Abstract
Selenium (Se) is one of the essential micronutrients for performing vital body functions. This study aims at examining the influence of dietary supplementation of garlic clove-based green-synthesized selenium nanoparticles (GBGS-SeNPs, 48–87 nm) on carcass minerals and trace elements, and growth, biochemical, enzymological, and gene expression analyses in the freshwater prawn, Macrobrachium rosenbergii post larvae (PL). The 96 h LC50 of this GBGS-SeNPs to M. rosenbergii PL was 52.23 mg L−1. Five different artificial diets without supplementation of GBGS-SeNPs (control, 0.0 mg kg−1) and with supplementations of GBGS-SeNPs starting from 100 times lower than the LC50 value (0.5, 1.0, 1.5, and 2.0 mg kg−1) were prepared and fed to M. rosenbergii PL for 90 days. A dose-dependent accumulation of Se was observed in the carcass of experimental prawns. GBGS-SeNPs, up to 1.5 mg kg−1 significantly influenced the absorption of other trace elements (Ca, Cu, and Fe) and mineral salts (K, Mg, Na, and Zn). GBGS-SeNPs-supplemented diets showed efficient food conversion ratio (FCR) of 1.32 g against 2.71 g, and therefore enhanced the survival rate (85.6% against 78.8% in control) and weight gain (WG) of 1.41 g against 0.46 g of control prawn. GBGS-SeNPs significantly elevated the activities of protease, amylase, and lipase, and the contents of total protein, essential amino acids (EAA), total carbohydrate, total lipid, monounsaturated fatty acids (MUFA), polyunsaturated fatty acids (PUFA), and ash. These indicate the growth promoting potential of GBGS-SeNPs in prawn. The insignificantly altered activities of glutamic oxaloacetate transaminase (GOT), glutamic pyruvate transaminase (GPT), superoxide dismutase (SOD), and catalase, and the content of malondialdehyde (MDA) up to 1.5 mg kg−1 suggest its acceptability in prawn. Moreover, a respective down- and upregulated myostatin (MSTN) and crustacean hyperglycemic hormone (CHH) genes confirmed the influence of GBGS-SeNPs on the growth of prawn. In contrast, 2.0 mg kg−1 GBGS-SeNPs supplementation starts to produce negative effects on prawn (FCR, 1.76 g; survival rate, 82.2%; WG, 0.84 g against respective values of 1.32 g, 85.6%; and 1.41 g observed in 1.5 mg kg−1 of GBGS-SeNPs-supplemented diet fed prawn). This study recommends a maximum of 1.5 mg kg−1 GBGS-SeNPs as dietary supplement to attain sustainable growth of M. rosenbergii. This was confirmed through polynomial and linear regression analyses.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The giant freshwater prawn, Macrobrachium rosenbergii, is an economically important species; its global production increased from 217.7 thousand ton in 2010 to 234.4 thousand ton in 2018 [1]. It possesses unique characteristics, such as faster growth, better tolerance to various environmental conditions, disease resistance, delightful meat quality, and high commercial value compared to other shrimps [2, 3]. It is represents a reasonable dietary source of proteins, essential amino acids, polyunsaturated fatty acids, and low in fat. Hence, it has long been used as a delicious, balanced food choice for human consumption [4].
Selenium (Se) is considered one of the essential micronutrients to maintain homeostasis in humans and animals, its effects on physiological functions are multifaceted, and it has functions of regulating endocrine and immunity [5, 6]. It generally acts as a co-factor of some enzymatic structures called selenoproteins, such as glutathione peroxidase and thioredoxin reductase, which protects body against oxidative stress [7, 8] and prevents various diseases, including arthritis, cardiovascular disease, cystic fibrosis, muscular dystrophy, Alzheimer disease, cancer, etc. [9,10,11,12]. Selenium deficiency has been reported to be associated with a variety of adverse health effects such as urinary problems and inflammation [13,14,15].
Selenium can be added in organic and inorganic forms in aquaculture systems. Selenomethionine and selenocystine are its organic forms, and selenite and selenate are the inorganic forms [16]. The organic Se species, such as selenium yeast (SeY) and selenium methionine (SeMet), are more readily absorbed and more potent in terms of bioavailability and effects than inorganic forms [17]. Organic Se can increase the body’s total Se accumulation in Chinese mitten crab, Eriocheir sinensis [18] with similar structure to sulfur-containing amino acids. In protein synthesis, seleno-amino acids are often replaced by sulfur-containing amino acids and incorporated into proteins, which increase the Se storage capacity [19]. In order to improve the uptake of Se in the digestive system of aquatic animals, selenium nanoparticles (SeNPs) have recently been suggested as an alternative form, since they provide higher bioavailability and lower toxicity [17, 20, 21].
Several authors have shown that dietary supplementation of SeNPs in fish species could improve their growth efficiency, immune response, antioxidant defense system, and muscle Se content [8, 20, 22,23,24]. However, information for Se requirements in crustaceans is yet to be fully understood, despite few studies have been reported on marron Cherax cainii [25], Chinese mitten crab E. sinensis [26], freshwater prawns M. rosenbergii [27, 28], Macrobrachium nipponense [29], and marine shrimp Litopenaeus vannamei [30, 31].
The line between the healthy and toxic level of SeNPs is very sharp, so it is necessary to address the problem when considering the role of Se in dietary supplements, as its higher level can create toxicity [30, 32, 33]. In this viewpoint, this study revealed information about the deleterious impact of garlic-based green synthesis of selenium nanoparticles (GBGS-SeNPs) and its dietary requirements for survival, growth, carcass mineral contents, activities of digestive enzymes, muscle biochemical compositions, activities of metabolic and antioxidant enzymes, and lipid peroxidation in M. rosenbergii post-larvae (PL). In addition, in order to see the growth regulation of prawn, the gene expressions of myostatin and crustacean hyperglycemic hormone were examined after GBGS-SeNPs supplementation. These results provide beneficial information for practical diet development for aquatic animals, especially prawn. Moreover, the ameliorative property of garlic in reduction of acute toxic levels of selenium in comparison with the available literature (LC50) would reveal the importance of GBGS-SeNPs in maintaining good physiological function.
Materials and Methods
Procurement of M. rosenbergii PL
The post-larvae (PL-7) of the giant freshwater prawn M. rosenbergii were procured from a nursery pond at Singanallur (10.59′′ N 77.88′′ E), Coimbatore, India. They were safely taken to the laboratory in carrier bags half filled by well-oxygenated farm water and acclimatized to ground water for a week in a 1000-L cement tank (6′ × 3′ × 3′). Ground water meets the following physicochemical parameters: temperature, 28 °C; pH, 7.10 ± 0.20; total dissolved solids (TDS), 0.96 ± 0.02 g L−1; dissolved oxygen, 7.35 ± 0.30 mg L−1; biological oxygen demand (BOD), 10.50 ± 1.10 mg L−1; chemical oxygen demand (COD), 65.50 ± 2.10 mg L−1; ammonia, 0.016 ± 0.003 mg L−1. The PLs were fed boiled egg albumin once a day and Artemia nauplii three times a day, and the aquarium water (3⁄4) was renewed daily and aerated appropriately to ensure an environment free of accumulated metabolic waste and sufficient oxygen. In order to keep the aquarium in optimum condition, the unfed feed, feces, exuvia, and dead prawn PL if any were removed by siphoning them.
Garlic-Based Green Synthesis of Selenium Nanoparticles
In our previous study [28], we have done GBGS-SeNPs from sodium selenite (Na2SeO3) of Sigma-Aldrich. Ultrapure water (Millipore) with resistance greater than 18 MΩ was used as the solvent. The pulp of garlic (Allium sativum) purchased from the local market was washed with sterile distilled water thoroughly and ground to an oil form. The garlic extract was filtered using Whatman No. 1 filter paper. To produce GBGS-SeNPs, 5 mL of the garlic extract was mixed with 50 mL of 20 mM Na2SeO3 solution and heated in magnetic stirrer with 150 rpm at 60 °C until the color changed to brick red from pale yellow, which designates the formation of colloidal GBGS-SeNPs. After 24 h of incubation, the preparation was centrifuged at 10,000 rpm for 30 min. The pellet was washed with double-distilled water then with absolute ethanol three times and dried under room temperature. The brick red GBGS-SeNPs were suspended in PBS (pH 7.4) by ultra-sonication and then centrifuged at 10,000 rpm for 15 min. The powder form of the GBGS-SeNPs was used for further studies.
