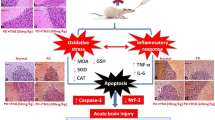
Abstract
Arsenic (As) as a neurotoxic environmental pollutant has attracted extensive attention. Curcumin (Cur) is a natural antioxidant that shows an excellent protective effect against arsenic trioxide (ATO)–induced toxicity in many animal organs. However, the mechanism of Cur against ATO-induced hypothalamic toxicity in ducks has not yet been fully elucidated. Here, ducks were treated with ATO and/or Cur during 28 days; the results showed that ATO exposure induced growth retardation, messy feathers, and abnormal posture in ducks. Moreover, ATO caused neuron vacuolar degeneration and disintegration in the hypothalamus of ducks. Simultaneously, ATO induced blood–brain barrier damage, downregulated the expression of ZO-1, Occludin, and mediated NF‐κB activation, resulting in an increase in inflammatory factors (TLR-4, NF-κB, TNF-α, IL-2, and IL-6). Furthermore, ATO increased the production of pyroptosis-related factors (Caspase-1, IL-18, IL-1), exacerbating the inflammatory damage through NLRP3-mediated inflammasome activation. Cur, on the other hand, exerted excellent inhibitory effects on inflammation and pyroptosis. In summary, our study revealed that ATO triggered inflammation and pyroptosis by modulating NF-κB/NLRP3 signaling pathways in the hypothalamus of ducks, and Cur can alleviate inflammation and pyroptosis caused by ATO. Therefore, as a plant extract, Cur has the potential to prevent and cure ATO-induced hypothalamus toxicity.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Arsenic (As) is a ubiquitous metalloid element found in numerous forms in the environment, like trivalent, pentavalent, and organic compounds [1]. As has been found to accumulate in chicken, rice, vegetables, and fish, which induced a potential risk to humans [2]. Arsenic trioxide (As2O3, ATO) has been linked to neurotoxicity, cardiotoxicity, and reproductive toxicity in numerous studies [3,4,5]. In general, toxicologists consider that As can be absorbed through the intestines and circulatory system, further inducing mitochondrial reactive oxygen species (ROS) generation in the target organs during the sub-chronic As exposure [6]. Researchers pointed out that long-term ATO exposure caused memory loss and movement disorders [7]. There is no doubt that the hypothalamus is an important organ of the central nervous system, and its function was influenced by long-term ATO exposure [8]. In animals, food intake and metabolism are highly dependent on hypothalamus regulation [9]. In addition, ducks are widely farmed as the potential protein source in many Asian countries, yet ATO-exposure induced hypothalamic toxicity inevitably disturbs normal physiological metabolism of ducks and finally posed a potential risk to human and animal healthy [10].
Arsenic can cross the blood–brain barrier (BBB) and then induce neurological diseases [11]. BBB plays an important role in regulating the movement of molecules and ions between the blood and the brain [12]. The structure and function of BBB are highly dependent on the tight junction proteins (ZO-1 and Occludin) [13]. Our previous study has also shown that the ATO exposure (8 mg/kg) led to a significant decrease in the expression of BBB-related protein [14]. Therefore, they are essential to be detected for evaluating the hypothalamic toxicity caused by ATO.
Numerous studies have shown that heavy metal exposure induced target organ dysfunction [15,16,17]. Inflammation is an adaptive response to organ injury caused by a negative stimulus. When inflammatory stimuli persist, unresolved inflammation leads to cancer [18]. Inflammation activation induced the nuclear factor-κB (NF-κB) to enter nucleus and phosphorylation, mediated the transcription of the pro-inflammatory factors such as interferon-gamma (IFN-γ) and tumor necrosis factor-alpha (TNF-α) [19, 20]. Furthermore, pyroptosis is a cell death phenomenon caused by excessive inflammation, characterizing with the formation of inflammasomes, Caspase-1 activation, and the release of inflammatory cytokines IL-18 and IL-1β [21]. It should be noted that the inflammasome is composed of three components: a sensor (NLRP 3), an adaptor (ASC), and an effector (Caspase-1) [6]. Activation of Caspase-1 can cut gasdermin D (GSDMD) into a 30-kDa fragment, lock onto the plasma membrane forming pores, and then cause cell death [22]. Zhong found that ATO (8 mg/kg) can induce inflammation and pyroptosis in the gut and liver of ducks [6]. The study has shown that NLRP3-mediated inflammasome activation leads to pyroptosis and exacerbates inflammation-induced hepatocyte fibrosis [23]. However, there is no report on whether ATO can induce inflammation and pyroptosis in the hypothalamus of ducks.
