Abstract
The supply of food for the world population that is increasing is one of the concerns of governments. The Food and Agriculture Organization of the United Nations assessment shows that the aquaculture industry could help meet food needs for human communities. The aquaculture industry also relies on providing a feed of high quality. Minerals are one essential component of an aquatic diet. Chromium (Cr) is a trace element that finds the form of Cr+3 (trivalent) and Cr+6 (hexavalent) in nature and food items. Studies show that exposure to Cr waterborne have toxicity effects on fish. However, oral exposure to Cr has a different impact on fish. Cr is usually involved in the metabolism of fats, carbohydrates, proteins, growth function, enzyme functions, etc. This element could play a significant role in fish nutrition and physiology. Cr as a dietary supplement can improve growth performance and adjust the metabolism of carbohydrates and lipids. However, high concentrations of Cr can be toxic to fish. Although the physiological effects of Cr on aquatic organisms are well known, there are still ambiguities in determining the appropriate concentration in the diet of some species. Maybe, the physiological response of fish depends on the concentration, origin, and chemical composition of Cr, as well as the biological and individual characteristics of the fish. Therefore, it is necessary to estimate the appropriate concentration of Cr in fish diets. This article aims to summarize the available information about the effect of Cr on various physiological indicators and fish growth. Therefore, this information may help to find the appropriate concentration of Cr in the diet.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heavy metals are a group of metals and metalloids that have a relatively high density and are very toxic to plants and animals [1]. If the heavy metals enter a body of organisms through the food chain, they can have a toxic effect on the body of animals [2]. Some heavy metals, such as chromium (Cr), are also known as trace elements [3, 4]. This metal finds in different forms, divalent (Cr+2), trivalent (Cr+3), and hexavalent (Cr+6) forms. Although Cr+3 and Cr+6 are the most dominant and stable forms [5], Cr + 3 is a natural and stable form of chromium that find in living organisms [6]. Also, Cr+3 as a cofactor could affect the metabolism of glucose, lipids, and proteins in various animal species [7]. Cr binds to an oligopeptide to form chromodulin, a low molecular weight. Next, chromodulin conjugates to insulin receptors and enhances insulin affinity for its receptor [8, 9]. The role of chromium in carbohydrate metabolism has been reported for turkeys [10] and humans [11]. Furthermore, Cr has an important role in the metabolism of protein, nucleic acid, and lipids [12]. Cr also reduces cholesterol and triglyceride [13, 14] and enhances the concentration of insulin and high-density lipoprotein (HDL) in serum [15, 16]. However, there is little information about the nutritional effects of dietary chromium on various fish (Tables 1, 2, 3, and 4).
In fish, Cr may be absorbed through the gills and gastrointestinal tract and then enter the tissues through the blood. However, its absorption and excretion mechanisms are unclear. Studies showed that Cr could be uptake from water and diet [35]. Although Cr could affect fish’s nutritional and physiological responses [36], Cr is not an essential biological element [37]. Previous studies showed that oral administration Cr-picolinate (Cr-Pic) increased the crude protein content in the carcass of Nile tilapia (Oreochromis niloticus), while decreasing crude fat [18]. A significant decrease was reported in the carcass fat content in the herbivorous carp (Ctenopharyngodon idellus) fed Cr-Pic dietary supplementations [15]. However, feeding zebrafish (Danio rerio) breeders with chromium-rich un-encapsulated Artemia cyst reduced larval survival rates [38].
Compared to birds and mammals, fish often rely on protein sources to supply energy and use fewer carbohydrates as energy sources. Therefore, fish need Cr much less than mammals and poultry [39,40,41]. Previous studies showed that Cr is involved in the metabolism of carbohydrates [4, 42] and act as a cofactor for insulin in transporting glucose from the bloodstream to peripheral tissues [43]. Consequently, Cr dietary supplementation can increase protein-sparing efficiency and make it possible to replace carbohydrates as an energy source for fish.
Moreover, studies revealed that fish’s physiological response to Cr supplements might depend on species, age, gender, concentrations of Cr, and Cr supplement origin. Furthermore, biological conditions, physicochemical quality of water and the breeding system, and test duration may also affect the results [44,45,46].
Research on the effects of Cr on fish health and growth is very limited and often includes studies on the toxicity of Cr. Therefore, there is a gap in information about the effects of Cr on fish. Therefore, in the present paper, a literature review on the impacts of Cr on fish has been done. Hence, studying its effects on aquatic health seems necessary to better understand the advantages and disadvantages of using Cr supplements in fish diets.
The Effects on Growth Rate
The effect of Cr on fish growth is highly contradictory and depends on the concentration of Cr in the diet, a chemical form of Cr, and species of fish. Feeding Cyprinus carpio with 0.5 mg kg−1 Cr increased growth performance after 65 days, while oral administration of 2 mg kg−1 Cr reduced growth after 30 days [17]. A significant increase was observed in the growth performance of grass carp (Ctenopharyngodon idellus) fed with dietary supplements containing 0.8 mg kg−1 for 10 weeks, while growth indexes decreased when Cr concentration increased [15]. Improvement in growth indices and an increase in hepatic glycogen stores were also observed in Nile tilapia [22], and hybrid tilapia (Oreochromis niloticus × O. aureus) [28] fed Cr supplementation. Feeding fingerlings L. rohita and grass carp, C. idellus with 800 μg kg−1 Cr-Pic significantly improved growth performance indexes. Results showed that alterations in growth performance were related to increasing protein assimilation [25].
Furthermore, Giri et al. [13] and Liu et al. [15] found that Cr-Pic could improve the efficiency of consuming carbohydrates as an energy source. Therefore, Cr-picolinate supplementation could affect the protein-sparing action of carbohydrates. Similarly, increased survival and growth rates were reported in fish fed with Cr supplementation [26, 47].
