (≤ 2.5 ng in 0.5 mL). In all rats the injection volume of 500 μL was administered intramuscularly via six injection sites (100 μL each into both M. quadriceps and M. gastrocnemius of the hind limbs and 50 μL each into both M. triceps of the front limbs).
Sample collection
Blood samples (approx. 300 µL) were collected from the lateral tail vein at pre-dose, and at day 1, 5, 10, 15, 20, 30, 45, 60, and 80 post-dose using K3-EDTA Multivette 600 collection tubes (Sarstedt, Nümbrecht) connected to a 23G cannula. Blood was centrifuged at 4 °C for 10 min at 3220×g. Plasma was pipetted into 1.5 mL microtubes and stored at − 20 °C. In all rats, at time of euthanasia [80 days p.i. (post-injection)] the right hemisphere of the brain, whole muscle M. triceps and whole humerus bone of the right front leg were dissected, transferred into 5 mL tubes, weighed, and stored at − 70 °C.
Bioanalytical method
Measures taken for contamination control and the bioanalytical method used for determination of total Al concentration in plasma and tissues (AAS) were as described in detail in a previous publication (Weisser et al. 2019). The whole pre-analytical and analytical process was designed and controlled for minimizing Al contamination. All determinations in the analytical laboratory were conducted in blinded manner. Al concentration in bone was determined as µg/g wet weight (ww), in muscle and brain samples as both µg/g ww and µg/g dry weight (dw).
Data analysis
Individual area under the curve (AUC) of Al in plasma from zero to day 80 (AUC(0–80 d)) was calculated by the linear trapezoidal rule (MS excel).
Individual Al concentration (µg/g) measured in muscle samples were multiplied by the wet weight of the muscle sample (g) to give the absolute Al amount in whole M. triceps (µg). Al dose “remaining” (%) was calculated as the ratio between Al amount in whole M. triceps (subtracted by vehicle group mean) and Al dose injected into M. triceps. Al dose “released” (%) was calculated as 100 - Al dose “remaining” (%). Under the assumption of equal absorption behavior in all six injection site muscles total absolute Al “release” in µg per rat was estimated as percentage Al dose “released” in M. triceps/100 × total Al dose injected on day 0. Individual negative ratios were not set to zero.
Statistical analysis
If not otherwise indicated, data are presented as means ± standard deviation (SD). Statistical tests were calculated for a two-sided significance level α = 0.05, adjusted for multiple comparisons where necessary.
Two plasma and one muscle sample showing implausible high Al concentrations were eliminated as outliers (confirmed by Dixon’s outlier test).
To investigate stability of Al plasma concentration in the vehicle group over time, a linear trend curve was fitted to the data from day 0 up to day 80 by means of a linear model for repeated measures (animal) with fixed factor day.
Testing for a significant difference of Al plasma exposure after treatment compared to vehicle group was done by comparison of total AUC(0–80 d) (Wilcoxon–Mann–Whitney test, two-sided). Percent remaining Al concentration at injection site was tested for a significant difference from 100% by the Wilcoxon signed rank test.
Al concentration in bone or brain samples was compared between groups using a linear model (ANOVA) with fixed factor “treatment” based on logarithmized values. The statistical analysis was performed with SAS®/STAT software, version 9.4, SAS System for Windows, and software R.
Linear regression and correlation (Pearson r) analysis were done by GraphPad Prism® (Version 7.04) software.
Results
All rats tolerated treatments well and did not show any sign of toxicity throughout the study.
Al in plasma
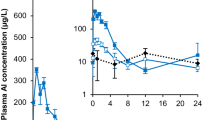
Mean total Al plasma concentrations over time up to day 80 and calculated plasma AUC(0–80 d) for all treatment groups are shown in Fig. 1 and Table 2.
Mean (+SD) Al plasma concentration–time course (a) and Al plasma AUC(0–80 d) (b) in rats after IM administration of pAH (filled circles), pAP (filled triangles), V1 (filled squares), V2 (asterisks), V3 (filled diamonds), or vehicle (open diamonds; dotted line). *p < 0.05 (Wilcoxon–Mann–Whitney test on difference to vehicle)
Mean pre-treatment levels of Al concentration in plasma were similar in all groups (overall mean 12.4 ± 7.8 µg/L). The mean concentration of the vehicle control group over 80 days was 19.8 µg/L (95% CI 14.4–25.3; CV 82%; geometric mean: 14.3 µg/L; 95% CI 10.8–19.0) showing a slightly positive slope of the time course (0.177, p = 0.0298).