Curcumin (Cur) is a kind of polyphenol compound derived from the rhizome of turmeric that has anti-inflammation properties [24]. As a natural antioxidant, Cur plays a role in treating metabolic disorders, degenerative diseases, and multiple malignant diseases [25, 26]. More importantly, Cur has been shown to have excellent effect against ATO toxicity [27]. Therefore, Cur may exert a protective effect in ATO-induced hypothalamus toxicity. Wu and Rao reported that ATO induced oxidative stress and inflammation in the brain of ducks, and Cur (400 mg/kg) played a considerable therapeutic effect [14]. However, the mechanism of ATO exposure in the hypothalamus of ducks, as well as whether Cur plays a certain role has not been fully elucidated. Thus, we designed the experiment based on previous research [28]. The factors of inflammation and pyroptosis were detected to evaluate the toxicity of ATO exposure on the hypothalamus of ducks and the protective role of Cur in this process.
Methods and Materials
Animal Treatment and Experimental Design
All the animal procedures and experimental methods in this research were authorized by the Ethics Committee of South China Agricultural University (Permit Number: 2020A004). Twenty-one Sanshui white ducks were purchased by the Huimin-poultry industry (Guangzhou, China). Ducks were housed in cages with a 12-h light–dark cycle under appropriate conditions (the temperature at 24 ± 2 °C, humidity at 50% ± 5%) for 28 days. Libitum access to water and basal food were provided; the composition of basal food is shown in Table 1 (supplementary materials). Ducks were randomly divided into three groups: the control group, the ATO group (8 mg/kg ATO, BW), and the ATO + Cur group (8 mg/kg ATO, BW + 400 mg/kg Cur). According to previous experiments, all ducks were administered to ATO orally, and Cur was mixed in the basal diet [6, 29].
Body and Hypothalamus Weight Measurement
Ducks were weighted diurnally, then sacrificed after 28 days, and hypothalamus of ducks was meticulously dissected, washed in a PBS buffer to scour off external blood, and weighted after being dried out with filter paper.
Histological Examination
Hypothalamus tissue specimens were fixed in 4% paraformaldehyde solution and embedded in paraffin. For histopathological analysis, the paraffin blocks were subsequently sectioned (5 μm), dewaxed, hydrated, and stained with hematoxylin and eosin. All the slices were examined under optical microscopy (Leica, Germany) in 200 × and 400 × .
Quantitative Real-time PCR Analysis
The Trizol reagent (Takara, Japan) was used to isolate and extract total RNA from the hypothalamus of ducks, following the manufacturer’s instructions. Reverse transcription of isolated RNA and synthesis of complementary DNA (cDNA) was performed with the Revert Aid cDNA Synthesis Kit (Takara, Japan). Real-time quantitative PCR was done on a PCR machine (LightCycler480II; Roche, USA) as per kit instructions (Roche, Basel, Switzerland), using ChamQ SYBR Color qPCR Master Mix (Vazyme, China) with verified qPCR primers for ZO-1, Occludin, TLR 4, NF-κB, TNF α, COX-2, IL-2, IL-4, IL-6, NLRP-3, ASC, Caspase-1, IL-18, IL-1β, and GAPDH. The experimental data were calculated using the 2−ΔΔCt method, and the housekeeping gene GAPDH was used as an internal reference for normalization for relative quantitation. Specific primer sequences are exhibited in Table 2 (supplementary materials).
Western Blotting Analysis
Total protein in the hypothalamus was extracted via the Total Protein Extraction Kit (Beyotime, China). The protein concentration was calculated using an enhanced BCA Protein Assay Kit (Vazyme, China). After that, equal amounts of extracted protein were resolved by 12.5% SDS–polyacrylamide gel electrophoresis (SDS-PAGE), transferred to a PVDF membrane, and blocked with 5% skim milk, followed by being incubated with primary antibodies at 4 °C overnight. The information on primary antibodies are shown in Table 3 (supplementary materials). After being washed with tris-buffered saline tween (TBST) in the thermostat oscillator, the membranes were incubated with the suitable secondary antibodies for 1 h at 37 °C. The integrated density of immunoreactive bands was determined by ImageJ software (National Institutes of Health, Washington, DC, USA).
Statistical Analysis
The results were collected from at least four independent experiments, and all the data were expressed as mean ± standard deviation (SD). Statistics were performed using Microsoft Excel 2016. SPSS25.0 (SPSS Inc., Chicago, IL, USA) was used to one-way analysis of variance (ANOVA) and least-significant difference (LSD) was used to analyze the significances of intergroup differences. All the bar groups were drawn by GraphPad Prism8.0 (GraphPad Inc., LaJolla, CA, USA), and P-values < 0.05 were considered significant.