In contrast, oral administration of Cr-Pic dietary supplement did not have a significant effect on the growth performance of O. niloticus [18], gilthead seabream (Sparus aurata) [19], and rainbow trout (O. mykiss) [20, 21].
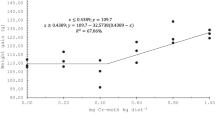
Growth performance decreased after young golden pompano (Trachinotus ovatus) fed 20 mg kg−1 Cr-nicotinate (Cr-Nic) supplementation [23]. Decreased growth was observed in Crocker (Larimichthys crocea) [47], tilapia [15, 48], and rainbow trout [49] fed with diets containing relatively high levels of Cr. A decrease in growth performance and feed efficiency may be due to the toxic effect of Cr. Oral administration of dietary supplements containing 8 mg kg−1 caused toxicity and reduced the growth performance of striped catfish [24]. In common carp (Cyprinus carpio), the concentration of 0.5 mg kg−1 of Cr supplement in the diet increased growth, while the concentration of 2 mg kg−1 decreased growth [17]. Previous studies showed that increasing concentrations of Cr could affect the feed taste [24]. Therefore, the administration of high concentrations of Cr supplementation could reduce fish’s appetite and growth performance [24].
The Effects of Cr on Carbohydrate Metabolism
Cr can affect glucose metabolism and helps insulin transport glucose into cells for energy production. Cr-Pic dietary supplements can regulate urea and glucose levels. Feeding C. carpio with CrCl2 supplementation decreased glucose levels in the serum [27]. The use of Cr-poly nicotinate in the diet of juvenile T. ovatus could increase the protein-sparing effect of carbohydrates [23]. A significant decrease in gluconeogenesis in the liver of Labeo rohita indicated that the administration of Cr could change the carbohydrate metabolism pathway [25]. Change in the enzyme activities involved in carbohydrate metabolism was observed in Catla catla [50], C. carpio [27], and hybrid tilapia [51] fed with Cr supplement. Increasing the catecholamine levels affect glycogenolysis in the liver of Anabas scandens fed with Cr supplement [52]. The effect of Cr on insulin functions may accelerate the assimilation of amino acids and protein biosynthesis [26, 53].
The Effects on Fat Metabolism
Chromium salts can accelerate the process of lipogenesis and affect glycogen accumulation in the presence of insulin [10, 54,55,56]. Insulin reduces fat lipolysis by reducing the adenylate cyclase activity and hormone-sensitive lipase [57]. The high body fat content of tilapia was significantly observed in glucose-containing diets in which chromium supplementation was used compared to chromium-free diets [28, 29]. The liver glycogen content of Atlantic salmon (Salmo salar L) increased at different levels of the presence of corn starch in the diet.
The reduction of crude fat in fish carcasses fed with high concentrations of Cr supplementation may be due to the toxicity of Cr [58]. However, administration of low levels of Cr-Pic supplementation (800 μg kg−1) could increase the fat content of fish carcasses [15]. It is hypothesized that the administration of low levels of Cr-Pic supplement may cause fish to use carbohydrates for energy and dietary lipids to accumulate in fish tissue [51].
Studies showed that high levels of Cr can reduce lipid storage by regulating lipogenesis [58]. However, there is no report on the effect of Cr supplementation on the activity of glucose-6-phosphate dehydrogenase (G6PDH), one of the enzymes involved in lipolysis [30,31,32]. Therefore, there was insufficient evidence to support the involvement of G6PDH in lipogenesis [25]. Cr supplementation may accelerate the conversion of glucose to Acetyl-CoA (necessary in the process of lipogenesis) [55, 56], possibly by increasing pyruvate dehydrogenase [59], Acetyl-CoA carboxylase, and citrate lyase activity [60] followed by promoting lipogenesis.
The Effect on Protein Metabolism
The fact that chromium is involved in protein metabolism has been well established [61] and improves the function of insulin to regulate amino acid metabolism [62]. Due to its lipophilic nature, Cr-Pic supplementation used in the diet can increase cell membrane fluidity and insulin uptake to accelerate insulin activity, and therefore amino acid transfer and protein synthesis may increase [63, 64]. Previous studies have shown that Cr in the diet positively affects crude carcass protein [65]. Cr-Pic dietary supplementation (up to 1200 µg L−1) significantly increased crude protein content and decreased ether extract content in Nile tilapia O. niloticus [18]. Moreover, a significant increase was reported in the crude protein content of Nile tilapia carcasses fed Cr supplement [18, 34]. Laboratory studies showed that Cr is involved in nucleic acid metabolism and biosynthesis of proteins in the liver [66]. Cr supplementation could reduce blood urea nitrogen content, indicating improved protein synthesis in fish [22].
The Effects on Enzymes
Enzymes are large biochemical molecules that monitor metabolic processes in living organisms, so a slight change in enzyme activity in the body can affect the condition of an organism [67]. Accordingly, by assessing the enzymatic activity in an organism, a metabolic disorder can be easily recognized. Enzymatic activities also prepare rapid screening methods to assess the health of various fish and can be used to estimate the initial lethal concentration of a toxin. Creatine kinase is found in various body tissues containing bones and muscles. It must catalyze the conversion reaction of creatine to phosphocreatine by dividing itself in the conversion of adenosine triphosphate (ATP). In one study, chromium supplementation in the diet significantly decreased creatine kinase activity under cold stress conditions [22]. Although Cr could not significantly affect creatine kinase activity in serum, its activity decreased in the kidney and liver of catfish [33].