Al plasma time courses after treatment did not exhibit profiles distinctive from that of the vehicle group, except the pAP curve showing an apparent peak on day 10 with a maximum Al difference to baseline of about 30 µg/L. Total Al plasma exposure in terms of AUC(0–80 d) was significantly enhanced in the pAP, but not in other groups, compared to vehicle with a mean absolute difference of 681 µg/L*d.
Al in tissues
Injection site muscle
None of the IM-treated animals showed palpable indurations at the injection sites throughout the study.
Results of total Al amounts measured in one injection site muscle (M. triceps) on day 80 and calculated fractions of Al dose “remaining” and “released” from M. triceps compared to the injected dose (1/10 of total Al dose) are shown in Table 2 and Fig. 2.
a Mean (+SD) Al amount (difference to vehicle group mean) found in injection site muscle M. triceps of rats 80 days after treatment (light/colored bars) compared to Al amount injected into this muscle on day 0 (black bars). *p < 0.05 (Wilcoxon signed rank test on difference to 100%). b Mean (+SD) extrapolated Al release from all injection site muscles per rat at day 80 p.i
After treatment with pAH total injected Al amount was completely recovered in M. triceps at day 80 (102.1%), whereas mean percentage Al “remaining” in the pAP group was 33.1% only. In contrast to V1 (77.7%), the percentage Al “remaining” was also significantly below 100% in groups V2 and V3 (68.2 and 14.5%, respectively; Fig. 2a).
The highest percentage Al dose “released” from the injection site was found in group V3 (85.5%) followed by pAP (66.9%). Due to the higher Al dose injected, the highest absolute Al amount released from all injection site muscles was estimated for pAP (836.8 µg) followed by 427 mg for V3 (Fig. 2b and Table 2).
Bone
In all treatment groups geometric mean Al bone concentration at day 80 p.i. was significantly higher than in the vehicle controls (all p values < 0.001; Table 2 and Fig. 3a). Variability in the treatment groups was low (CV 11.6–62.4%). Maximum geometric mean Al concentration found was 3.33 µg/g ww (pAP group) which amounts to an absolute difference of 3.28 µg/g ww compared to GM in vehicle controls (0.05 µg/g ww). Absolute GM differences were 2–15 times lower (1.40, 1.23, 0.76, and 0.22 µg/g ww) in V3, V2, pAH, and V1 group, respectively.
Brain
Geometric mean Al concentration in the right brain hemisphere was below 0.3 µg/g ww (1 µg/g dw) in all groups with low inter-individual variability (CV < 36%; Table 2 and Fig. 3b). In three groups (V1, V2, and V3) statistically significant differences to vehicle were observed (Table 2).
Relationship between estimated Al release and plasma/tissue exposure
A positive relationship was found between estimated Al amount released from all injection sites and exposure observed in plasma and bone in all adjuvant treated rats (Fig. 4). For both plasma AUC(0–80 d) (y = 0.57x + 1737; r = 0.35; Fig. 4a) and bone Al concentration (y = 0.0025x + 0.61; r = 0.78; Fig. 4b), a linear increase with total Al release was found.
Discussion
To our knowledge this are the first data demonstrating systemic increase of Al concentrations, particularly in bone, after IM administration of marketed Al-adjuvanted human vaccines in vivo. Though Flarend et al. (1997) investigated short-term plasma and various tissue Al concentrations in two rabbits, they did not evaluate Al levels in bone and used intramuscular (IM) injection of plain self-prepared 26Al-adjuvants (Masson et al. 2018). Their results indicated an increase in Al plasma levels of 1–2 µg/L after a dose of 0.28 mg Al/kg in rabbits. Going beyond, we administered the highest Al adjuvant dose allowed in human vaccines (1.25 mg; WHO 2016; Ph. Eur. 2018) as well as full human doses of marketed human vaccines in rats reaching much higher Al doses in relation to body weight (1.4–3.6 mg/kg). Furthermore, we measured Al in bone being the major storage compartment of Al in both animals and humans (Yokel and McNamara 2001; Priest 2004; Krewski et al. 2007).