Results
Body and Hypothalamus Weight in Ducks
The weight of the body and hypothalamus in each group is shown in Fig. 1(A and B). Compared with the control group, the body weight decreased significantly after ATO exposure (P < 0.01), but the hypothalamus quality after ATO exposure decreased with insignificance. However, when compared to the ATO group, the ATO + Cur group’s body and hypothalamus weights were much higher (P < 0.01 or P < 0.001).
The effects of ATO and/or Cur on the body weight, hypothalamic weight, and hypothalamic appearance of ducks. (A) Bodyweight. (B) Hypothalamus weight. (C) Hypothalamic appearance. Data are presented as mean ± SD (n = 6). “*” indicates a significant difference from the control group (*P < 0.05, **P < 0.01, and ***P < 0.001). “#” indicates a significant difference from the ATO group (#P < 0.05, ##P < 0.01, and.###P < 0.001). The same scheme also applies to the remaining figures
Histological Alterations in Hypothalamus Tissues
Hematoxylin and eosin (H&E) stains were used to examine the histopathological alterations in hypothalamus tissues of ducks. The hypothalamus in the Con group showed the structure with regular cellular morphology (Fig. 2). The ATO group showed an increase in the number of disintegrated neurons (black arrows) and microglial cells (red arrows) at 200 × . More neuron vacuolar degeneration (yellow arrows) and red cells (green arrows) were found in 400 × . However, in the ATO + Cur group, the damage caused by ATO was significantly alleviated compared with the ATO group.
The Protein and mRNA Change of the Blood–Brain Barrier in the Hypothalamus
The mRNA of BBB-related were detected by qPCR. As shown in Fig. 3(A), the mRNA expression level of Occludin and ZO-1 are downregulated with insignificance after ATO exposure compared with the control group (P > 0.05). But in the ATO + Cur group, the above mRNA expression was significantly upregulated (P < 0.05). The expression of BBB-related protein is shown in Fig. 3(B and C). In the ATO group, the protein levels of Occludin and ZO-1 were reduced (P < 0.05). But after being treated with Cur, the protein level of Occludin increased markedly (P < 0.01). Therefore, Cur (400 mg/kg) has a certain protective effect against ATO (8 mg/kg)-induced BBB degradation.
The Change of Inflammation-Related Factors in Hypothalamus of Ducks
To confirm whether Cur can alleviate ATO-induced inflammatory damage in the hypothalamus of ducks, we detected mRNA and protein expression related to the TLR-4/NF-κB pathway. As described in Fig. 4(A and B), the mRNA expression levels of TLR-4, NF-κB, TNF-α, COX-2, IL-2, and IL-6 were upregulated prominently in the ATO group compared with the control group (P < 0.01, P < 0.001), but the mRNA expression level of I-κB was decreased (P < 0.001). However, an opposite result was found in the ATO + Cur group, the expression of TLR-4, NF-κB, TNF-α, COX-2, IL-2, and IL-6 were decreased (P < 0.01, P < 0.001), but then I-κB was upregulated compared with the control group (P < 0.01). In relevance to the western blot assay of the TLR-4/NF κB signaling pathway (Fig. 4C and D), protein expression of TLR-4, P-NF-κB/NF-κB, P-I-κB/I-κB, TNF-α were upregulated in the ATO group compared with the control group (P < 0.05, P < 0.01), but after they were downregulated after Cur supplementation compared to the ATO group (P < 0.01, P < 0.001).
The Change of Pyroptosis-Related Factors in Hypothalamus of Ducks
The expression of pyroptosis-related mRNA and protein (NLRP 3, ASC, GSDMD, Caspase-1, IL-18, IL-1β) are shown in Fig. 5(A). Compared with the control group, the pyroptosis-related mRNA and protein were upregulated in the ATO group (P < 0.05, P < 0.01, P < 0.001), whereas CUR treatment significantly decreased the related mRNA expression (NLRP3, Caspase-1. IL-18) (P < 0.05, P < 0.01). Protein expression in the ATO group were upregulated compared to the control group (P < 0.05) (Fig. 5B and C). Notwithstanding, after treatment with Cur, they were downregulated compared to the ATO group except NLRP3 (P < 0.05, P < 0.01).