Cr is a metal element that significantly affects the activity of various enzymes in the body. Cr supplementation in the diet increases liver enzymes such as glycolytic enzymes and lipogenic enzymes associated with an early section of glycolysis and lipogenesis pathways [68], which elucidate the mechanisms regulating carbohydrate intake [69, 70]. Cr supplementation could significantly affect blood glucose homeostasis by regulating the gene expression of enzymes involved in glucose regulation (phosphoenolpyruvate carboxykinase PEPCK, pyruvate kinase PK, glycogen synthase GS, and glucose-6-phosphatase G6Pase) [34]. Moreover, Cr dietary can also regulate lipid levels in mRNA levels of lipogenesis genes in Blunt Snout Bream (Megalobrama amblycephala) [34].
In a previous study, the effect of Cr on glucokinase (GK), pyruvate kinase (PK), hexokinase (HK) and 6-phosphofructokinase (PFK) activities, which are key enzymes in the glycolysis process, were assessed [23]. GK is the first glycolytic pathway enzyme that plays an essential role in catalyzing the conversion of glucose to glucose-6-phosphate, which is an intermediate metabolite and can be used in various catabolic metabolic pathways (glycogenesis and pentose phosphate) [71,72,73,74]. In some fish, such as rainbow trout, carp, goldfish, and gilthead seabream, liver GK enzyme activity is strongly provoked after the use of carbohydrates, and this action is communicated to the high expression of the liver GK enzyme gene [71,72,73,74]
In one analysis, the activities of GK and PFK enzymes in the groups that received Cr-Nic in their diet were not significantly different from the control groups. On the other hand, Cr-Nic in the diet naturally increased HK and PK activities compared to control groups [23]
Glycolysis is the only pathway for glucose catabolism in various organisms, including fish [75], and involves the progressive oxidation of one molecule of glucose (6C) to two molecules of pyruvate (3C). HK and PK are key enzymes that regulate the glycolytic pathway. The HK enzyme catalyzes the first glycolysis reaction, which involves the phosphorylation of glucose to glucose-6-phosphate, a molecule that may be used in other metabolic and catabolic pathways such as glycogenesis and the pentose-phosphate pathway [76,77,78]
PK catalyzes the last stage of glycolysis, which converts phosphoenolpyruvate to pyruvate [79,80,81,82,83]. A study found that Cr-Nic supplementation possibly regulated glycolytic processes by increasing HK and PK activity, thereby increasing growth performance and feed efficiency in fish-fed diets containing chromium supplements. In addition, hepatic glycogen was significantly lower in the food groups that have Cr-Nic supplements than in the control group. The various digestive organs of the body are highly sensitive to food composition and cause immediate changes in the activity of digestive enzymes [84,85,86]. Increased activity of digestive enzymes indicates better utilization of nutrients in the diet and thus better growth. The study found that amylase, protease, and lipase activity in intestinal tissue was significantly increased in diets containing 800 μg kg−1 of Cr-Pic supplementation, indicating an improvement in nutrient utilization, and the result is higher growth performance [25].
The Effect of Chromium on Cortisol
Cr supplement could decrease stress by reducing cortisol in serum [87,88,89,90,91,92]. Stressors increase plasma cortisol levels, including cold exposure [88] and short-term heat exposure [89]. How chromium affects cortisol production is unknown. Glucocorticoids inhibit insulin excretion [93]. Because chromium boosts insulin function, it may inhibit cortisol secretion reversely. In a study on tilapia, serum cortisol levels decreased following dietary chromium supplementation [22].
Toxicity of Chromium
High levels of Cr in diet and water cause tissue changes in the intestine, gills, liver, and kidneys, but the mechanism of toxicity is not yet known [35, 94]. The toxicity of Cr+3 is very low. However, Cr+6 is more toxic than the Cr+3 form due to its easy permeability through cell membranes [95]. Toxic Cr+6 readily crosses cell membranes and then decreases when converted to the trivalent form. The Cr3+ form combines with numerous macromolecules, including genetic material, within the cytosol, eventually resulting in changes due to the toxic and mutagenic form of chromium [96].
Kim and Kang [97] found that dietary chromium (Cr+6) exposure could induce oxidative damage and block the catalytic domain of acetylcholine esterase [97]. The bioaccumulation capacity of Cr in fish has been studied [98,99,100,101]. Kumar et al. [102] showed that the toxicity of Cr in fish depends on the pH and temperature of the water [102]. Also, Lunardelli et al. [103] detected a significant correlation between Cr concentrations in the environment and alteration in oxidative biomarkers in the Neotropical fish, Prochilodus lineatus [103]. Similar changes in the antioxidant enzymes were reported in the liver of eel fish (Anguilla anguilla) [104].
Moreover, Mohamed et al. [105] reported histopathological and hematological changes in O. niloticus exposed to Cr+6 [105]. Similarly, growth performance and a decrease in CYP450 and GST gene expression. Furthermore, the metallothionein gene expression increased in the liver of juvenile rockfish Sebastes schlegelii after oral exposure to Cr [6].
Histopathological damage to gills, increased mucus secretion, and increased blood lactate were observed in Colisa fasciatus following exposure to Cr [106].
Conclusion
Although Cr is not the essential biological element, it can affect the physiology and health of fish. Fish can uptake Cr from diet and water. However, oral administration of Cr may have contradictory impacts on fish. Exposure to waterborne Cr could have toxicity effects on fish. Literature reviews showed that Cr could play a role in the metabolism of fats, carbohydrates, and proteins and affect the function of enzymes and some parameters. Also, the suitable levels of Cr in fish diets could improve growth performance. In contrast, high concentrations of Cr could have toxic effects on fish and decrease fish carcasses’ growth rate and quality.