The formation of GBGS-SeNPs absorption spectrum was determined using UV–Vis spectra under the wavelength range of 200–700 nm at a resolution of 1 nm using JASCO V 650 spectrophotometer. The high-resolution images of surface topography with excellent depth of field were examined by field emission scanning electron microscopy (FEI Quantax-250 SEM) attached with EDX. For understanding the crystalline structure of GBGS-SeNPs, the XRD patterns of calcined GBGS-SeNPs were recorded on an XPERT–PRO diffractometer system for a range of 2 h = 10–80° using Cu-Kα radiation of wavelength, 1.5406 Å. The chemical composition (functional groups involved in formation of GBGS-SeNPs) and physical state of GBGS-SeNPs was further characterized by Fourier transform infrared spectroscopy (Shimadzu FT-IR PC(S) 8201 spectrometer in kBr with absorption in cm−1).
Garlic clove extract was a clear solution and appeared as brown in color. Na2SeO3 appeared as a colorless solution. In addition of garlic clove extract, the Na2SeO3 solution changed to pale yellow within a minute and finally attained brick red within 45 min. This indicated GBGS-SeNPs formation. The characterization of GBGS-SeNPs revealed uniform, mono-dispersive and highly stable spherical shaped particles within the size range of 48–87 nm [28].
Bio Assay of GBGS-SeNPs on Prawn PL
In order to understand the 96 h LC50 of GBGS-SeNPs, the static renewal acute toxicity test was performed after conformity with the guidance of American Society for Testing and Materials [34]. Preliminary exploratory tests were conducted to find out the broad range of concentrations of GBGS-SeNPs which could be relevant to the test. The toxic range was determined by adding a graded series of ten different concentrations of GBGS-SeNPs (10, 20, 30, 40, 50, 60, 70, 80, 90, and 100 mg L−1) from the stock solution to ten different aquaria, each contained 5 L of tap water and housed 10 individuals of experimental prawn PL for a duration of 96 h. During the toxicity test, the prawns were neither fed nor aerated. A control aquarium was maintained simultaneously. The mortality data were subjected to probit analysis Finney et al. [35].
Formulation of Artificial Diets
A basal diet was formulated artificially in the laboratory by using locally available ingredients, such as fishmeal and soy meal as protein sources, wheat bran and tapioca flour as carbohydrate sources, cod liver oil as lipid source, and egg albumin and tapioca used as binding agents; moreover, vitamin B complex with vitamin C “Becosules Capsules” (Manufactured by Pfizer Ltd., Mumbai, India), GBGS-SeNPs free mineral mixture and a pinch of table salt were also added (Table 1). The proximate composition (crude protein, crude fat, crude fiber, total ash, moisture, total nitrogen free extract sand and silica, and gross energy) of this basal diet was analyzed following the methods prescribed in AOAC [36]. The values are presented in the footnote of Table 1. This was done by using the outsourcing service available at Tamilnadu Veterinary and Animal Science University, Namakkal, India.
The micro pulverized and sieved basal ingredient mixture including tapioca flour were steam cooked for 15 min at 95–100 °C and allowed to cool at room temperature. Then vitamin B-complex with vitamin C, egg albumin, and sunflower oil were added. The garlic-based SeNPs was supplemented as a source of Se at different concentrations (starting from 100 times lower than the LC50 value, 0.5, 1.0, 1.5, and 2.0 mg kg−1) with the basal diet. The dough was prepared with 10% boiled water and 3 mm diameter diets were pelletized in a manual pelletizer. The feed pellets were dried under room temperature until they reached moisture content below 10%. Then the diets were stored at – 20 °C and used.
Feeding Experiment
In this study, five groups of M. rosenbergii PL (1.76 ± 0.05 cm length and 0.23 ± 0.02 g weight) were used for 90 days feeding trials. Each group consisted of forty numbers of PL in a 40 L plastic tank and maintained in triplicate (40 × 3 = 120 PLs × 5 = 600 PLs). One group was served as a control that was fed with the basal diet (‘0’ concentration of GBGS-SeNPs supplemented). The remaining four groups were fed ad libidum (in order to avoid voracious cannibalistic behavior of prawns) with 0.5, 1.0, 1.5, and 2.0 mg/kg of GBGS-SeNPs-supplemented diets, respectively. A mild aeration was continuously given throughout the experimental period of 90 days. On the 90th day, the prawns were sacrificed and analyzed by following parameters.
Estimations of Carcass Trace Elements and Mineral Contents
The whole-body carcass mineral contents, such as Se, Cr, Ca, Cu, Fe, K, Mg, Na, and Zn, were analyzed using an ICP-MS (Thermo scientific Xserious II, USA) by adopting triple-acid digestion method AOAC [37]. To achieve this, sacrificed prawns were digested by using HNO3, H2SO4, and HClO4 (9:3:1) in a hotplate at 400 °C for 2 h. The digested samples were allowed to cool at room temperature and diluted with double distilled water.
Survival, Growth Performance, and Nutritional Indices
The food indices parameters such as survival rate (SR), length gain (LG), weight gain (WG), feed intake (FI), specific growth rate (SGR), feed conversion ratio (FCR), and protein efficiency ratio (PER) were calculated according to the calculation described by Tekinay and Davies [38] as below:
Survival rate [SR (%)] = no. of live prawns/ no. of prawns introduced × 100.
Length gain [LG (cm)] = final length (cm)-initial length (cm).
Weight gain [WG (g)] = final weight (g)-initial weight (g).
Feed intake [FI (g day−1)] = feed eaten (g)/total number of days.
Specific growth rate [SGR (%)] = log final weight (g)—log initial weight (g) / total number of days × 100.
Feed conversion ratio [FCR (g)] = feed intake (g)/ weight gain (g).
Protein efficiency ratio [PER (g)] = weight gain (g)/protein intake (g).
Assays of Digestive Enzymes
The entire digestive tract along with hepatopancreas was homogenized using ice-cold double-distilled water and centrifuged at 9300 × g for 20 min under 4 °C. The supernatant was used as a primitive source of the enzyme. Protease activity was determined by the casein-hydrolysis method of Furne et al. [39], where one unit of enzyme activity represents the amount of enzyme required to liberate 1 μg of tyrosine per minute under assay conditions. Amylase activity was determined by the starch-hydrolysis method. The specific activity of amylase was calculated as milligrams of maltose liberated per gram of protein per hour [40]. Lipase activity was assayed by the method of Furne et al. [39]. One unit of lipase activity was defined as the quantity of free fatty acid released from triacylglycerol per unit of time estimated by the quantity of NaOH required to maintain constant pH and represented as mille alkali equivalents consumed.
Estimation of Basic Biochemical Constituents
The methods described by Lowry et al. [41], Moore and Stein [42], and Roe [43] were applied to estimate the respective concentration of muscle’s total protein, total amino acids, and total carbohydrate. The total lipid was extracted by the method of Folch et al. [44] and its content was estimated by the method of Barnes and Blackstock [45].
Analyses of Amino Acids and Fatty Acids Profiles
The profiles of amino acids were analyzed on the samples from the control and the prawns fed with 1.5 mg kg−1 of GBGS-SeNPs-supplemented diet, by using the high-performance thin-layer chromatography (HPTLC) as prescribed by Hess and Sherma [46]. Similarly, the profiles of fatty acids were analyzed by using the gas chromatography (GC) as prescribed by Nichols et al. [47]. These were outsourced by Sastra Deemed University, Thanjavur, Tamilnadu, India.
Assays of Metabolic Enzymes
The tissues of muscle and hepatopancreas were separately homogenized in 0.25 M sucrose and centrifuged at 3300 g for 20 min in a high-speed cooling centrifuge at 4 °C. The supernatant was used as the enzyme source. The metabolic enzymes such as glutamic oxaloacetate transaminase (GOT) and glutamic pyruvate transaminase (GPT) were analyzed according to the method of Reitman and Frankel [48] using a kit (Medsource Ozone Biomedicals Pvt. Ltd. Haryana, India). l-Aspartic acid (pH 7.4) was used as a substrate and sodium pyruvate (160 U/L) was used as a calibrator for GOT assay. Buffered l-Alanine, 2-Oxoglutarate (pH 7.4) was used as substrate and sodium pyruvate (170 U/L) was used as a calibrator for GPT assay. The activities of GOT and GPT were expressed as U/L.
Assays of Antioxidant Enzymes and Lipid Peroxidation
The tissues of muscle and hepatopancreas was separately homogenized in ice-cold 50 mM Tris buffer (pH 7.4), centrifuged at 9300 g for 20 min at 4 °C and the supernatant was used to assay the enzyme activities. Superoxide dismutase (SOD) activity was measured using pyrogallol (10 mM) autoxidation in Tris buffer (50 mM, pH 7.0) by adopting the method of Marklund and Marklund [49] and its specific activity was expressed in U/mg protein. Catalase (CAT) activity was measured using hydrogen peroxide (H2O2) as the substrate in phosphate buffer by following the method of Sinha [50] and its specific activity was expressed as μ moles of H2O2 consumed/min/mg protein.