Discussion
ATO is a neurotoxic environmental pollutant that induced neuronal oxidative stress, apoptosis, and inflammation in animals [8, 30]. Cur as a natural antioxidant have been found alleviated arsenic poising in animals [27]. However, ducks are very sensitive to arsenic poisoning, and hypothalamus plays an important role in regulating duck metabolism. It is a good measure to prevent arsenic poisoning by adding Cur in basal food to relieve the hypothalamic toxicity induced by arsenic. Our previous experiment confirmed that Cur (400 mg/kg) could alleviate ATO (8 mg/kg) induced neurotoxicity, nephrotoxicity, and skeletal muscle toxicity in ducks [14, 28, 29]. In this study, Cur mixed in the basal food does alleviate oral ATO-induced inflammation and pyroptosis in the hypothalamus of ducks. It demonstrated that the bioactivity of Cur is enough to protect against ATO poisoning. Although the doses of arsenic in this experiment (8 mg/kg) were much higher than reported, the following reasons should be considered. On one hand, ducks ingested arsenic orally once a day, accompanied by 24 h of arsenic metabolism; on the other hand, higher than average doses of arsenic were used, which can better simulate environmental exposure in areas with severe arsenic pollution [31, 32].
Body weight and organ coefficients are commonly used to evaluate toxicology experiments [33]. In this research, we found that ATO (8 mg/kg) can inhibit weight gain and organ development in ducks. However, Cur (400 mg/kg) alleviated ATO-induced duck body weight and hypothalamic developmental disorders. Interestingly, Cur seems to boost duck appetite and feed conversion efficiency, resulting in considerable weight gain in the Cur group. This may be another effect of Cur added to ducks, which can significantly increase weight gain and this result has also been found in other articles [28]. Otherwise, we also found that ducks in the ATO group had messy feathers and abnormal posture, but no obvious abnormalities in ducks after being treated with Cur. While the potential differences in the images of the hypothalamus are difficult to perceive, they may exist at the molecular level. Numerous studies found that heavy metals (cadmium and molybdenum) can cause changes in the number of neurons, microglia, and neuronal vacuolar degeneration in the hypothalamus of ducks [10]. Meanwhile, after the treatment with antioxidants, the morphological changes caused by exogenous stimuli have been significantly alleviated in the hypothalamus of hens [34]. In this study, ATO-intoxicated animals proved to exist more disintegrated neurons and neuron vacuolar degeneration, while those were relieved after the Cur therapy. These results are consistent with the previous studies mentioned above. This indicates that our model is acceptable to explore Cur’s preventive impact against ATO-induced hypothalamus toxicity.
BBB maintains the exchange of substances between the brain and blood vessels and prevents harmful substances from entering the central nervous system (CNS) [35]. Our experiments found that the mRNA and proteins of ZO-1 and Occludin were downregulated in the ATO group, indicating that ATO can cross the BBB and cause neurotoxicity. The reason may be that ATO induced ROS accumulation and further led to endothelial cell permeability increase [36]. However, these phenomena were significantly altered after treatment with Cur, suggesting that Cur may attenuate ATO-induced BBB damage through some potential mechanism. This phenomenon may be attributed to Cur’s exceptional capacity to scavenge ROS [37].
ATO can induce inflammation, which is accompanied by the activation of NF-κB and targeted phosphorylation, and subsequently induces the expression of inflammatory factors, such as IFN-γ, TNF-α, COX-2, IL-2, and IL-6 [38]. It is worth noting that targeting Toll-like receptor 4 (TLR-4) could induce neuroinflammation through the overproduction of inflammatory mediators [39]. Furthermore, IL-4, an important anti-inflammatory cytokine, plays an essential role in regulating the inflammatory response [40]. Our studies are in line with previous findings [41]. Cur can play an excellent role in the anti-inflammatory process; a study reported that Cur might alleviate lipopolysaccharide (LPS)-induced inflammation by modulating the NF-κB signaling pathway [42]. As mentioned above, we found similar results. It suggested that Cur supplementation can mitigate ATO-induced inflammation via the regulation of the NF-κB signaling pathway in the hypothalamus of duck. The potential mechanism may be that Cur alleviates ROS generation caused by ATO and further inhibits NF-κB-mediated inflammation activation [43].
Chen et al. found that activation of the NF-κB induced NLRP3-mediated inflammasome formation, which aggravated LPS-induced BBB damage and pyroptosis [44]. The research found that NLRP3, GSDMD, and Caspase-1 were upregulated in inflammatory bowel disease (IBD) [45]. Additionally, IL-18 and IL-1β will initiate inflammatory signaling cascades and then induce neuronal injury in the CNS [46]. In this work, we found the activation of NLRP3 and the increased expression of GSDMD, Caspase-1, ASC, IL-18, and IL-1β in the hypothalamus of ducks in the ATO group. These results implied that ATO induced pyroptosis in the hypothalamic of ducks. Yu found out that Cur alleviated doxorubicin-induced pyroptosis by inhibiting NLRP3 in animals [46]. In the ATO + Cur group, pyroptosis induced by the NLRP3 pathway was inhibited, suggesting that Cur possessed an excellent role in combating ATO-induced pyroptosis.