Overall, the chemical structure, origin, concentration, and bioavailability of Cr play a decisive role in its effects on fish. Furthermore, individual characteristics, species, gender, and environmental conditions also play a role in the physiological response of fish exposed to Cr. This information suggests that knowledge of the non-toxic concentrations of Cr in the food and environment is essential for different fish species. As a result, this report may help to design a complete diet containing chromium supplements.
Data Availability
The data that support the findings of this study are available from Shiraz University but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of Shiraz University.
References
Aslam S, Yousafzai AM (2017) Chromium toxicity in fish: a review article. Journal of Entomology and Zoology Studies 5(3):1483–1488
Coban MZ, Eroğlu M, Canpolat O, Calta M, Şen D (2013) Effect of chromium on scale morphology in scaly carp (Cyprinuscarpio L.). J Anim Plant Sci 23(5):1455–1459
Kabata-Pendias A, Pendias H 2001 Trace elements in soils and plants, 3rd edn CRC Press. Boca Raton, FL, USA
Mertz W (1993) Chromium in human nutrition: a review. J Nutr 123:626–633. https://doi.org/10.1093/jn/123.4.626
Velma V, Vutukuru SS, Tchounwou PB (2009) Ecotoxicology of hexavalent chromium in fresh water fish: a critical review. Rev Environ Health 24(2):129–145. https://doi.org/10.1515/REVEH.2009.24.2.129
Friberg L, Kjellström T, Nordberg G, Piscator M 1979 Cadmium. Handbook on the Toxicology of Metals. Edited by L. Friberg et al. Nordberg L, Vouk GF, VB: 355–381
Steven JD, Davies LJ, Stanley EK, Abbott RA, Ihnat M, Bidstrup L, Jaworski JF (1976) Effects of chromium in the Canadian environment. Nat Res Coun Canada, NRCC No 15017:168
Boyd M (2013) The role of supplemental chromium on glucose intolerance and insulin resistance. Top Clin Nutr 28:171–180. https://doi.org/10.1097/TIN.0b013e31828d7bb1
Hua Y, Clark S, Ren J, Sreejayan N (2012) Molecular mechanisms of chromium in alleviating insulin resistance. J of Nutr Biochem 23:313–319
Rosebrough RW, Steele NC (1981) Effect of supplemental dietary chromium or nicotinic acid on carbohydrate metabolism during basal, starvation, and refeeding periods in poults. Poult Sci 60(2):407–417. https://doi.org/10.3382/ps.0600407
Levine RA, Streeten DH, Doisy RJ (1968) Effects of oral chromium supplementation on the glucose tolerance of elderly human subjects. Metab 17:114–125. https://doi.org/10.1016/0026-0495(68)90137-6
Amata IA (2013) Chromium in livestock nutrition: a review. Glob Adv Res J Agric Sci 2:289–306
Giri AB, Sahu NP, Saharan N, Dash G (2014) Effect of dietary supplementation of chromium on growth and biochemical parameters of Labeo rohita (Hamilton) fingerlings. Indian J Fish 61:73–81
Jain SK, Rains JL, Croad JL (2007) Effect of chromium niacinate and chromium picolinate supplementation on lipid peroxidation, TNF- a, IL-6, CRP, glycated hemoglobin, triglycerides, and cholesterol levels in blood of streptozotocin-treated diabetic rats. Free Radical Biol Med 43:1124–1131. https://doi.org/10.1016/j.freeradbiomed.2007.05.019
Liu T, Wen H, Jiang M, Yuan D, Gao P, Zhao Y, Wu F, Liu W (2010) Effect of dietary chromium picolinate on growth performance and blood parameters in grass carp fingerling Ctenopharyngodon idellus. Fish Physiol Biochem 36(3):565–572. https://doi.org/10.1007/s10695-009-9327-5
Sahin K, Kucuk O, Sahin N, Ozbey O (2001) Effect of dietary chromium picolinate supplementation on egg production, egg quality and serum concentrations of insulin, corticosterone and some metabolites of Japanese quails. Nutr Res 21:1315–1321. https://doi.org/10.1016/S0271-5317(01)00330-X
Lin YH, Liu JM, Fu HG, Liang ZL, Zhao SM, Ma JJ (2003) Effect of chromium on growth and plasma biochemical indexes of Cyprinus carpio juveniles. J Dalian Fish Univ 18:48–51
El-Sayed EH, Hassanein EI, Soliman MH, El-Khatib NR 2010 The effect of dietary chromium picolinate on growth performance, blood parameters and immune status in Nile tilapia (Oreochromis niloticus). Proceedings of the 3rd Global Fisheries and Aquaculture Research Conference, November 29 - December 1: 51–63.