Lipid peroxidation (LPO) was measured by estimating the content of malondialdehyde (MDA), a product of LPO by following the method of Ohkawa et al. [51], and it was expressed as nmoles of MDA/mg protein.
Gene Expression Analysis of Growth Factors
Total RNA in the muscles of the prawn was extracted using the TRIzol reagent. The RNA quantity and quality were then determined by spectrophotometry using a NanoDrop ND-2000 (Thermo Scientific, Wilmington, DE, USA) and 1.2% agarose gel electrophoresis, respectively. Subsequently, 1 μg of RNA was reverse transcribed into cDNA using PrimeScript™ RT Reagent kit (Takara, China).
Real-time fluorescent quantitative PCR detected the relative expression of myostatin (MSTN) and crustacean hyperglycemic hormone (CHH) mRNA in prawn muscle. The reference gene, β-actin, is used as a housekeeping gene. The primers used are listed below. β-actin: 5′-ACCACCGAAATTGCTCCCATCCTCT-3′; 3′-ACGGTCACTTGTTCACCATCGGCATT-5′ [52]; MSTN: 5′-ACTGCGCTGTGTTGATTGTAGCTG-3′; 3′-ACAACAGTACGTGTTCACGGGTCT-5′ [53]; CHH: 5′-CAGGTTCTTTTTCCCCCTTT-3′; 3′-ATCAACGCGAAAGCCTCAT-5′ [52]. Real-time PCR was conducted in a Quant Studio 5 (Applied Biosystems, Foster City, CA, USA) with a total volume of 18 µL, containing SYBR Fast qPCR Mix (2 ×), special primers (10 mM), cDNA, and sterilized double-distilled water. The PCR program was run at 95 °C for 10 min followed by 40 cycles of 95 °C for 15 s and 60 °C for 60 s. Melting curve analysis was carried out after the amplification phase for confirmation. Each sample was run in triplicate. The gene expression levels of MSTN and CHH were studied using the 2−ΔΔCT method.
Statistical Analysis
For each biochemical and enzyme parameter, tissues from five prawns were pooled together, and three such pooled samples were taken for analysis. The data were analyzed by one-way analysis of variance (ANOVA) using SPSS (16.0), followed by Duncan's multiple range test (DMRT) to compare the differences among treatments where significant differences (p < 0.05) were observed. Data were expressed as mean ± S.D. All the parameters were subjected to polynomial and linear regression analysis SPSS (16.0). For each parameter, the observed lower and upper values and probit (expected) values of lower and upper limits are noted.
Results
Acute Toxicity of GBGS-SeNPs to Prawn PL
The 96 h LC50 of GBGS-SeNPs to M. rosenbergii PL was found to be 52.28 mg L−1. The 95% upper and lower confidence limits were 56.67 mg L−1 and 47.82 mg L−1, respectively as shown in Table 2. During the toxicity test, the experimental prawn PL exhibited behavioral abnormalities, such as fast jerking, frequent jumping, erratic swimming, spiraling, and tendency to escape from the aquaria in concentration 40–100 mg L−1. Following this state of hyper-excitability, the prawn PL became inactive and showed muscular spasms and loss of orientation. There was loss of equilibrium and paralysis, which ultimately caused the death of the experimental prawn PL. The mortality was faster and quicker in higher concentrations (> 50 mg L−1) than that of lower concentrations.
Carcass Trace Elements and Mineral Contents
In this study, a dose-dependent increase in accumulation Se in the carcass of the whole body was recorded in prawn PL fed with GBGS-SeNPs-supplemented diet when compared with control (Table 3). The content of other trace elements (Ca, Cu, and Fe) and mineral salts (K, Mg, Na, and Zn) were significantly increased (p < 0.05) in prawns fed with GBGS-SeNPs-supplemented diets up to 1.5 mg kg−1 when compared with control. Whereas, the level of these trace elements and mineral salts absorption was started to decrease in 2.0 mg kg−1 of GBGS-SeNPs-supplemented diet fed prawns.
Supplementary Table 1 represents the paired sample “t” test for concentrations of trace elements and mineral salts presented in Table 3. The values of Se (Table 3) was found to be increased in all the treatments when compared with control individually (including the values between 1.5 and 2.0 mg kg−1 of GBGS SeNPs), which showed the “t”values with negative signs (Supplementary Table 1). This indicates the fact that all the values of treatments were higher than that of control (Table 3). Therefore, in this study, the uptake of Se was a continuous process. However, the “t” values of trace elements (except Se in 2.0 mg kg−1 of GBGS SeNPs) between 1.5 and 2.0 mg kg−1 of GBGS SeNPs were shown with positive signs (Table 3 and Supplementary Table 1). This indicates the fact that the values in 2.0 mg kg−1 of GBGS SeNPs were lower than that of the respective values in 1.5 mg kg−1 of GBGS SeNPs (Table 3 and Supplementary Table 1).
Survival and Growth Performance of Prawn PL Upon Feeding with GBGS-SeNPs
The survival rate (SR), morphometric data (length and weight), and nutritional indices, such as length gain (LG), weight gain (WG), feed intake (FI), specific growth rate (SGR), and protein efficiency ratio (PER), were found to be significantly increased (p < 0.05) in prawn PL fed with GBGS-SeNPs-supplemented diets up to 1.5 mg kg−1 when compared with control. Whereas, 2.0 mg kg−1 of GBGS-SeNPs-supplemented diet fed prawns showed the decreased values of all these parameters. The food conversion ratio (FCR) was found dose-dependent decrease up to 1.5 mg kg−1 of GBGS-SeNPs-supplemented diets fed prawns when compared with control. In the case of 2.0 mg kg−1 of GBGS-SeNPs-supplemented diet fed prawns, the FCR was found to increase when compared with 0.5 mg kg−1 of GBGS-SeNPs (Table 4).
Supplementary Table 2 represents paired sample “t” test for nutritional indices presented in Table 4. The “t” values of all the parameters of nutritional indices (except FCR) were found to be increased when compared with control individually (Table 4 and Supplementary Table 2), which were shown with negative signs (Supplementary Table 2). However, the trends were just opposite between 1.5 and 2.0 mg kg−1 of GBGS SeNPs. This indicates the fact that the values in 2.0 mg kg−1 of GBGS SeNPs were lower (except FCR) than that of the respective values in 1.5 mg kg−1 of GBGS SeNPs (Table 4 and Supplementary Table 2). This suggests that 2.0 mg kg−1 of GBGS SeNPs produced negative effects.
Activities of Digestive Enzymes
Activities of digestive enzymes such as protease, amylase, and lipase were significantly increased (p < 0.05) in prawns fed with up to 1.5 mg kg−1 of GBGS-SeNPs-supplemented diet when compared with control. However, in the case of 2.0 mg kg−1 of GBGS-SeNPs-supplemented diet fed prawns, the activities of these digestive enzymes started to decrease when compared with 0.5 mg kg−1 of GBGS-SeNPs (Table 5).
Supplementary Table 3 represents paired sample “t” test for activities of digestive enzymes presented in Table 5. The “t” values of digestive enzymes were found to be increased when compared with control individually (Table 7 and Supplementary Table 3), which were showed with negative sings (Supplementary Table 3). However, the trends were just opposite between 1.5 and 2.0 mg kg−1 of GBGS SeNPs. This indicates the fact that the values in 2.0 mg kg−1 of GBGS SeNPs were lower than that of the respective values in 1.5 mg kg−1 of GBGS SeNPs (Table 5 and Supplementary Table 3). This suggests that 2.0 mg kg−1 of GBGS SeNPs produced negative effects.
Concentrations of Basic Biochemical Constituents
Concentrations of muscle biochemical constituents, such as total protein, amino acids, carbohydrate, lipid, and ash, were significantly increased (p < 0.05) in prawns fed with GBGS-SeNPs-supplemented diets up to 1.5 mg kg−1 when compared with control. Whereas, 2.0 mg kg−1 of GBGS-SeNPs-supplemented diet fed prawns, the content of these parameters started to decrease (Table 6). In the case of moisture content, just the reverse trend was observed.