Although this study proves that oral ATO can activate the NF-κB/NLRP-3 pathway and induce inflammation and pyroptosis in the hypothalamus of ducks, some major limitations should be addressed. Firstly, many scientists have noticed that natural arsenic exposure routes include skin contact, inhalation, and oral ingestion [48]. Secondly, although the high doses of ATO were exposed to ducklings, 28 days may not be long enough to observe obvious toxic effects [49]. Thirdly, it is meaningful to investigate different concentrations and the time of As-induced toxicity in ducks. Therefore, there is more to explore the effects of arsenic exposure on animals.
Conclusion
Taken together, this experiment revealed the protective mechanism of Cur against ATO-induced hypothalamic toxicity in the ducks. Specifically, Cur can counteract ATO-induced BBB damage and NF-κB/NLRP3 activation and further alleviate hypothalamic toxicity in ducks.
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Jiang X, Yu W, Wu S, Tang L, Zhong G, Wan F, Lan J, Zhang H, Pan J, Tang Z, Zhang X, Hu L, Huang R (2021) Arsenic (III) and/or Antimony (III) induced disruption of calcium homeostasis and endoplasmic reticulum stress resulting in apoptosis in mice heart. Ecotoxicol Environ Saf 220:112394. https://doi.org/10.1016/j.ecoenv.2021.112394
Das HK, Mitra AK, Sengupta PK, Hossain A, Islam F, Rabbani GH (2004) Arsenic concentrations in rice, vegetables, and fish in Bangladesh: a preliminary study. Environ Int 30(3):383–387. https://doi.org/10.1016/j.envint.2003.09.005
Karri V, Schuhmacher M, Kumar V (2016) Heavy metals (Pb, Cd, As and MeHg) as risk factors for cognitive dysfunction: a general review of metal mixture mechanism in brain. Environ Toxicol Pharmacol 48:203–213. https://doi.org/10.1016/j.etap.2016.09.016
Shao YZ, Zhao HJ, Wang Y, Liu JJ, Li JL, Luo LY, Xing MW (2018) The apoptosis in arsenic-induced oxidative stress is associated with autophagy in the testis tissues of chicken. Poult Sci 97(9):3248–3257. https://doi.org/10.3382/ps/pey156
Moon KA, Oberoi S, Barchowsky A, Chen Y, Guallar E, Nachman KE, Rahman M, Sohel N, D’Ippoliti D, Wade TJ, James KA, Farzan SF, Karagas MR, Ahsan H, Navas-Acien A (2018) A dose-response meta-analysis of chronic arsenic exposure and incident cardiovascular disease. Int J Epidemiol 47(3):1013. https://doi.org/10.1093/ije/dyy073
Zhong G, Wan F, Lan J, Jiang X, Wu S, Pan J, Tang Z, Hu L (2021) Arsenic exposure induces intestinal barrier damage and consequent activation of gut-liver axis leading to inflammation and pyroptosis of liver in ducks. Sci Total Environ 788:147780. https://doi.org/10.1016/j.scitotenv.2021.147780
Sharma A, Kumar S (2019) Arsenic exposure with reference to neurological impairment: an overview. Rev Environ Health 34(4):403–414. https://doi.org/10.1515/reveh-2019-0052
Garza-Lombo C, Pappa A, Panayiotidis MI, Gonsebatt ME, Franco R (2019) Arsenic-induced neurotoxicity: a mechanistic appraisal. J Biol Inorg Chem 24(8):1305–1316. https://doi.org/10.1007/s00775-019-01740-8
Adlanmerini M, Nguyen HC, Krusen BM, Teng CW, Geisler CE, Peed LC, Carpenter BJ, Hayes MR, Lazar MA (2021) Hypothalamic REV-ERB nuclear receptors control diurnal food intake and leptin sensitivity in diet-induced obese mice. J Clin Invest 131(1) https://doi.org/10.1172/JCI140424
Cui T, Jiang W, Yang F, Luo J, Hu R, Cao H, Hu G, Zhang C (2021) Molybdenum and cadmium co-induce hypothalamus toxicity in ducks via disturbing Nrf2-mediated defense response and triggering mitophagy. Ecotoxicol Environ Saf 228:113022. https://doi.org/10.1016/j.ecoenv.2021.113022