Gatta PP, Piva A, Paolini M, Testi S, Bonaldo A, Antelli A, Mordenti A (2001) Effects of dietary organic chromium on gilthead seabream (Sparusaurata L.) performances and liver microsomal metabolism. Aquac Res 32 suppl.1:60–69. https://doi.org/10.1046/j.1355-557x.2001.00005.x
Bureau DP, Kirkland JB, Cho CY (1995) The effects of dietary chromium picolinate supplementation performance, carcass yield and blood glucose of rainbow trout (Oncorhynchus mykiss) fed two practical diets. ASAS Annual Meeting Orlando Florida. J Anim Sci 7(3):194. https://doi.org/10.1006/fsim.2000.0323
Selcuk Z, Tiril SU, Alagil F, Belen V, Salman M (2010) Effects of dietary L-carnitine and chromium picolinate supplementations on performance and some serum parameters in rainbow trout (Oncorhynchus mykiss). Aquac Int 18:213–221. https://doi.org/10.1007/s10499-008-9237-z
Li H, Meng X, Wan W, Liu H, Sun M, Wang H, Wang J (2018) Effects of chromium picolinate supplementation on growth, body composition, and biochemical parameters in Nile tilapia(Oreochromis niloticus). Fish Physiol Biochem 44(5):1265–1274. https://doi.org/10.1007/s10695-018-0514-0
Wang J, Gatlin DM III, Li L, Wang Y, Jin N, Lin H, Guo Y (2019) Dietary chromium polynicotinate improves growth performance and feed utilization of juvenile golden pompano (Trachinotus ovatus) with starch as the carbohydrate. Aquaculture 505:405–411.https://doi.org/10.1016/j.aquaculture.2019.02.060
Shaheen T, Farhat J (2015) Effect of various doses of chromium hexahydrate on survival & growth of Cyprinus carpio. Pak J Zool 47(4):913–919
Giri AK, Sahu NP, Dash G (2021) Improvement in the growth status and carbohydrate utilization of Labeo rohita (Hamilton, 1822) fingerlings with dietary supplementation of chromium picolinate. Fish Physiol Biochem 47(2):599–616. https://doi.org/10.1007/s10695-021-00934-9
Asad F, Mubarik MS, Ali T, Zahoor MK, Ashrad R, Qamer S (2019) Effect of organic and in-organic chromium supplementation on growth performance and genotoxicity of Labeorohita. Saudi J biol Sci 26(6):1140–1145. https://doi.org/10.1016/j.sjbs.2018.12.015
Hertz Y, Mader Z, Hepher B, Gertlera A (1989) Glucose metabolism in the common carp (Cyprinus carpio L.): the effect of cobalt and chromium. Aquaculture 76:255–267. https://doi.org/10.1016/0044-8486(89)90079-3
Shiau SY, Lin SF (1993) Effects of supplementation dietary chromium and vanadium on the utilization of different carbohydrates in tilapia, (Oreochromis niloticus x O. aureus). Aquaculture 110:321–330. https://doi.org/10.1016/0044-8486(93)90379-D
Hemre GI, Torrissen O, Krogdahl Å, Lie Ø (1995) Glucose tolerance in Atlantic salmon Salmo salar L dependence on adaption to dietary starch and water tem- perature. Aquac Nutr 1(2):69–75. https://doi.org/10.1111/j.1365-2095.1995.tb00021.x
Kubrak OI, Lushchak OV, Lushchak JV, Torous IM, Storey JM, Storey KB, Lushchak VI (2010) Chromium effects on free radical processes in goldfish tissues: comparison of Cr(III) and Cr(VI) exposures on oxidative stress markers, glutathione status and antioxidant enzymes. Comp Biochem Physiol Part C 152(3):360–370. https://doi.org/10.1016/j.cbpc.2010.06.003
Lushchak OV, Kubrak OI, Lozinsky OV, Storey JM, Storey KB, Lushchak VI (2009) Chromium(III) induces oxidative stress in goldfish liver and kidney. Aquat Toxicol 93:45–52. https://doi.org/10.1016/j.aquatox.2009.03.007
Lushchak OV, Kubrak OI, Torous IM, Nazarchuk TY, Storey KB, Lushchak VI (2009) Trivalent chromium induces oxidative stress in goldfish brain. Chemosphere 75:56–62. https://doi.org/10.1016/j.chemosphere.2008.11.052
Ovie KS, Kabir MA, Isioma JO, Kori-Siakpere O (2012) Sublethal effects of chromium on enzymatic activities of the African catfish: Clariasgariepinus. Notulae Scientia Biologicae 4(1):24–30. https://doi.org/10.15835/nsb417208
Ren M, Mokrani A, Liang H, Ji K, Xie J, Ge X, Liu B (2018) Dietary chromium picolinate supplementation affects growth, whole-body composition, and gene expression related to glucose metabolism and lipogenesis in Juvenile Blunt snout bream. Megalobrama amblycephala Biol Trace Elem Res 185(1):205–215. https://doi.org/10.1007/s12011-018-1242-0
Reid SD (2011) Molybdenum and chromium. In Fish physiology (Vol. 31, pp. 375-415). Academic Press
Kucukbay FZ, Yazlak H, Sahin N, Cakmak MN (2006) Effects of dietary chromium picolinate supplementation on serum glucose, cholesterol and mineral of rainbow trout (Oncorhynchus mykiss). Aquac Int 14:259–266. https://doi.org/10.1007/s10499-005-9030-1
Di Bona KR, Love S, Rhodes NR, McAdory D, Sinha SH, Kern N, Kent J, Strickland J, Wilson A, Beaird J, Ramage J, Rasco JF, Vincent JB (2011) Chromium is not an essential trace element for mammals: effects of a “low-chromium” diet. J Biol Inorg Chem 16(3):381–390. https://doi.org/10.1007/s00775-010-0734-y
Tye MT, Montgomery JE, Hobbs MR, Vanpelt KT, Masino MA (2018) An adult zebrafish diet contaminated with chromium reduces the viability of progeny. Zebrafish 15(2):179–187. https://doi.org/10.1089/zeb.2017.1514
Furuichi M, Yone Y (1980) Effect of dietary dextrin levels on the growth and feed efficiency, the chemical composition of liver and dorsal muscle and the absorption of dietary protein and dextrin in fishes. Bull Jpn Soc Sci Fish 46:225–229. https://doi.org/10.2331/suisan.46.225
Wilson RP, Poe WE (1987) Apparent inability of channel catfish to utilize dietary mono- and di-saccharides as energy sources. J Nutr 117:280–285. https://doi.org/10.1093/jn/117.2.280
National Research Council 2011. Nutrient Requirements of Fish and Shrimp.National Academy Press, Washington, DC.