The treatment preparations comprised plain suspensions of the two adjuvant types AH and AP which are commonly used in vaccine production (HogenEsch et al. 2018) as well as three authorized vaccine products either solely based on AH (V1) or AP (V3), or both (V2). As these products contain the natural 27Al-isotope, our study was designed to monitor Al “baseline” levels in plasma and tissues resulting from dietary Al intake by use of a control group throughout the whole study period.
After IM application of adjuvanted preparations, only the group treated with plain AP adjuvant showed a significant increase in total Al plasma AUC(0–80 d) which is a robust quantitative measure of plasma exposure. The mean 80d-baseline plasma level of 19.8 µg/L in our control rats is somewhat higher than that expected in healthy humans (0.5–8 µg/L; Krewski et al. 2007). A lower Al baseline level might have been desirable for the purpose of higher sensitivity to detect AUC differences after treatment. However, we decided against dietary depletion of Al in order not to unbalance the Al equilibrium in the body. The observed slight trend of the baseline towards an increase in slope over time did not have impact on our results, since statistical evaluation in plasma was based on comparison of total AUCs between treatment and control group.
The apparent peak (“Cmax”) observed at day 10 after pAP injection is not considered compatible with simple first order absorption kinetics as attempts to estimate an absorption rate constant for pAP by adjusting ka_IM in the recently established model for IM administration of Al citrate (Weisser et al. 2019) was not successful. However, the input process of Al3+ions after injection of insoluble adjuvant particles is probably not characterized by a single kinetic function describing dissolution of the Al complex. Several processes may be involved in parallel [e.g., lymphatic transport of undissolved particles, Al release from immune cells after phagocytosis (He et al. 2015)] causing a substantial delay in the absorption process.
In line with its increase of plasma AUC the pAP group also showed the highest increase of Al concentration in bone (3.28 µg/g ww). However, in contrast to plasma, bone results also indicated systemic availability of Al, though at least twofold less, for all other (including AH-based) formulations. Bone Al levels in the vehicle group (0.05 ± 0.06 µg/g) were extremely low compared to the reference value of 0.53 µg/g ww for healthy rats (mean for all ages; Hirayama et al. 2011). The estimate for the y-intercept of the linear relationship found between Al release and bone Al concentration (0.61 µg/g) suggests a higher “true” control level more in line with the reference value.
A more visible increase in bone exposure rather than plasma is not surprising: fast renal Al plasma clearance prevents a sharp rise of plasma levels above a relatively high baseline level, whereas elimination of Al from bone is very slow, thus, Al amounts reaching bone build a long-term deposit which facilitates detection (Yokel and McNamara 2001; Priest 2004; Krewski et al. 2007).
The findings in plasma and bone were confirmed by the injection site release results as an indirect measure of bioavailability up to day 80. A high Al release was noticed for plain AP (66.9%) and AP-adjuvanted V3 (85.5%) in contrast to very small dose fractions of the Alhydrogel®-adjuvanted preparations pAH and V1 (0 and 22.3%, resp.). In accordance with its mixed composition V2 showed a degree of release between both extremes (31.8%). Thus, we observed a remarkable difference in the degree of Al release up to day 80 between AP and AH after injection of plain adjuvants as well as vaccines containing the respective adjuvant type. Crude linear extrapolation from 100% on day 0 through the mean dose fraction of V1 remaining at the injection site on day 80 (77.7%) predicts that complete absorption of Al from AH-adjuvanted vaccines will take at least 350 days (1 year). In contrast, linear extrapolation through the remaining dose fraction for V3 (14.5%) suggests that Al from AP-adjuvanted vaccines might be completed much earlier after ca. 120 days.
Our results are in line with injection site muscle measurements after vaccination in macaques by Verdier et al. (2005) who still observed substantial Al concentration in M. quadriceps after injection of the AH-adjuvanted vaccine at 6 months p.i., in contrast to low but significant Al concentrations above control at 3 months (90 d) but no longer at 6 months (180 d) after injection of an AP-adjuvanted vaccine. In contrast to Verdier et al., we collected the whole injected muscle being able to quantify the percentage of injected dose. Our quantitative differences suggest a 3- to 4-fold higher rate of systemic availability for AP than AH. The results are fully in line with the threefold Al plasma AUC(0–28 d) found after self-prepared plain AP compared to AH in rabbits (Flarend et al. 1997). We could demonstrate that this difference also applies to marketed adjuvanted vaccines.