Supplementary Table 4 represents paired sample “t” test for concentrations of basic biochemical constituents presented in Table 6. The “t” values of concentrations of basic biochemical constituents (except moisture) were found to be increased when compared with control, individually (Table 6 and Supplementary Table 4), which showed negative signs. However, the trends were just opposite between 1.5 and 2.0 mg kg−1 of GBGS SeNPs. This indicates the fact that the values in 2.0 mg kg−1 of GBGS SeNPs were lower (except moisture) than that of the respective values in 1.5 mg kg−1 of GBGS SeNPs (Table 6 and Supplementary Table 4). This suggests that 2.0 mg kg−1 of GBGS SeNPs produced negative effects.
Profiles of Amino Acids
Eighteen amino acids were detected in control and the best concentration of GBGS-SeNPs (1.5 mg kg−1) supplemented feed fed prawns. Of which, ten were essential amino acids (EAA), arginine, histidine, leucine, isoleucine, lysine, methionine, phenylalanine, threonine, tryptophan and valine, and the remaining were non-essential amino acids (NEAA). The concentrations of all EAA were found to be significantly higher (p < 0.05) in test prawns when compared with control (Table 7).
Profiles of Fatty Acids
Ten fatty acids were detected in control and the best concentration of GBGS-SeNPs (1.5 mg kg−1) supplemented feed fed prawns. Of which, five were saturated fatty acids (SFA), lauric acid, myristic acid, palmitic acid, stearic acid, and arachidic acid; two were mono unsaturated fatty acids (MUFA), palmitoleic acid and oleic acid; and three were polyunsaturated fatty acids (PUFA), linoleic acid (n-6), EPA (n-3), and DHA (n-3). The concentrations of MUFA and PUFA were found to be significantly higher (p < 0.05) in test prawns when compared with control. The MUFA were predominantly higher than that of PUFA (Table 8).
Activities of Metabolic and Antioxidant Enzymes, and Lipid Peroxidation
In this study, there were no significant alterations observed in the activities of metabolic (GOT and GPT) and antioxidant (SOD and catalase) enzymes, and the content of malondialdehyde (MDA) produced during lipid peroxidation (LPO) in the hepatopancreas of prawns fed with up to 1.5 mg kg−1 of GBGS-SeNPs-supplemented diets when compared with control. However, significant elevation (p < 0.05) was recorded in GOT, GPT, SOD, catalase, and LPO in prawns fed with 2.0 mg kg−1 of GBGS-SeNPs-supplemented diet when compared with control (Table 9).
Supplementary Table 5 represents paired sample “t” test for activities of GOT, GPT, SOD, and CAT and concentration of MDA presented in Table 9. The “t” values obtained for GOT, GPT, SOD, and CAT and MDA were found to be not in a proper trend up to 1.5 mg kg−1 of GBGS SeNPs when compared with control, individually (Table 9 and Supplementary Table 5). Therefore, depending upon the alteration, the sign has appeared. However, in 2.0 mg kg−1 of GBGS SeNPs, there was steady increase in “t” values when compared with control and with 1.5 mg kg−1 of GBGS SeNPs (Table 9). Therefore, all the “t” values appeared in negative sign (Supplementary Table 5). This suggests that 2.0 mg kg−1 of GBGS SeNPs produced negative effects.
Relative Gene Expression
Among the four concentrations of GBGS-SeNPs (0.5, 1.0, 1.5, and 2 mg kg−1) supplemented diets, the diet with 1.5 mg kg−1 GBGS-SeNPs was performed well (produced the least FCR value); therefore, the gene expressions studies were performed in this diet fed prawns only. The quantity of total RNA isolated from the muscle of prawn is presented in Supplementary Table 6. In the control prawn, the total RNA level was found about 2170 ng/µL. The test prawn fed with 1.5 mg kg−1 of GBGS-SeNPs showed higher quantum of total RNA (3561 ng/µL) than that of control. The qualitative assay of total RNA in 1.2% agarose gel electrophoresis (AGE) showed three bands (28S, 18S, 5.8S). The intensity of 28S and 18S were intense in test prawns fed with GBGS-SeNPs when compared with control (Supplementary Fig. 1).
Figure 1 represents transcript expression levels of MSTN and CHH genes in M. rosenbergii. These genes were significantly influenced by the supplementation of GBGS-SeNPs. The MSTN gene expression was downregulated, whereas the CHH was upregulated (Fig. 1). Supplementary Table 7 represents the RQ values for the expression levels of MSTN and CHH genes. The expression level of the MSTN gene was downregulated, whereas the CHH was upregulated.
The quality of RT-PCR products (m-RNA) resolved in 1.0% AGE is presented in Supplementary Fig. 2. The house-keeping gene (β actin) resolved at 100 bp region. The MSTN gene was resolved at1500 bp region with better intensity in control than that of prawns fed with GBGS-SeNPs, which showed the downregulation of MSTN in test prawns. The CHH gene was resolved at just above 100 bp region with better intensity in test prawns fed with GBGS-SeNPs than that of control, which showed the upregulation of CHH in test prawns (Supplementary Fig. 2).
In this study, the overall results indicate that up to 1.5 mg kg−1 of GBGS SeNPs produced positive effects and not the case with 2.0 mg kg−1 of GBGS SeNPs. These were further confirmed by the structure of the polynomial curves and linear lines obtained (Figs. 2, 3, 4, 5, 6, 7, and 8). The break points of the polynomial curve by the linear line are at below 0.5 mg kg−1 of GBGS SeNPs as lower level, and above 1.5 mg kg−1 of GBGS SeNPs as upper level, but not reached 2.0 mg kg−1 of GBGS SeNPs in all the parameters including Se uptake (previously, which was described as a continuous process (Table 3)). However, the optimum range of 0.5–1.5 mg kg−1 of GBGS-SeNPs can be extended slightly in both ways (0.25–1.75 mg kg−1 of GBGS-SeNPs) as per polynomial and regression analyses results shown. At the outset, Se concentration influences the uptake of other trace elements (Ca, Cu, and Fe) and mineral salts (K, Mg, Na, and Zn). Similarly, it influences the survival, growth, nutritional, biochemical, and enzymological parameters (Tables 3, 4, 5, 6, 7, 8, 9; Supplementary Tables 1, 2, 3, 4, 5; Figs. 2, 3, 4, 5, 6, 7, 8).
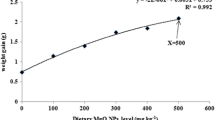
Regression (polynomial and linear with probit) values of trace elements in the control and GBGS-SeNPs-supplemented feeds fed prawns. a Selenium (with straight polynomial curve), b calcium, c copper, d iron. GBGS SeNPs garlic-based green-synthesized Se nanoparticles. The “0” on the “X” axis represents the control value
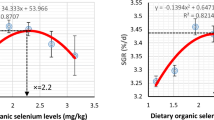
Regression (polynomial and linear with probit) values of survival, growth performance, and nutritional indices in the control and GBGS-SeNPs-supplemented feeds fed prawns. a Survival rate, b weight gain, c specific growth rate, d feed conversion ratio. GBGS SeNPs garlic-based green-synthesized Se nanoparticles. The “0” on the “X” axis represents the control value
Regression (polynomial and linear with probit) values of basic biochemical constituents in the control and GBGS-SeNPs-supplemented feeds fed prawns. a Protein, b amino acid, c carbohydrate, d lipid. GBGS SeNPs garlic-based green-synthesized Se nanoparticles. The “0” on the “X” axis represents the control value
Regression (polynomial and linear with probit) values of GOT and GPT in the control and GBGS-SeNPs-supplemented feeds fed prawns. a Glutamic oxaloacetic transaminase. b Glutamic pyruvic transaminase. GBGS SeNPs garlic-based green-synthesized Se nanoparticles, GOT glutamic oxaloacetate transaminase, GPT glutamic pyruvate transaminase. The “0” on the “X” axis represents the control value
Regression (polynomial and linear with probit) values of SOD, CAT, and concentration of MDA in the control and GBGS-SeNPs-supplemented feeds fed prawns. a Superoxide dismutase, b catalase. c lipid peroxidation. GBGS SeNPs garlic-based green-synthesized Se nanoparticles, SOD superoxide dismutase, CAT catalase, MDA malondialdehyde. The “0” on the “X” axis represents the control value
Discussion
Nanomaterials have significant beneficial effects on the growth of animals in aquaculture, as they serve as feed additives [54]. Nanoscale dietary minerals enter cell membranes faster than bulk particles and thus, improving their assimilation, which will not only increase survival and growth but also improve overall health. Nanoparticles show elevated ability over their conventional form, as they have an intense surface region that combines rapidly with other organic molecules. It has been proposed that diets supplemented with nanoparticles improve the capacity to digest in the gut [55]. The usage of nano-selenium in aqua diets showed improved bioavailability, low toxicity besides enhancing the immune response and antioxidant protection in cultured fishes [17, 20].