National Research Council (1989) Recommended dietary allowances. National Academy Press, Washington, DC
Anderson R, Mertz AW (1997) Glucose tolerance factor: an es- sential dietary agent. Trends Biochem Sci 2:277–284. https://doi.org/10.1016/0968-0004(77)90280-8
Akter S, Jahan N, Rohani MF, Akter Y, Shahjahan M (2021) Chromium supplementation in diet enhances growth and feed utilization of striped catfish (Pangasianodonhypophthalmus). Biol Trace Elem Res 1:9
Asad F, Qamer S, Behzad A, Ali T, Ashraf A (2017a) Growth performance and chemical composition of Cirrhinusmrigala (mori) under the effect of chromium chloride hexahydratre. Pure Appl Biol 6(4):1226–1233. https://doi.org/10.19045/bspab.2017.600130
Asad FF, Naseem N, Ashraf A, Ali T, Behzad A (2017b) Chemical composition and growth performance of Labeorohita under the influence of Chromium chloride hexahydrate marker. Int J Biosci 10(1):186–194. https://doi.org/10.12692/ijb/10.1.186-194
Wang J, Ai Q, Mai K, Xu H, Zuo R (2014) Dietary chromium polynicotinate enhanced growth performance, feed utilization and resistance to Cryptocaryon irritansin juvenile large yellow croaker (Larmichthys crocea). Aquaculture 432:321–326. https://doi.org/10.1016/j.aquaculture.2014.05.027
Shiau SY, Liang HS (1995) Carbohydrate utilization and digestibility by tilapia (Oreochromisniloticusx O. aureus) are affected by chromium oxide inclusion in the diet. J Nutr 125:976–982. https://doi.org/10.1093/jn/125.4.976
Tacon AGJ, Beveridge MM (1982) Effects of dietary trivalent chromium on rainbow trout. Nutr Rep Int 25:49–56
Yengkokpam S, Sahu NP, Pal AK, Mukherjee SC, Debnath D (2007) Gelatinized carbohydrates in the diet of Catlacatla fingerlings: effect of levels and sources on nutrient utiliza- tion, body composition and tissue enzyme activities. Asian- Aust J Anim Sci 20(1):89–99. https://doi.org/10.5713/ajas.2007.89
Shiau SY, Chen MJ (1993) Carbohydrate utilization by Tilapia Oreochromis niloticus × O. aureus as influenced by different chromium sources. J Nutr 123:1747–1753. https://doi.org/10.1093/jn/123.10.1747
Venugopal NBRK, Reddy SLN (1992) Nephrotoxic and hepato- toxic effects of hexavalent and trivalent chromium in a fresh water teleost Anabas scandens, biochemical and environ- mental changes. Ecotoxicol Environ Safety 24:287–293. https://doi.org/10.1016/0147-6513(92)90004-M
Hastuti S, Subandiyono S (2014) Production performance of African catfish (Clarias gariepinus burch) were rearing with Biofloc technology. SAINTEK PERIKANAN: Indones J Fish Sci Tech 10(1):37–42. https://doi.org/10.14710/ijfst.10.1.37-42
Glinsmann WH, Mertz W (1966) Effects of trivalent chromium on glucose tolerance. Metab Clin Exp 15:510–520. https://doi.org/10.1016/0026-0495(66)90111-9
Steele NC, Rosebrough RW (1981) Effects of trivalent chromium on hepatic lipogenesis by the turkey poult. Poult Sci 60:617–622. https://doi.org/10.3382/ps.0600617
Steele NC, Rosebrough RW (1981) Effects of trivalent chromium on hepatic glucose tolerance test. Bull Jpn Soc Sci Fish 47:761–764. https://doi.org/10.1016/S0044-8486(96)01491-3
Lambert B, Jacquemin C (1979) Inhibition of epinephrine induced lipolysis in isolated white adipocytes of aging rabbits by increased alpha adrenergic responsiveness. J Lipid Res 20:208–216
Gang X, Zirong X, Si HW, Shijiang C (2001) Effects of chromium picolinate on growth performance, carcass characteristics, serum metabolites and metabolism of lipid in pigs. Asian Aust J Anim 14(2):258–262. https://doi.org/10.5713/ajas.2001.258
Denton RM, Halestrap AP (1979) Regulation of pyruvate metabolism in mammalian tissue. Essays Biochem 15:37–77
Brownsey RW, Edgell NJ, Hepkirk TJ, Denton RM (1984) Studies of insulin stimulated phosphorylation of acetyl-CoA carboxylase, ATP citrate lyase and other proteins in rat epididymal adipose tissue. Biochem J 218(3):733–743. https://doi.org/10.1042/bj2180733
Anderson R (1987) Chromium. In: Trace elements in human and animal nutrition, Mertz, M. (Ed.). 5th Edn, Academic Press Inc, San Diego, CA 225–244. ISBN-10: 012491252.