The disparity is most probably attributed to well-known physicochemical differences between AP and AH, mainly the degree of crystallinity, chemical composition and surface charge: AH consists of crystalline Al-oxyhydroxide (AlOOH), whereas AP is chemically composed of Al(OH)x(PO4)y in which the ratio of hydroxyls to phosphate depends on the precipitation conditions. As a consequence, AP is non-crystalline (amorphous), because the incorporation of phosphate interferes with the crystallization process, and, in contrast to AH, has a negative surface charge at neutral pH (HogenEsch et al. 2018; Powell et al. 2015; He et al. 2015). Higher solubility of AP compared to AH is clearly seen in dissolution experiments with adjuvants in vitro (Seeber et al. 1991; personal unpublished data). Thus, we conclude that our finding is mainly attributed to these physicochemical differences favoring release and dissolution of Al from AP adjuvant.
A further reason for the high recovery of AH-adjuvants 80 days after injection could be the development of granuloma as a foreign body reaction subsequently preventing Al dissolution. Although more commonly seen after SC application of AH-adjuvants, development of persistent granuloma at the injection site has also been reported after IM application, often accompanied by Al contact allergy (Netterlid et al. 2013). Since IM granuloma is less palpable, occurrence might be underestimated. For example, a 100% frequency of granuloma was observed in the neck of 31 pigs after IM injection of AH-adjuvanted vaccines (Valtulini et al. 2005). Also in mice a high number was found after IM injection of Alhydrogel® or HBV Engerix® vaccine (93% at day 45 decreasing to still 35% at day 270 p.v.; Crépeaux et al. 2015).
The highest estimate of absolute Al release from all injection sites for pAP is fully consistent with the highest increase in plasma AUC and bone Al concentration found for this group. Corresponding correlations obtained for all rats between estimated Al amount released from the injection site and both plasma and bone Al exposure confirm that Al release can be interpreted as systemically available amount and increase in bone and plasma exposure are fairly proportional to this amount. However, we cannot exclude overestimation of systemically available amounts as the total Al release might include a fraction of still undissolved Al particles phagocytosed and transported to the draining lymph node by antigen-presenting immune cells (He et al. 2015).
The highest total bone Al concentration measured in our rats (3.35 µg/g ww) is far below levels of toxicological concern. Studies conducted by Sun et al. (2015, 2016) indicated that rats with bone Al concentrations up to 15 µg/g (ww) were without abnormal findings, whereas above 20 µg/g (ww) bone formation markers decreased and oxidative stress markers increased, and in groups > 30 µg/g (ww) bone mineral density (BMD) decreased significantly.
Also in humans bone Al levels below 10–15 µg/g are not associated with “Al-overload” or any signs of bone toxicity (Klein 2019; Hellström et al. 2005, 2006; Van Landeghem et al. 1998).
Extrapolating the Al increase found in humerus bone to the whole rat skeleton (using 25 g skeleton weight for a 350 g rat [Brown et al. 1997; O’Flaherty 1991)], a mean treatment-related Al amount “added” to the skeleton of 82.6, 7.1, 31.3, and 35.4 µg per rat is estimated for groups pAP, V1, V2, and V3, respectively. These amounts represent 5.3–12.0% of the corresponding total Al amounts released from the injection site (Table 2). These percentages are in line with dose fractions of 3–20% found in rat skeleton during 1 year after a single IV dose of 26Al-chloride (Steinhausen 1997).
Very low brain Al concentrations were observed in all groups. Geometric mean level in the control group (0.12 µg/g ww) was well in line with reported control levels in rat brain of 0.02–0.8 µg/g ww (Ogasawara et al. 2002; Veiga et al. 2013; Lin et al. 2015). Statistical significance of brain Al levels in the vaccine groups is not consistent with the ranking of the products regarding Al release from injection site or Al concentration in bone. Of note, despite its highest bioavailable Al amount and highest increase in bone and plasma Al exposure pAP did not show any increase in Al concentration in brain. From 26Al-kinetic data in rats it is known that in contrast to bone only a very small fraction of dose (< 0.01%) retains in brain (Yokel and McNamara 2001; Walker et al. 1994; Yumoto et al. 1997). Several animal studies demonstrated that brain has much lower Al concentrations than many other tissues, also in normal human beings (Yokel and McNamara 2001). A fraction of 0.01% of the highest bioavailable amount in our study (836.8 µg) would correspond to 0.084 µg Al as the maximum amount supposed to have reached brain. Equal distribution in a rat brain weighing 2 g (estimate for a male 350 g rat; Brown et al. 1997) would lead to a maximum brain concentration increase of 0.042 µg/g ww. Considering our control group mean level (0.13 ± 0.04 µg/g ww), this small difference is unlikely to be detected. Overall, this rather supports the notion that the small increases in brain Al concentration found for V1–V3 are chance findings.