The toxicity of a chemical in an aquatic animal depends upon several factors such as species, size, weight, water quality parameters, local environmental condition, biological variability, and chemical formulations [56, 57]. In this study, the 96 h LC50 value of GBGS-SeNPs was 52.28 mg L−1 for M. rosenbergii PL. This is the first report to describe the lethal concentration of GBGS-SeNPs to this species of prawn. Moreover, no report is available on the LC50 of selenium in any of the Macrobrachium species. At this lethal concentration, the prawn exhibited some behavioral changes, such as inactiveness, muscular spasms, and loss of orientation. There was loss of equilibrium and paralysis, which ultimately led to death. Several authors have reported the lethal concentrations of Se in different organisms. The LC50 of selenite was 2.9 mg L−1 in fathead minnow fry (Pimephales promelas Rafinesque), 7.3 mg L−1 in fathered minnow juvenile, 9.1 mg L−1 in juvenile flag fish (Jordanella floridae Goode and Bean), 14.3 mg L−1 in brook trout (Salvelinus fontinalis Mitchill) adult, 18.2 mg L−1 in juvenile channel catfish (Ictalurus punctatus Rafinesque), and 36.6 mg L−1 in juvenile goldfish (Carassius auratus L.) [58]. Mal et al. [59] found that the LC50 for Se-NPs was 1.77 mg L−1 in zebrafish (Danio rerio) embryos. Since Se is an essential trace element for several metabolic and enzymatic functions, it has a significant role in determining the dosage of nutritional supplementation [32]. It has been reported that the organic nano-selenium such as selenometheonine (SeMet) and selenocysteine (SeCys) showed less toxic and comparable efficacy in upregulating seleno-enzymes and tissue selenium levels when compared with bulk materials [32, 60, 61]. In this study, the recorded LC50 value of GBGS-SeNPs was found to be higher than that of the reported values. This may be due to several factors, like duration of exposure, condition of water quality parameters, life stage, health of the individual, etc. Moreover, the ameliorative property of garlic may reduce the toxicity of Se, since this was garlic-based green-synthesized SeNPs.
In this study, up to 1.5 mg kg−1 GBGS-SeNPs has the potency to produce the positive effect on physiological, biochemical, enzymological, and gene expressions in M. rosenbergii PL, but at 2.0 mg kg−1, it showed toxic effect. A dose-dependent increase in concentration of whole body Se was observed from 0.5 to 2.0 mg kg−1 following exposure to GBGS-SeNPs-supplemented diets, which indicated that the prawn body was able to become enriched with Se from the diet. It has been reported that the integument, antennal glands, gills, and gastro-intestinal tract of crustaceans readily takes up Se [62, 63]. Presumably, excess of these nanoparticles might cost more energy in osmotic and ionic regulation and interact with other nutrients utilization. The optimized concentration of GBGS-SeNPs (1.5 mg kg−1) facilitated the maximum absorption of other trace elements (Ca, Cu, and Fe) and minerals (K, Mg, Na, and Zn) available in the formulated experimental diet, by the prawns.
Similarly, it has been reported that the whole body Se concentration increased with increasing dietary selenium levels in gibel carp Carassius auratus gibelio, cobia Rachycentron canadum, yellowtail kingfish Seriola lalandi, oriental river prawn M. nipponense, and white leg prawn L. vannamei [29, 31, 64, 65]. It is likely that prawns could have the potential to sustain selenium homeostasis. Wang et al. [66] reported that excess Se-yeast in the diet of E. sinensis reduced oxidative stress and did not limit the growth. Yuan et al. [18] also reported that Se-biofortified corn fed to mitten crab was biologically active while being less toxic. However, higher GBGS-SeNPs showed the tendency of affecting the growth of prawn by generating excessive free radicals and cause oxidative damage as seen in S. lalandi [65], M. nipponense [29], Japanese abalone, Haliotis discus hannai [29], and rainbow trout, Oncorhynchus mykiss [31].
Though the absorption of Se was continuous in all four levels (0.5, 1.0, 1.5, and 2.0 mg kg−1 of GBGS-SeNPs), only up to 1.5 mg kg−1 of GBGS-SeNPs showed positive response/regulation on overall growth and survival of M. rosenbergii PL. The lowest FCR observed in 1.5 mg kg−1 of GBGS-SeNPs-supplemented diet fed prawns reflects the superior quality of the diet prepared. It has been reported that nano-Se, dietary Se, and SeMet-supplemented feed fed fish O. mykiss, C. auratus gibelio, blunt snout bream Megalobrama amblycephala, marine fish species including grouper Epinephelus malabaricus, cobia R. canadum, S. lalandi, common carp Cyprinus carpio, E. sinensis, and L. vannamei produced significant improvement in survival and growth [8, 22, 26, 29,30,31, 65, 67]. In this study, the growth of prawns was significantly inhibited in 2.0 mg kg−1 of GBGS-SeNPs-supplemented diet. This is consistent with the report of [29] in M. nipponense, where excessive Se inhibits growth. Selenium limits may vary by the influence of various environmental factors and life stages of the individual species.
Almost in all organisms, Se acts as a cofactor for various enzymes [5]. Food digestion is one of the most essential roles of the body’s metabolism to obtain nutrients for various activities. Digestive enzymes in crustacean play a vital role in nutritional physiology, and regulate the growth and moult cycle by maintaining general health [68]. In this study, the noticed increase in the activity of protease, amylase, and lipase in 1.5 mg kg−1 GBGS-SeNPs-supplemented feed fed prawns indicated that the macromolecules, protein, carbohydrate, and lipid might be broken down into amino acids, monosaccharides, and fatty acids, respectively. The increased activities of digestive enzymes contributed to improved food intake and food conversion, which eventually led to better survival and growth of M. rosenbergii. It has been reported that dietary Se supplementations increased protease, amylase, and lipase activities in grass carp Ctenopharyngodon idella, sea bream Pagrus major, and marron Cherax cainii [69, 70]. It has also been reported that optimum amounts of dietary bulk and nano form of Zn, Cu, Fe, Mg, and Mn have increased digestive enzyme activity in M. rosenbergii PL [71,72,73,74,75,76]. It has also been reported that NaCl dietary supplement increased protease, amylase, and lipase activities in M. rosenbergii, Cirrhinus mrigala, and C. carpio [77]. In this study, the 2.0 mg kg−1 of GBGS-SeNPs supplementation resulted in decreased activities of these digestive enzymes, which indicates its toxic limit to M. rosenbergii PL.
In this study, the increased levels of total protein, total amino acids, EAA, NEAA, total carbohydrate and total lipid, SFA, MUFA, and PUFA recorded in 1.5 mg kg−1 of GBGS-SeNPs-supplemented diet fed prawns suggest their increased synthesis due to metabolic influence of Se. It has been reported that Se- and SeNPs-supplemented feeds influence the synthesis and storage of protein and lipid in rainbow trout O. mykiss, common carp C. carpio, African catfish Clarias gariepinus, grass carp Ctenopharyngodon idellus, and red sea bream P. major [6, 8, 22, 67, 70]. It has also been reported that optimum amounts of dietary bulk and nano form of Zn, Cu, Fe, Mg, and Mn have also increased the basic biochemical constituents, and essential amino acids and fatty acids in M. rosenbergii PL [71,72,73,74,75,76]. In this study, the decrease recorded in basic biochemical constituents by 2.0 mg kg−1 of GBGS-SeNPs supplementation indicates its toxicity to M. rosenbergii PL.
The dietary requirement of minerals can be optimized by assessing the survival, growth, activities of GOT, GPT, SOD, and CAT, and MDA production [71,72,73,74,75,76, 78, 79]. One can assess the state of stress in any animal by the decreased or increased activities of these enzymes. The antioxidant enzymes, SOD and CAT, are responsible for scavenging superoxide radicals, are involved in protective mechanisms within tissue injury following oxidative process, phagocytosis, and LPO [15, 24]. In this study, the significant elevation observed in activities of GOT, GPT, SOD, and CAT, and the level of LPO in M. rosenbergii PL particularly in 2.0 mg kg−1 of GBGS-SeNPs fed prawns indicates its toxicity. Below this level, there was no significant alteration in any of these parameters. Such a state suggests that there was no hepatotoxicity, thereby not producing the LPO and oxidative stress. Therefore, these suggest that 1.5 mg kg−1 GBGS-SeNPs was safe and accelerated growth in M. rosenbergii PL. Similar observations have been reported in M. rosenbergii PL against optimum concentrations of dietary bulk and nano form of Zn, Cu, Fe, Mg, and Mn [71,72,73,74,75,76]. Sodium selenite and Se-yeast have also produced higher glutathione peroxidase (GPx) activity in fish [80]. It has also been reported that nano-Se and organic sources of Se could enhance antioxidant capacity and oxidative stress resistance in fishes [22, 24, 67] and in L. vannamei [31].