Vincent JB (2000) The biochemistry of chromium. J Nutr 130(4):715–718. https://doi.org/10.1093/jn/130.4.715
Newshome EA 1974 Regulation in metabolism. Press, New York, Arrowsmith Co
Evans GW, Bowman TD (1992) Chromium picolinate increases membrane fluidity and rate of insulin internalization. J Inorg Biochem 46:243–250. https://doi.org/10.1016/0162-0134(92)80034-s
Mooney K, Cromwell G (1997) Efficacy of chromium picolinate and chromium chloride as potential carcass modifiers in swine. J Anim Sci 75:2661–2671. https://doi.org/10.2527/1997.75102661x
Okada S, Tsukada H, Ohba H (1984) Enhancement of nucleo RNA synthesis by chromium (III) in regenerating rat liver. J Inorg Biochem 21:113–119
Roy SS (2002). Some toxicological aspects of chlorpyrifos to the intertidal fish Boleophthalmusdussumieri. PhD. Thesis. India. University of Mumbai
Ahmad AR, Awadesh NJ, Simon JD (2012) The effect of dietary organic chromium on specific rate, tissue chromium concentration, enzymes activities and histology in common carp (Cyprinus carpio). Biol Trace Elem Res 149(3):362–370. https://doi.org/10.1007/s12011-012-9436-3
Enes P, Peres H, Couto A, Oliva-Teles A (2010) Growth performance and metabolic utilization of diets including starch, dextrin, maltose or glucose as carbohydrate source by gilthead sea bream (Sparus aurata) juveniles. Fish Physiol Biochem 36:903–910. https://doi.org/10.1007/s10695-009-9366-y
Carvalho CS, Fernandes MN (2008) Effect of copper on liver key enzymes of anaerobic glucose metabolism from freshwater tropical fish Prochilodus lineatus. Comp Biochem Physiol A Mol Integr Physiol 151:437–442. https://doi.org/10.1016/j.cbpa.2007.04.016
Tranulis MA, Dregni O, Christophersen B, Krogdahl A, Borrebaek B (1996) A glucokinase-like enzyme in the liver of Atlantic salmon (Salmo salar). Comp Biochem Physiol 114B:35–39. https://doi.org/10.1016/0305-0491(95)02119-1
Panserat S, Médale F, Blin C, Bréque J, Vachot C, Plagnes-Juan E, Gomes E, Krishnamoorthy R, Kaushik S (2000) Hepatic glucokinase is induced by dietary carbohydrates in rainbow trout, gilthead seabream and common carp. Am J Phys Regul Integr Comp Phys 278:1164–1170. https://doi.org/10.1152/ajpregu.2000.278.5.R1164
Borrebaek B, Christophersen B (2001) Activities of glucose phosphorylation, glucose-6- phosphatase and lipogenic enzymes in the liver of perch, Perca fluviatilis, after dif- ferent dietary treatment. Aquac Res 32:221–224
Moreira IS, Peres H, Couto A, Enes P, Oliva-Teles A (2008) Temperature and dietary carbohydrate levels effects on performance and metabolic utilisation of diets in European sea bass (Dicentrarchus labrax) juveniles. Aquaculture 274:153–160. https://doi.org/10.1016/j.aquaculture.2007.11.016
Cowey CB, Walton MJ (1989) Intermediary metabolism. In: Halver JE (ed) Fish Nutrition. Academic Press, San Diego, pp 260–329. https://doi.org/10.1016/0300-9629(95)00035-6
Borrebaek B, Christophersen B, Sundby A (2003) Metabolic function of hepatic hex- okinase in perch, Perca fluviatilis. Aquac Res 34:235–239. https://doi.org/10.1046/j.1365-2109.2003.00809.x
Capilla E, Médale F, Panserat S, Vachot C, Rema P, Gomes E, Kaushik S, Navarro I, Gutiérrez J (2004) Response of hexokinase enzymes and the insulin system to dietary carbohydrates in the common carp, Cyprinus carpio. Reprod Nutr Dev 44:233–242
Kirchner S, Seixas P, Kaushik S, Panserat S (2005) Effects of low protein intake on extra-hepatic gluconeogenic enzyme expression and peripheral glucose phosphor- ylation in rainbow trout (Oncorhynchus mykiss). Comp Biochem Physiol 140B:333–340. https://doi.org/10.1016/j.cbpc.2004.10.019
Cowey CB, Knox D, Walton MJ, Adron JW (1977) The regulation of gluconeo- genesis by diet and insulin in rainbow trout (Salmo gairdneri). Br J Nutr 38:463–470. https://doi.org/10.1079/bjn19770111
Knox D, Walton MJ, Cowey CB (1980) Distribution of enzymes of glycolysis and gluconeogenesis in fish tissues. Mar Biol 56:7–10. https://doi.org/10.1007/BF02337889
Petersen TDP, Hochachka PW, Suarez RK (1987) Hormonal control of gluconeogenesis in rainbow trout hepatocytes: regulatory role of pyruvate kinase. J Exp Zool 243:173–180. https://doi.org/10.1002/jez.1402430202
Panserat S, Capilla E, Gutierrez J, Frappart PO, Vachot C, Plagnes-Juan E, Aguirre P, Brèque J, Kaushik S (2001) Glucokinase is highly induced and glucose-6-phosphatase poorly repressed in liver of rainbow trout (Oncorhynchus mykiss) by a single meal with glucose. Comp Biochem Physiol 128B:275–283. https://doi.org/10.1371/journal.pone.0105548
Panserat S, Plagnes-Juan E, Kaushik S (2001) Nutritional regulation and tissue specificity of gene expression for proteins involved in hepatic glucose metabolism in rainbow trout (Oncorhynchus mykiss). J Exp Biol 204:2351–2360
Moutou KA, Panagiotaki P, Mamuris Z (2004) Effects of salinity on digestive protease activity in the euryhaline sparid, Sparus aurata L.: a preliminary study. Aquac Res 35:912–914. https://doi.org/10.1111/j.1365-2109.2004.01068.x
Bolasina S, Perez A, Yamashita Y (2006) Digestive enzymes activity during ontogenetic development and effect of star- vation in Japanese flounder, Paralichthys olivaceus. Aquaculture 252:503–515. https://doi.org/10.1016/j.aquaculture.2005.07.015