As we determined Al concentration in a whole brain hemisphere Al clusters due to focal accumulation which have been reported for human brain tissues (House et al. 2012) could not be missed. Furthermore, as determination by AAS comprises dissolved Al3+ ions as well as insoluble Al species, our results would also capture any Al particles transported into the brain by macrophages which has been postulated by some authors (Gherardi et al. 2015; Crépeaux et al. 2015; Shardlow et al. 2018). Based on our results, we conclude that contribution of such particulate Al amounts, if any, are marginal.
In summary, the present study for the first time revealed systemically available Al from IM injected adjuvants and adjuvanted vaccines in vivo through increase of Al levels mainly in bone. The findings were corroborated by significant correlations with total Al release from the injection site. Moreover, our results clearly indicate that the rate of systemic availability of Al is markedly higher from AP- than from AH-adjuvanted vaccines. We are aware that tissue determination on day 80 is only a cross-sectional view and that different bone levels might only reflect different rates of absorption. This would imply that, once Al absorption is completed, two products with comparable Al doses might reach comparable cumulative Al concentrations in bone, however, at different time points.
Increases of Al exposure in plasma and bone observed in rats cannot one-to-one be translated to humans, this is especially true for bone allometry with inter-species differences in bone architecture and remodeling (Bagi et al. 2011; Barak et al. 2013). In relation to body weight the doses applied to our rats (mean body weight 350 g) were 170 times higher compared to application to a 60 kg human adult. Considering an allometric scaling factor of 6.2 which is usually applied for dose conversion on mg/kg basis between rats and humans in pharmacology (FDA 2005; Nair and Jacob 2018), this ratio is still 27. Thus, we may expect that after a single vaccination in adults Al levels in bone, and even more valid in plasma and brain, will be indistinguishable from baseline levels. With respect to children simple allometric dose scaling is not adequate, in particular for infants below 2 years of age due to complex age-related developmental changes (Lu and Rosenbaum 2014). For that purpose, physiology-based modeling is required as it is increasingly used in pediatric drug development and toxicologic evaluations (Sharma and McNeill 2009; Barrett et al. 2012). The results of this study will be highly valuable for establishment of a physiology-based toxicokinetic (PBTK) model for Al exposure from adjuvants (Weisser et al. 2017).
References
Bagi CM, Berryman E, Moalli MR (2011) Comparative bone anatomy of commonly used laboratory animals: implications for drug discovery. Comp Med 61:76–85
Barak MM, Lieberman DE, Hublin JJ (2013) Of mice, rats and men: trabecular bone architecture in mammals scales to body mass with negative allometry. J Struct Biol 183:123–131
Barrett JS, Della Casa Alberighi O, Läer S, Meibohm B (2012) Physiologically based pharmacokinetic (PBPK) modeling in children. Clin Pharmacol Ther 92:40–49
Brown RP, Delp MD, Lindstedt SL, Rhomberg LR, Beliles RP (1997) Physiological parameter values for physiologically based pharmacokinetic models. Toxicol Ind Health 13:407–484
Crépeaux G, Eidi H, David MO, Tzavara E, Giros B, Exley C, Curmi PA, Shaw CA, Gherardi RK, Cadusseau J (2015) Highly delayed systemic translocation of aluminum-based adjuvant in CD1 mice following intramuscular injections. J Inorg Biochem 152:199–205
FDA/CDER guidance for industry: Estimating the maximum safe starting dose in initial clinical trials for therapeutics in adult healthy volunteers (2005) https://www.fda.gov/downloads/drugs/guidances/ucm078932.pdf. Accessed 13 Sept 2019