Appropriate amounts of nanoparticles (in this study, 1.5 mg kg−1 of GBGS-SeNPs) decrease the quantum of free radicals in the body, while a high amount (in this study, 2.0 mg kg−1 of GBGS-SeNPs) increases the levels of free radicals [67, 81]. High concentrations of free radicals can react with some biological macromolecules, such as protein, DNA, and lipids, which can damage the body, triggering toxic effects, in addition to hindering the activity of oxygen free radical scavengers [66, 82, 83].
Myostatin, a member of the transforming growth factor (TGF)-β super family, inhibits the differentiation and proliferation of myoblasts and negatively correlates with the growth performance of animals [84]. The mechanisms of MSTN negatively regulate muscle growth, due to suppression of protein synthesis and stimulation of protein degradation taken simultaneously, which leads to reduction in skeletal muscle mass [85, 86]. In this study, the gene expression of myostatin in M. rosenberii was decreased in the optimum concentration of GBGS-SeNPs (1.5 mg kg−1). Thus, the low level of myostatin expression observed in M. rosenberii indicates good growth, as it is negatively correlated with growth performance. Similar hypothesis has been proved in L. vannamei [87] and Fenneropenaeus chinensis [88].
It has been reported that CHH participates in many biological processes, such as growth, molting, reproduction, glucose, carbohydrate and lipid metabolism, morphogenesis, and osmoregulation [52, 89,90,91,92,93,94]. In this study, CHH expression produced better growth performance in M. rosenberii at 1.5 mg kg−1 of GBGS-SeNPs supplementation, which suggests that the optimized concentration of SeNPs increases metabolic activity.
Conclusion
In this study, the optimized concentration of GBGS-SeNPs (1.5 mg kg−1) produced significantly higher survival, growth, activities of digestive enzymes, concentrations of biochemical constituents including essential amino acids, and unsaturated fatty acids. Thus GBGS-SeNPs supplementation could influence the improvement of the general health of the prawn. The downregulation of the negative growth regulator, MSTN, accelerates the survival and growth of M. rosenbergii when fed with GBGS-SeNPs at 1.5 mg kg−1. This was further confirmed by the upregulation of the positive growth regulator, the CHH gene. The optimized concentration of 1.5 mg kg−1 of GBGS-SeNPs improves the growth performance in prawns because it is an important component of the antioxidant enzymes, which can reduce the stress, improve antioxidant capacity, enhance the immune function, reduce the mortality, and consequently promotes growth. The 2.0 mg kg−1 of GBGS-SeNPs supplementation starts to produce negative effects on M. rosenbergii PL. This was confirmed further through the break points of the polynomial curve by the linear line. These are below 0.5 mg kg−1 of GBGS SeNPs as lower level, and above 1.5 mg kg−1 of GBGS SeNPs as upper level, but not reached 2.0 mg kg−1 of GBGS SeNPs. The increase in activities of antioxidant enzymes and increased LPO indicate that the prawns were able to detoxify the excess Se. Thus, among the four concentrations of GBGS SeNPs tested in this study, any level between 0.5 and 1.5 mg kg−1 of GBGS-SeNPs can be recommended for attaining the sustainable growth of M. rosenbergii. However, the optimum range can also be extended slightly in both ways (0.25–1.75 mg kg−1 of GBGS-SeNPs) as per polynomial and regression analyses shown.
References
FAO (2020) The state of world fisheries and aquaculture 2020. Sustainability in action. Rome 1–206. https://doi.org/10.4060/ca9229en
New MB (2005) Freshwater prawn farming: Global status, recent research and a glance at the future. Aquac Res 36:210–230. https://doi.org/10.1111/j.1365-2109.2005.01237.x
Nair CM, Salin KR (2012) Current status and prospects of farming the giant river prawn Macrobrachium rosenbergii (De Man) and the monsoon river prawn Macrobrachium malcolmsonii (Edwards HM) in India. Aquac Res 2:26–33. https://doi.org/10.1111/j.1365-2109.2011.03074.x
Bhavan PS, Ruby SA, Poongodi R, Seenivasan C, Radhakrishnan S (2010) Efficacy of cereals and pulses as feeds for the post-larvae of the freshwater prawn Macrobrachium rosenbergii. J Ecobiotech 2(5):9–19
Khan KU, Zuberi A, Fernandes JBK, Ullah I, Sarwar H (2017) An overview of the ongoing insights in selenium research and its role in fish nutrition and fish health. Fish Physiol Biochem 43(6):1689–1705. https://doi.org/10.1007/s10695-017-0402-z
Harsij M, Gholipour Kanani H, Adineh H (2020) Effects of antioxidant supplementation (nano-selenium, vitamin C and E) on growth performance, blood biochemistry, immune status and body composition of rainbow trout (Oncorhynchus mykiss) under sub-lethal ammonia exposure. Aquacul 521:734–942. https://doi.org/10.1016/j.aquaculture.2020.734942
Yu HJ, Liu JQ, Bock A, Li J, Luo GM, Shen JC (2005) Engineering glutathione transferase to a novel glutathione peroxidase mimic with high catalytic efficiency incorporation of selenocysteine into a glutathione-binding scaffold using an auxotrophic expression system. J Biol Chem 280(12):11930–11935. https://doi.org/10.1074/jbc.M408574200
Naderi M, Keyvanshokooh S, Salati AP, Ghaedi A (2017) Combined or individual effects of dietary vitamin E and selenium nanoparticles on humoral immune status and serum parameters of rainbow trout (Oncorhynchus mykiss) under high stocking density. Aquacul 474:40–47. https://doi.org/10.1016/j.aquaculture.2017.03.036
Skalickova S, Milosavljevic V, Cihalova K, Horky P, Richtera L, Adam V (2017) Selenium nanoparticles as a nutritional supplement. Nutrition 33:83–90. https://doi.org/10.1016/j.nut.2016.05.001
Li GZ, Liu F, Xu C, Li JY, Xu YJ (2018) Selenium and zinc against Aβ25–35-induced cytotoxicity and tau phosphorylation in PC12 cells and inhibits γ-cleavage of APP. Biol Trace Elem Res 184:442–449. https://doi.org/10.1007/s12011-017-1162-4
Abd-Rabou AA, Shalby AB, Ahmed HH (2019) Selenium nanoparticles induce the chemo-sensitivity of fluorouracil nanoparticles in breast and colon cancer cells. Biol Trace Elem Res 187:80–91. https://doi.org/10.1007/s12011-018-1360-8
Kutuk SG, Naziroglu M (2020) Selenium diminishes docetaxel-induced cell death, oxidative stress, and inflammation in the laryngotracheal epithelium of the mouse. Biol Trace Elem Res 196:184–194. https://doi.org/10.1007/s12011-019-01914-0
Chen CJ, Xiao P, Chen Y, Fang R (2019) Selenium deficiency affects uterine smooth muscle contraction through regulation of the RhoA/ROCK signalling pathway in mice. Biol Trace Elem Res 192:277–286. https://doi.org/10.1007/s12011-019-01677-8
Qu KC, Li HQ, Tang KK, Wang ZY, Fan RF (2019) Selenium mitigates cadmium-induced adverse effects on trace elements and amino acids profiles in chicken pectoral muscles. Biol Trace Elem Res 193:234–240. https://doi.org/10.1007/s12011-019-01682-x
Zhang Y, Cui J, Lu Y, Huang C, Liu H, Xu S (2020) Selenium deficiency induces inflammation via the iNOS/NF-κB pathway in the brain of pigs. Biol Trace Elem Res 196:103–109. https://doi.org/10.1007/s12011-019-01908-y
Wang C, Lovell RT (1997) Organic selenium sources, selenomethionine and selenoyeast, have higher bioavailability than an inorganic selenium source, sodium selenite, in diets for channel catfish (Ictalurus punctatus). Aquacul 152:223–234. https://doi.org/10.1016/S0044-8486(96)01523-2