Shan X, Xiao Z, Huang W, Dou S (2008) Effect of photoperiod on growth, mortality and digestive enzymes in miiuy croaker larvae and juveniles. Aquaculture 281:70–76. https://doi.org/10.1016/j.aquaculture.2008.05.034
Yousef MK, Johnson HD (1967) Calorigenesis of cattle as influenced by hydrocortisone and environmental temperature. J Anim Sci 26(5):1087–1093. https://doi.org/10.2527/jas1967.2651087x
Sasaki Y, Weekes TEC 1986 Metabolic response to cold. In: Milligan LP, Grovum WL, Dobson A (eds) Control of digestion and metabolism in ruminants. Prentice-Hall, Englewood Cliffs, p 326
Christison GI, Johnson HD (1972) Cortisol turnover in heat- stressed cows. J Anim Sci 35:1005–1010. https://doi.org/10.2527/jas1972.3551005x
Chang X, Mowat DN (1992) Supplemental chromium for stressed and growing feeder calves. J Anim Sci 70:559–567. https://doi.org/10.2527/1992.702559x
Chang X, Mowat DN, Spiers GA (1992) Carcass characteristics and tissue mineral contents of steers fed supplemental chro- mium. Can J Anim Sci 72:663–668
Moonsie-Shager S, Mowat DN (1993) Effect of level of supple- mental chromium on performance, serum constituents, and immune status of stressed feeder calves. J Anim Sci 71:232–240. https://doi.org/10.2527/1993.711232x
Munck A, Guyre P, Holbrook N (1984) Physiological functions of glucocorticoids in stress and their relation to pharmacological actions. Endocr Rev 5:25–44. https://doi.org/10.1210/edrv-5-1-25
Bakshi A, Panigrahi AK (2018) A comprehensive review on chromium induced alterations in fresh water fishes. Toxicol Rep 5:440–447. https://doi.org/10.1016/j.toxrep.2018.03.007
Ahmed MK, Kundu GK, Al-Mamun MH, Sarkar SK, Akter MS, Khan MS (2013) Chromium (VI) induced acute toxicity and genotoxicity in freshwater stinging Catfish, Heteropneutes fossilis. Ecotoxicol Environ Saf 92:1–7. https://doi.org/10.1016/j.ecoenv.2013.02.008
Kim JH, Kang JC (2017) Effects of dietary chromium exposure to rockfish, Sebastes schlegelii are ameliorated by ascorbic acid. Ecotoxicol Environ Saf 139:109–115. https://doi.org/10.1016/j.ecoenv.2017.01.029
Kim JH, Kang JC (2016) The chromium accumulation and its physiological effects in juvenile rockfish, Sebastes schlegelii, exposed to different levels of dietary chromium (Cr6+) concentrations. Environ Toxicol Pharmacol 41:152–158. https://doi.org/10.1016/j.etap.2015.12.001
Van der Oost R, Beyer J, Vermeulen NP (2003) Fish bioaccumulation and biomarkers in environmental risk assessment: a review. Environl Toxicol Pharmacol 13(2):57–149. https://doi.org/10.1016/s1382-6689(02)00126-6
Gholamhosseini A, Shiry N, Soltanian S, Banaee M (2021) Bioaccumulation of metals in marine fish species captured from the northern shores of the Gulf of Oman. Iran. Reg Stud Mar Sci 41:101599. https://doi.org/10.1016/j.rsma.2020.101599
Naeem S, Ashraf M, Babar ME, Zahoor S, Ali S (2021) The effects of some heavy metals on some fish species. Environ Sci Pollut Res Int 28(20):25566–25578. https://doi.org/10.1007/s11356-021-12385-z
Yin J, Zhang F, Wang L, Li S, Huang T, Zhang X (2021) A kinetic study on accumulation and depuration of hexavalent chromium in crucian carp (Carassius auratus) reveals the potential health risk of fish head consumption. Food Control 130:108291. https://doi.org/10.1016/j.foodcont.2021.108291
Kumar N, Bhushan S, Patole PB, Gite A (2022) Multi-biomarker approach to assess chromium, pH and temperature toxicity in fish. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology, 109264 https://doi.org/10.1016/j.cbpc.2021.109264
Lunardelli B, Cabral MT, Vieira CE, Oliveira LF, Risso WE, Meletti PC, Martinez CB (2018) Chromium accumulation and biomarker responses in the Neotropical fish Prochilodus lineatus caged in a river under the influence of tannery activities. Ecotoxicol Environ Saf 153:188–194. https://doi.org/10.1016/j.ecoenv.2018.02.023
Pacheco M, Santos MA, Pereira P, Martínez JI, Alonso PJ, Soares MJ, Lopes JC (2013) EPR detection of paramagnetic chromium in liver of fish (Anguilla anguilla) treated with dichromate (VI) and associated oxidative stress responses—contribution to elucidation of toxicity mechanisms. Comp Biochem Physiol C: Toxicol Pharmacol 157(2):132–140. https://doi.org/10.1016/j.cbpc.2012.10.009
Mohamed AAR, El-Houseiny W, AbdElhakeem EM, Ebraheim LL, Ahmed AI, Abd El-Hakim YM (2020) Effect of hexavalent chromium exposure on the liver and kidney tissues related to the expression of CYP450 and GST genes of Oreochromisniloticus fish: Role of curcumin supplemented diet. Ecotoxicol Environ Saf 188:109890. https://doi.org/10.1016/j.ecoenv.2019.109890
Nath K, Kumar N (1987) Effects of hexavalent chromium on the carbohydrate meta- bolism of a fresh water tropical teleost Colisa fasciatus. Bull Inst Zool Acad Sin 26:245–248
Author information
Authors and Affiliations
Contributions
Bagheri and Gholamhosseini collected manuscripts data and wrote the manuscript. Banaee contributed to the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bagheri, S., Gholamhosseini, A. & Banaee, M. Investigation of Different Nutritional Effects of Dietary Chromium in Fish: A Literature Review. Biol Trace Elem Res 201, 2546–2554 (2023). https://doi.org/10.1007/s12011-022-03326-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-022-03326-z