Flarend RE, Hem SL, White JL, Elmore D, Suckow MA, Rudy AC, Dandashli EA (1997) In vivo absorption of aluminium-containing vaccine adjuvants using 26Al. Vaccine 15:1314–1318
Gherardi RK, Eidi H, Crépeaux G, Authier FJ, Cadusseau J (2015) Biopersistence and brain translocation of aluminum adjuvants of vaccines. Front Neurol 6:4
He P, Zou Y, Hu Z (2015) Advances in aluminum hydroxide-based adjuvant research and its mechanism. Hum Vaccin Immunother 11:477–488
Hellström HO, Mjöberg B, Mallmin H, Michaëlsson K (2005) The aluminum content of bone increases with age, but is not higher in hip fracture cases with and without dementia compared to controls. Osteoporos Int 16:1982–1988
Hellström HO, Mjöberg B, Mallmin H, Michaëlsson K (2006) No association between the aluminium content of trabecular bone and bone density, mass or size of the proximal femur in elderly men and women. BMC Musculoskelet Disord 7:69
Hem SL, HogenEsch H (2007) Relationship between physical and chemical properties of aluminum-containing adjuvants and immunopotentiation. Expert Rev Vaccines 6:685–698
Hirayama M, Iijima S, Iwashita M, Akiyama S, Takaku Y, Yamazaki M, Omori T, Yumoto S, Shimamura T (2011) Aging effects of major and trace elements in rat bones and their mutual correlations. J Trace Elem Med Biol 25:73–84
HogenEsch H, O’Hagan DT, Fox CB (2018) Optimizing the utilization of aluminum adjuvants in vaccines: you might just get what you want. NPJ Vaccines 3:51
HogenEsch H (2013) Mechanism of immunopotentiation and safety of aluminum adjuvants. Front Immunol 3:406
House E, Esiri M, Forster G, Ince PG, Exley C (2012) Aluminium, iron and copper in human brain tissues donated to the Medical research council’s cognitive function and ageing study. Metallomics 4:56–65
Immunization Safety Review (2001) Thimerosal-containing vaccines and neurodevelopmental disorders. Institute of Medicine (US) Immunization safety Review Committee. National Academies Press (US), Washington
Immunization Safety Review (2004) Vaccines and Autism. Institute of Medicine (US) Immunization safety review committee. Washington (DC): National Academies Press (US)
Klein GL (2019) Aluminum toxicity to bone: A multisystem effect? Osteoporos Sarcopenia 5:2–5
Krewski D, Yokel RA, Nieboer E, Borchelt D, Cohen J, Harry J et al (2007) Human health risk assessment for aluminium, aluminium oxide, and aluminium hydroxide. J Toxicol Environ Health B Crit Rev 10(Suppl 1):1–269
Lin WT, Chen RC, Lu WW, Liu SH, Yang FY (2015) Protective effects of low-intensity pulsed ultrasound on aluminum-induced cerebral damage in Alzheimer’s disease rat model. Sci Rep 5:9671
Lu H, Rosenbaum S (2014) Developmental pharmacokinetics in pediatric populations. J Pediatr Pharmacol Ther 19:262–276
Masson JD, Crépeaux G, Authier FJ, Exley C, Gherardi RK (2018) Critical analysis of reference studies on the toxicokinetics of aluminum-based adjuvants. J Inorg Biochem 181:87–95
Nair AB, Jacob S (2016) A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm 7:27–31
Netterlid E, Hindsén M, Siemund I, Björk J, Werner S, Jacobsson H, Güner N, Bruze M (2013) Does allergen-specific immunotherapy induce contact allergy to aluminium? Acta Derm Venereol 93:50–56
O’Flaherty EJ (1991) Physiologically based models for bone-seeking elements. I. Rat skeletal and bone growth. Toxicol Appl Pharmacol 111:299–312
Ogasawara Y, Sakamoto T, Ishii K, Takahashi H, Tanabe S (2002) Effects of the administration routes and chemical forms of aluminum on aluminum accumulation in rat brain. Biol Trace Elem Res 86:269–278
Ph. Eur. 9.6, Monograph 0153: Vaccines for human use (07/2018)
Powell BS, Andrianov AK, Fusco PC (2015) Polyionic vaccine adjuvants: another look at aluminum salts and polyelectrolytes. Clin Exp Vaccine Res 4:23–45
Priest ND (2004) The biological behaviour and bioavailability of aluminium in man, with special reference to studies employing aluminium-26 as a tracer: review and study update. J Environ Monit 6:375–403