Prakash C, Soni M, Kumar V (2016) Mitochondrial oxidative stress and dysfunction in arsenic neurotoxicity: a review. J Appl Toxicol 36(2):179–188. https://doi.org/10.1002/jat.3256
Yu S, Fu L, Lu J, Wang Z, Fu W (2020) Xiao-Yao-San reduces blood-brain barrier injury induced by chronic stress in vitro and vivo via glucocorticoid receptor-mediated upregulation of Occludin. J Ethnopharmacol 246:112165. https://doi.org/10.1016/j.jep.2019.112165
Daneman R, Prat A (2015) The blood-brain barrier. Cold Spring Harb Perspect Biol 7(1):a20412. https://doi.org/10.1101/cshperspect.a020412
Wu S, Rao G, Wang R, Pang Q, Zhang X, Huang R, Li T, Tang Z, Hu L (2021) The neuroprotective effect of curcumin against ATO triggered neurotoxicity through Nrf2 and NF-kappaB signaling pathway in the brain of ducks. Ecotoxicol Environ Saf 228:112965. https://doi.org/10.1016/j.ecoenv.2021.112965
Adedara IA, Abiola MA, Adegbosin AN, Odunewu AA, Farombi EO (2019) Impact of binary waterborne mixtures of nickel and zinc on hypothalamic-pituitary-testicular axis in rats. Chemosphere 237:124501. https://doi.org/10.1016/j.chemosphere.2019.124501
Wang C, Nie G, Yang F, Chen J, Zhuang Y, Dai X, Liao Z, Yang Z, Cao H, Xing C, Hu G, Zhang C (2020) Molybdenum and cadmium co-induce oxidative stress and apoptosis through mitochondria-mediated pathway in duck renal tubular epithelial cells. J Hazard Mater 383:121157. https://doi.org/10.1016/j.jhazmat.2019.121157
Wang X, Cao H, Fang Y, Bai H, Chen J, Xing C, Zhuang Y, Guo X, Hu G, Yang F (2022) Activation of endoplasmic reticulum-mitochondria coupling drives copper-induced autophagy in duck renal tubular epithelial cells. Ecotoxicol Environ Saf 235:113438. https://doi.org/10.1016/j.ecoenv.2022.113438
Moeini A, Torrecilla S, Tovar V, Montironi C, Andreu-Oller C, Peix J, Higuera M, Pfister D, Ramadori P, Pinyol R, Sole M, Heikenwalder M, Friedman SL, Sia D, Llovet JM (2019) An immune gene expression signature associated with development of human hepatocellular carcinoma identifies mice that respond to chemopreventive agents. Gastroenterology 157(5):1383–1397. https://doi.org/10.1053/j.gastro.2019.07.028
Baldwin AJ (1996) The NF-kappa B and I kappa B proteins: new discoveries and insights. Annu Rev Immunol 14:649–683. https://doi.org/10.1146/annurev.immunol.14.1.649
Kwon DJ, Bae YS, Ju SM, Youn GS, Choi SY, Park J (2014) Salicortin suppresses lipopolysaccharide-stimulated inflammatory responses via blockade of NF-kappaB and JNK activation in RAW 264.7 macrophages. Bmb Rep 47(6):318–323. https://doi.org/10.5483/bmbrep.2014.47.6.200
Deng M, Guo H, Tam JW, Johnson BM, Brickey WJ, New JS, Lenox A, Shi H, Golenbock DT, Koller BH, Mckinnon KP, Beutler B, Ting JP (2019) Platelet-activating factor (PAF) mediates NLRP3-NEK7 inflammasome induction independently of PAFR. J Exp Med 216(12):2838–2853. https://doi.org/10.1084/jem.20190111
Bertheloot D, Latz E, Franklin BS (2021) Necroptosis, pyroptosis and apoptosis: an intricate game of cell death. Cell Mol Immunol 18(5):1106–1121. https://doi.org/10.1038/s41423-020-00630-3
Gaul S, Leszczynska A, Alegre F, Kaufmann B, Johnson CD, Adams LA, Wree A, Damm G, Seehofer D, Calvente CJ, Povero D, Kisseleva T, Eguchi A, Mcgeough MD, Hoffman HM, Pelegrin P, Laufs U, Feldstein AE (2021) Hepatocyte pyroptosis and release of inflammasome particles induce stellate cell activation and liver fibrosis. J Hepatol 74(1):156–167. https://doi.org/10.1016/j.jhep.2020.07.041
Vallee A, Lecarpentier Y (2020) Curcumin and endometriosis. Int J Mol Sci 21(7). https://doi.org/10.3390/ijms21072440