Kohshahi AJ, Sourinejad I, Sarkheil M, Johari SA (2019) Dietary cosupplementation with curcumin and different selenium sources (nanoparticulate, organic, and inorganic selenium): influence on growth performance, body composition, immune responses, and glutathione peroxidase activity of rainbow trout (Oncorhynchus mykiss). Fish Physiol Biochem 45(2):793–804. https://doi.org/10.1007/s10695-018-0585-y
Yuan L, Zhang R, Ma X, Yang L, Zheng Q, Chen D (2018) Selenium accumulation, antioxidant enzyme levels, and amino acids composition in Chinese mitten crab (Eriocheir sinensis) fed selenium-biofortified corn. Nutrients 10(3):318. https://doi.org/10.3390/nu10030318
Schram E, Pedrero Z, Camara C, Van Der Heul JW, Luten JB (2008) Enrichment of African catfish with functional selenium originating from garlic. Aquac Res 39:850–860. https://doi.org/10.1111/j.1365-2109.2008.01938.x
Saffari S, Keyvanshokooh S, Zakeri M, Johari SA, Pasha-Zanoosi H, Mozanzadeh MT (2018) Effects of dietary organic, inorganic, and nanoparticulate selenium sources on growth, hemato-immunological, and serum biochemical parameters of common carp (Cyprinus carpio). Fish Physiol Biochem 44(4):1087–1097. https://doi.org/10.1007/s10695-018-0496-y
Ghazi S, Diab AM, Khalafalla MM, Mohamed RA (2021) Synergistic effects of selenium and zinc oxide nanoparticles on growth performance, hemato-biochemical profile, immune and oxidative stress responses, and intestinal morphometry of Nile tilapia (Oreochromis niloticus). Biol Trace Elem Res 1–11. https://doi.org/10.1007/s12011-021-02631-3
Ashouri S, Keyvanshokooh S, Salati AP, Johari SA, Pasha-Zanoosi H (2015) Effects of different levels of dietary selenium nanoparticles on growth performance, muscle composition, blood biochemical profiles and antioxidant status of common carp (Cyprinus carpio). Aquacult 446:25–29. https://doi.org/10.1016/j.aquaculture.2015.04.021
Kumar N, Singh NP (2019) Effect of dietary selenium on immuno-biochemical plasticity and resistance against Aeromonas veronii biovar sobria in fish reared under multiple stressors. Fish Shellfish Immunol 84:38–47. https://doi.org/10.1016/j.fsi.2018.09.065
Neamat-Allah ANF, Mahmoud EA, Abd El Hakim Y (2019) Efficacy of dietary Nano-selenium on growth, immune response, antioxidant, transcriptomic profile and resistance of Nile tilapia, Oreochromis niloticus against Streptococcus iniae infection. Fish Shellfish Immunol 94:280–287. https://doi.org/10.1016/j.fsi.2019.09.019
Nugroho RA (2002) Fotedar R (2014) Comparing the effects of dietary selenium and mannan oligosaccharide supplementation on the growth, immune function, and antioxidant enzyme activity in the cultured marron Cherax cainii (Austin. Aquac Int 22(2):585–596. https://doi.org/10.1007/s10499-013-9682-1
Tian WJ, Li EC, Chen LQ, Sun LM, Chen YL, Li M, Jiang X, Du ZY (2014) Growth, body composition and anti-oxidative status of juvenile Chinese mitten crabs, Eriocheir sinensis fed different dietary selenium levels. J Fishery Sci China 21(1):92–100
Chiu ST, Hsieh SL, Yeh SP, Jian SJ, Cheng W, Liu CH (2010) The increase of immunity and disease resistance of the giant freshwater prawn, Macrobrachium rosenbergii by feeding with selenium enriched-diet. Fish Shellfish Immunol 29:623–629. https://doi.org/10.1016/j.fsi.2010.06.012
Satgurunathan T, Bhavan PS, Komathi S (2017) Green synthesis of selenium nanoparticles from sodium selenite using garlic extract and its enrichment on Artemia nauplii to feed the freshwater prawn Macrobrachium rosenbergii post-larvae. Res J Chem Environ 21:1–12
Kong Y, Ding Z, Zhang Y, Ye J, Du Z (2017) Dietary selenium requirement of juvenile oriental river prawn Macrobrachium nipponense. Aquacul 476:72–78. https://doi.org/10.1016/j.aquaculture.2017.04.010
Yu Q, Fu Z, Huang M, Xu C, Wang X, Qin JG, Li E (2021) Growth, physiological, biochemical, and molecular responses of Pacific white shrimp Litopenaeus vannamei fed different levels of dietary selenium. Aquacul 535:736393. https://doi.org/10.1016/j.aquaculture.2021.736393
Wang L, Li X, Lu K, Song K, Wang G, Zhang C (2021) Dietary hydroxyl methionine selenium supplementation enhances growth performance, antioxidant ability and nitrite tolerance of Litopenaeus vannamei. Aquacul 537:736513. https://doi.org/10.1016/j.aquaculture.2021.736513
Li H, Zhang J, Wang T, Luo W, Zhou Q, Jiang G (2008) Elemental selenium particles at nano-size (Nano-Se) are more toxic to medaka (Oryzias latipes) as a consequence of hyper-accumulation of selenium: a comparison with sodium selenite. Aquat Toxicol 89:251–256. https://doi.org/10.1016/j.aquatox.2008.07.008
Kapur M, Soni K, Kohli K (2017) Green synthesis of selenium nanoparticles from broccoli, characterization, application and toxicity. Adv Tech Biol Med 1–7. https://doi.org/10.4172/2379-1764.1000198.05
ASTM (2007) Standard guide for conducting acute toxicity tests on test materials with fishes, macro invertebrates, and amphibians. West Conshohocken, PA, United States. 0.1520/E0729-96
Finney (1971) Probit analysis. Cambridge University Press, London
AOAC (2005) Determination of moisture, ash, protein and fat. Official method of analysis of the association of analytical chemists. 18th Edition, AOAC, Washington DC
AOAC (2002) AOAC Official Method 999.11 Determination of Lead, Cadmium, Copper, Iron, and Zinc in foods. Off Methods Anal AOAC Int 11:1–3
Tekinay AA, Davies SJ (2001) Dietary carbohydrate level influencing feed intake, nutrient utilisation and plasma glucose concentration in the rainbow trout, Oncorhynchus mykiss. Turkish J Vet Anim Sci 25(5):657–666
Furne M, Hidalgo MC, Lopez A, Garcia-Gallego M, Morales AE, Domezain A, Domezaine J, Sanz A (2005) Digestive enzyme activities in Adriatic sturgeon, Acipenser naccarii and rainbow trout Oncorhynchus mykiss. A comparative study Aquacul 250(1–2):391–398. https://doi.org/10.1016/j.aquaculture.2005.05.017
Bernfeld P (1955) Amylases, α and β. Methods Enzymol 149–158. https://doi.org/10.1016/0076-6879(55)01021-5
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193(1):265–275. https://doi.org/10.1016/0922-338X(96)89160-4
Moore S, Stein WH (1948) Photometric ninhydrin method for use in the chromatography of amino acids. J Biol Chem 176:367–388
Roe JH (1955) The determination of sugar in blood and spinal fluid with anthrone reagent. J Biol Chem 212:335–343
Folch J, Lees M, Sloane Stanley GH (1957) A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 226(1):497–509. https://doi.org/10.3989/scimar.2005.69n187
Barnes H, Blackstock J (1973) Estimation of lipids in marine animals and tissues: detailed investigation of the sulphophosphovanilun method for “total” lipids. J Exp Mar Bio Ecol 12(1):103–118. https://doi.org/10.1016/0022-0981(73)90040-3
Hess B, Sherma J (2004) Quantification of arginine in dietary supplement tablets and capsules by silica gel high-performance thin-layer chromatography with visible mode densitometry. Acta Chromatogr 60–69
Nichols DS, Nichols PD, McMeekin TA (1993) Polyunsaturated fatty acids in Antarctic bacteria. Antarct Sci 5:149–160
Reitman S, Frankel S (1957) A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol 28:56. https://doi.org/10.1093/ajcp/28.1.56
Marklund S, Marklund G (1974) Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem 47(3):469–474
Sinha AK (1972) Colorimetric assay of catalase. Anal Biochem 47(2):389–394. https://doi.org/10.1016/0003-2697(72)90132-7
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95(2):351–358. https://doi.org/10.1016/0003-2697(79)90738-3
Thanh NM, Barnes AC, Mather PB, Li Y, Lyons RE (2010) Single nucleotide polymorphisms in the actin and crustacean hyperglycemic hormone genes and their correlation with individual growth performance in giant freshwater prawn Macrobrachium rosenbergii. Aquacul 301:7–15. https://doi.org/10.1016/j.aquaculture.2010.02.001