Seeber SJ, White JL, Hem SL (1991) Solubilization of aluminum-containing adjuvants by constituents of interstitial fluid. J Parenter Sci Technol 45:156–159
Shardlow E, Mold M, Exley C (2018) Unraveling the enigma: elucidating the relationship between the physicochemical properties of aluminium-based adjuvants and their immunological mechanisms of action. Allergy Asthma Clin Immunol 14:80. https://doi.org/10.1186/s13223-018-0305-2
Sharma V, McNeill JH (2009) To scale or not to scale: the principles of dose extrapolation. Br J Pharmacol 157:907–921
Steinhausen C (1997) Untersuchung der Aluminiumbiokinetik mit 26Al und Beschleunigermassenspektrometrie. Dissertation, Technical University of Munich, Germany
Sun X, Liu J, Zhuang C, Yang X, Han Y, Shao B et al (2016) Aluminum trichloride induces bone impairment through TGF-β1/Smad signaling pathway. Toxicology 371:49–57
Sun X, Cao Z, Zhang Q, Liu S, Xu F, Che J et al (2015) Aluminum trichloride impairs bone and downregulates Wnt/β-catenin signaling pathway in young growing rats. Food Chem Toxicol 86:154–162
Valtulini S, Macchi C, Ballanti P, Cherel Y, Laval A, Theaker JM, Bak M, Ferretti E, Morvan H (2005) Aluminium hydroxide-induced granulomas in pigs. Vaccine 23:3999–4004
Van Landeghem GF, D’Haese PC, Lamberts LV, Djukanovic L, Pejanovic S, Goodman WG et al (1998) Low serum aluminum values in dialysis patients with increased bone aluminum levels. Clin Nephrol 50:69–76
Veiga M, Bohrer D, Banderó CR, Oliveira SM, do Nascimento PC, Mattiazzi P et al (2013) Accumulation, elimination, and effects of parenteral exposure to aluminum in newborn and adult rats. J Inorg Biochem 128:215–220
Verdier F, Burnett R, Michelet-Habchi C, Moretto P, Fievet-Groyne F, Sauzeat E (2005) Aluminium assay and evaluation of the local reaction at several time points after intramuscular administration of aluminium containing vaccines in the Cynomolgus monkey. Vaccine 23:1359–1367
Walker VR, Sutton RA, Meirav O, Sossi V, Johnson R, Klein J et al (1994) Tissue disposition of 26aluminium in rats measured by accelerator mass spectrometry. Clin Invest Med 17:420–425
Weisser K, Göen T, Oduro JD, Wangorsch G, Hanschmann KMO, Keller-Stanislawski B (2019) Aluminium toxicokinetics after intramuscular, subcutaneous, and intravenous injection of Al citrate solution in rats. Arch Toxicol 93:37–47
Weisser K, Stübler S, Matheis W, Huisinga W (2017) Towards toxicokinetic modelling of aluminium exposure from adjuvants in medicinal products. Regul Toxicol Pharmacol 88:310–321
WHO Expert Committee on Biological Standardization, sixty-sixth report. Geneva: World Health Organization; 2016 (WHO technical report series; no. 999)
WHO (2012) Global advisory committee for vaccine safety“(GACVS), Report of Meeting Held 6–7 June 2012. WHO Weekly Epidemiological Record
Yokel RA, McNamara PJ (2001) Aluminium toxicokinetics: an updated minireview. Pharmacol Toxicol 88:159–167
Yumoto S, Nagai H, Imamura M, Matsuzaki H, Hayashi K, Masuda A et al (1997) 26Al uptake and accumulation in the rat brain. Nucl Instrum Methods Phys Res B 123:279–282
Acknowledgements
The authors thank Barbara Verhoeven for her technical assistance and Daniela Golomb for preparation of the treatment formulations.
Funding
The project was funded by the German Ministry of Health (ZMVI1-2515-FSB-772).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Author Jennifer D. Oduro declares that she is employee at preclinics GmbH, a contract research organization that has received payment for conducting the animal study. All other authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution (preclinics GmbH, Germany) at which the studies were conducted.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Weisser, K., Göen, T., Oduro, J.D. et al. Aluminium in plasma and tissues after intramuscular injection of adjuvanted human vaccines in rats. Arch Toxicol 93, 2787–2796 (2019). https://doi.org/10.1007/s00204-019-02561-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-019-02561-z