Abdel-Daim MM, El-Tawil OS, Bungau SG, Atanasov AG (2019) Applications of antioxidants in metabolic disorders and degenerative diseases: mechanistic approach. Oxid Med Cell Longev 2019:4179676. https://doi.org/10.1155/2019/4179676
Salehi B, Stojanovic-Radic Z, Matejic J, Sharifi-Rad M, Anil KN, Martins N, Sharifi-Rad J (2019) The therapeutic potential of curcumin: a review of clinical trials. Eur J Med Chem 163:527–545. https://doi.org/10.1016/j.ejmech.2018.12.016
Bahrami A, Sathyapalan T, Moallem SA, Sahebkar A (2020) Counteracting arsenic toxicity: curcumin to the rescue? J Hazard Mater 400:123160. https://doi.org/10.1016/j.jhazmat.2020.123160
Lan J, Tang L, Wu S, Huang R, Zhong G, Jiang X, Tang Z, Hu L (2022) Curcumin alleviates arsenic-induced injury in duck skeletal muscle via regulating the PINK1/Parkin pathway and protecting mitochondrial function. Toxicol Appl Pharmacol 434:115820. https://doi.org/10.1016/j.taap.2021.115820
Wu S, Yu W, Jiang X, Huang R, Zhang X, Lan J, Zhong G, Wan F, Tang Z, Hu L (2021) Protective effects of curcumin on ATO-induced nephrotoxicity in ducks in relation to suppressed autophagy, apoptosis and dyslipidemia by regulating oxidative stress. Ecotoxicol Environ Saf 219:112350. https://doi.org/10.1016/j.ecoenv.2021.112350
Mochizuki H (2019) Arsenic neurotoxicity in humans. Int J Mol Sci 20(14). https://doi.org/10.3390/ijms20143418
Flora SJ (2011) Arsenic-induced oxidative stress and its reversibility. Free Radic Biol Med 51(2):257–281. https://doi.org/10.1016/j.freeradbiomed.2011.04.008
Li X, Yi H, Wang H (2018) Sulphur dioxide and arsenic affect male reproduction via interfering with spermatogenesis in mice. Ecotoxicol Environ Saf 165:164–173. https://doi.org/10.1016/j.ecoenv.2018.08.109
Piao Y, Liu Y, Xie X (2013) Change trends of organ weight background data in Sprague Dawley rats at different ages. J Toxicol Pathol 26(1):29–34. https://doi.org/10.1293/tox.26.29
Zhao Y, Zhuang Y, Shi Y, Xu Z, Zhou C, Guo L, Liu P, Wu C, Hu R, Hu G, Guo X, Xu L (2021) Effects of N-acetyl-l-cysteine on heat stress-induced oxidative stress and inflammation in the hypothalamus of hens. J Therm Biol 98:102927. https://doi.org/10.1016/j.jtherbio.2021.102927
Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ (2010) Structure and function of the blood-brain barrier. Neurobiol Dis 37(1):13–25. https://doi.org/10.1016/j.nbd.2009.07.030
Bao L, Shi H (2010) Arsenite induces endothelial cell permeability increase through a reactive oxygen species-vascular endothelial growth factor pathway. Chem Res Toxicol 23(11):1726–1734. https://doi.org/10.1021/tx100191t
Pulido-Moran M, Moreno-Fernandez J, Ramirez-Tortosa C, Ramirez-Tortosa M (2016) Curcumin and health. Molecules 21(3):264. https://doi.org/10.3390/molecules21030264
Didonato JA, Mercurio F, Karin M (2012) NF-kappaB and the link between inflammation and cancer. Immunol Rev 246(1):379–400. https://doi.org/10.1111/j.1600-065X.2012.01099.x
Leitner GR, Wenzel TJ, Marshall N, Gates EJ, Klegeris A (2019) Targeting Toll-like receptor 4 to modulate neuroinflammation in central nervous system disorders. Expert Opin Ther Targets 23(10):865–882. https://doi.org/10.1080/14728222.2019.1676416
Opal SM, Depalo VA (2000) Anti-inflammatory cytokines. Chest 117(4):1162–1172. https://doi.org/10.1378/chest.117.4.1162
Zhang K, Zhao P, Guo G, Guo Y, Tian L, Sun X, Li S, He Y, Sun Y, Chai H, Zhang W, Xing M (2016) Arsenic trioxide attenuates NF-kappaB and cytokine mRNA levels in the livers of cocks. Biol Trace Elem Res 170(2):432–437. https://doi.org/10.1007/s12011-015-0455-8
Zhong W, Qian K, Xiong J, Ma K, Wang A, Zou Y (2016) Curcumin alleviates lipopolysaccharide induced sepsis and liver failure by suppression of oxidative stress-related inflammation via PI3K/AKT and NF-kappaB related signaling. Biomed Pharmacother 83:302–313. https://doi.org/10.1016/j.biopha.2016.06.036
Zhang J, Zheng Y, Luo Y, Du Y, Zhang X, Fu J (2019) Curcumin inhibits LPS-induced neuroinflammation by promoting microglial M2 polarization via TREM2/ TLR4/ NF-kappaB pathways in BV2 cells. Mol Immunol 116:29–37. https://doi.org/10.1016/j.molimm.2019.09.020
Chen S, Tang C, Ding H, Wang Z, Liu X, Chai Y, Jiang W, Han Y, Zeng H (2020) Maf1 ameliorates sepsis-associated encephalopathy by suppressing the NF-kB/NLRP3 inflammasome signaling pathway. Front Immunol 11:594071. https://doi.org/10.3389/fimmu.2020.594071
Chen X, Liu G, Yuan Y, Wu G, Wang S, Yuan L (2019) NEK7 interacts with NLRP3 to modulate the pyroptosis in inflammatory bowel disease via NF-kappaB signaling. Cell Death Dis 10(12):906. https://doi.org/10.1038/s41419-019-2157-1
Voet S, Srinivasan S, Lamkanfi M, van Loo G (2019) Inflammasomes in neuroinflammatory and neurodegenerative diseases. Embo Mol Med 11(6). https://doi.org/10.15252/emmm.201810248
Yu W, Qin X, Zhang Y, Qiu P, Wang L, Zha W, Ren J (2020) Curcumin suppresses doxorubicin-induced cardiomyocyte pyroptosis via a PI3K/Akt/mTOR-dependent manner. Cardiovasc Diagn Ther 10(4):752–769. https://doi.org/10.21037/cdt-19-707
Palma-Lara I, Martinez-Castillo M, Quintana-Perez JC, Arellano-Mendoza MG, Tamay-Cach F, Valenzuela-Limon OL, Garcia-Montalvo EA, Hernandez-Zavala A (2020) Arsenic exposure: a public health problem leading to several cancers. Regul Toxicol Pharmacol 110:104539. https://doi.org/10.1016/j.yrtph.2019.104539
Xiao J, Jiang X, Zhou Y, Sumayyah G, Zhou L, Tu B, Qin Q, Qiu J, Qin X, Zou Z, Chen C (2022) Results of a 30-day safety assessment in young mice orally exposed to polystyrene nanoparticles. Environ Pollut 292(Pt B):118184. https://doi.org/10.1016/j.envpol.2021.118184
Author information
Authors and Affiliations
Contributions
Conceptualization: Rao Gan, Haiyan Liu; methodology: Gan Rao, Shaofeng Wu; formal analysis and investigation: Gan Rao, Ning Zhang, Riming Huang; writing—original draft preparation: Lianmei Hu; writing—review and editing: Lianmei Hu, Riming Huang; funding acquisition: Zhaoxin Tang, Lianmei Hu; resources: Lianmei Hu; supervision: Lianmei Hu.
Corresponding authors
Ethics declarations
Author Agreement
We the undersigned declare that this manuscript is original, has not been published before and is not currently being considered for publication elsewhere.
We confirm that the manuscript has been read and approved by all named authors and that there are no other persons who satisfied the criteria for authorship but are not listed.
We further confirm that the order of authors listed in the manuscript has been approved by all of us. We understand that the Corresponding Author is the sole contact for the Editorial process. He/she is responsible for communicating with the other authors about progress, submissions of revisions and final approval of proofs.
Signed by all the authors.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Oral intake of ATO-triggered hypothalamic toxicity in ducks.
• ATO cross BBB and lead to inflammation and pyroptosis in hypothalamic of ducks.
• Cur alleviated ATOinduced poising in hypothalamic of ducks.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gan, R., Liu, H., Wu, S. et al. Curcumin Alleviates Arsenic Trioxide–Induced Inflammation and Pyroptosis via the NF-κB/NLRP3 Signaling Pathway in the Hypothalamus of Ducks. Biol Trace Elem Res 201, 2503–2511 (2023). https://doi.org/10.1007/s12011-022-03321-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-022-03321-4