Sarasvathi E, Bhassu S, Maningas MB, Othman RY (2015) Myostatin: a potential growth-regulating gene in giant river prawn Macrobrachium rosenbergii. J World Aquac Soc 46(6):624–634. https://doi.org/10.1111/jwas.12238
Onuegbu CU, Aggarwal A, Singh NB (2018) ZnO nanoparticles as feed supplement on growth performance of cultured African catfish fingerlings. J Sci Ind Res 77:213–218
El Basuini MF, El-Hais AM, Dawood MAO, Abou-Zeid AES, El-Damrawy SZ, Khalafalla MMES, Koshio S, Ishikawa M, Dossou S (2017) Effects of dietary copper nanoparticles and vitamin C supplementations on growth performance, immune response and stress resistance of red sea bream Pagrus major. Aquac Nutr 23(6):1329–1340. https://doi.org/10.1111/anu.12508
Kumar N, Krishnani KK, Singh NP (2018) Comparative study of selenium and selenium nanoparticles with reference to acute toxicity, biochemical attributes, and histopathological response in fish. Environ Sci Pollut Res 25(9):8914–8927. https://doi.org/10.1007/s11356-017-1165-x
Selmani A, Ulm L, Kasemets K, Kurvet I, Erceg I, Barbir R, Vrcek T (2020) Stability and toxicity of differently coated selenium nanoparticles under model environmental exposure settings. Chemosphere 250:126–265. https://doi.org/10.1016/j.chemosphere.2020.126265
Cardwell RD, Foreman DG, Payne TR, Wilbur DJ (1976) Acute toxicity of selenium dioxide to freshwater fishes. Arch Environ Contam Toxicol 4(1):129–144. https://doi.org/10.1007/BF02221018
Mal J, Veneman WJ, Nancharaiah YV, van Hullebusch ED, Peijnenburg WJGM, Vijver MG, Lens PNL (2017) A comparison of fate and toxicity of selenite, biogenically, and chemically synthesized selenium nanoparticles to zebrafish (Danio rerio) embryogenesis. Nanotoxicol 11(1):87–97. https://doi.org/10.1080/17435390.2016.1275866
Wang H, Zhang J, Yu H (2007) Elemental selenium at nano size possesses lower toxicity without compromising the fundamental effect on selenoenzymes: comparison with selenomethionine in mice, Free Radic. Biol Med 42:1524–1533. https://doi.org/10.1016/j.freeradbiomed.2007.02.013
Zhang J, Wang X, Xu TT (2008) Elemental selenium at nano size (Nano-Se) as a potential chemopreventive agent with reduced risk of selenium toxicity: Comparison with se-methylselenocysteine in mice. Toxicol Sci 101(1):22–31. https://doi.org/10.1093/toxsci/kfm221
Wilder MN, Ikuta K, Atmomarsono M, Hatta T, Komuro K (1998) Changes in osmotic and ionic concentrations in the hemolymph of Macrobrachium rosenbergii exposed to varying salinities and correlation to ionic and crystalline composition of the cuticle. Comp Biochem Physiol - A Mol Integr Physiol 119(4):941–950. https://doi.org/10.1016/S1095-6433(98)00008-7
Furriel RPM, McNamara JC, Leone FA (2000) Characterization of (Na+, K+)-ATPase in gill microsomes of the freshwater shrimp Macrobrachium olfersii. Comp Biochem Physiol - B Biochem Mol Biol 126(3):303–315. https://doi.org/10.1016/S0305-0491(00)00184-X
Han D, Xie S, Liu M, Xiao X, Liu H, Zhu X, Yang Y (2011) The effects of dietary selenium on growth performances, Oxidative stress and tissue selenium concentration of gibel carp (Carassius auratus gibelio). Aquac Nutr 17(3):741–749. https://doi.org/10.1111/j.1365-2095.2010.00841.x
Le KT, Fotedar R (2014) Bioavailability of selenium from different dietary sources in yellowtail kingfish (Seriola lalandi). Aquacul 420:57–62. https://doi.org/10.1016/j.aquaculture.2013.10.034
Wang X, Shen Z, Wang C, Li E, Qin JG, Chen L (2019) Dietary supplementation of selenium yeast enhances the antioxidant capacity and immune response of juvenile Eriocheir sinensis under nitrite stress. Fish Shellfish Immunol 87:22–31. https://doi.org/10.1016/j.fsi.2018.12.076
Liu GX, Jiang GZ, Lu KL, Li XF, Zhou M, Zhang DD, Liu WB (2017) Effects of dietary selenium on the growth, selenium status, antioxidant activities, muscle composition and meat quality of blunt snout bream Megalobrama amblycephala. Aquac Nutr 23(4):777–787. https://doi.org/10.1111/anu.12444
Lovett DL, Felder DL (1990) Ontogenetic change in digestive enzyme activity of larval and postlarval white shrimp Penaeus setiferus (Crustacea, Decapoda, Penaeidae). Biol Bull 178(2):144–159. https://doi.org/10.2307/1541973
Nugroho RA (2002) Fotedar R (2015) Effects of dietary organic selenium on immune responses, total selenium accumulation and digestive system health of marron, Cherax cainii (Austin. Aquac Res 46(7):1657–1667. https://doi.org/10.1111/are.12320
Dawood MAO, Koshio S, Zaineldin AI, Van Doan H, Moustafa EM, Abdel-Daim MM, Angeles Esteban M, Hassaan MS (2019) Dietary supplementation of selenium nanoparticles modulated systemic and mucosal immune status and stress resistance of red sea bream (Pagrus major). Fish Physiol Biochem 45(1):219–230. https://doi.org/10.1007/s10695-018-0556-3
Muralisankar T, Bhavan PS, Radhakrishnan S, Seenivasan C, Manickam N, Srinivasan V (2014) Dietary supplementation of zinc nanoparticles and its influence on biology, physiology and immune responses of the freshwater prawn Macrobrachium rosenbergii. Biol Trace Elem Res 160(1):56–66. https://doi.org/10.1007/s12011-014-0026-4
Muralisankar T, Bhavan PS, Radhakrishnan S, Seenivasan C, Srinivasan V, Santhanam P (2015) Effects of dietary zinc on the growth, digestive enzyme activities, muscle biochemical compositions, and antioxidant status of the giant freshwater prawn Macrobrachium rosenbergii. Aquacul 448:98–104. https://doi.org/10.1016/j.aquaculture.2015.05.045
Muralisankar T, Bhavan PS, Radhakrishnan S, Seenivasan C, Srinivasan V (2016) The effect of copper nanoparticles supplementation on freshwater prawn Macrobrachium rosenbergii post larvae. J Trace Elem Med Biol 34:39–49. https://doi.org/10.1016/j.jtemb.2015.12.003
Srinivasan V, Bhavan PS, Rajkumar G, Satgurunathan T, Muralisankar T, Bhavan S (2016) Effects of dietary iron oxide nanoparticles on the growth performance, biochemical constituents and physiological stress responses of the giant freshwater prawn Macrobrachium rosenbergii post-larvae. Int J Fish Aquat Stud 4:170–182
Srinivasan V, Bhavan PS, Rajkumar G, Satgurunathan T, Muralisankar T (2017) Dietary supplementation of magnesium oxide (MgO) nanoparticles for better survival and growth of the freshwater prawn Macrobrachium rosenbergii Post-larvae. Biol Trace Elem Res 177(1):196–208. https://doi.org/10.1007/s12011-016-0855-4
Asaikkutti A, Bhavan PS, Vimala K, Karthik M, Cheruparambath P (2016) Dietary supplementation of green synthesized manganese-oxide nanoparticles and its effect on growth performance, muscle composition and digestive enzyme activities of the giant freshwater prawn Macrobrachium rosenbergii. J Trace Elem Med Biol 35:7–17. https://doi.org/10.1016/j.jtemb.2016.01.005
Nandeesha MC, Gangadhar B, Keshavanath P, Varghese TJ (2000) Effect of dietary sodium chloride supplementation on growth, biochemical composition and digestive enzyme activity of young Cyprinus carpio (Linn.) and Cirrhinus mrigala (Ham.). J Aquacult Trop 15(2):135–144
Wang FB, Luo L, Lin SM, Li Y, Chen S, Wang YG, Wen H, Hu CJ (2011) Dietary magnesium requirements of juvenile grass carp, ctenopharyngodon idella. Aquac Nutr 17(3):691–700. https://doi.org/10.1111/j.1365-2095.2010.00829.x
Shao XP, Liu W, Bin LuK, Le Xu WN, Zhang WW, Wang Y, Zhu J (2012) Effects of tribasic copper chloride on growth, copper status, antioxidant activities, immune responses and intestinal microflora of blunt snout bream (Megalobrama amblycephala) fed practical diets. Aquacul 338:154–159. https://doi.org/10.1016/j.aquaculture.2012.01.018
Cotter PA, Craig SR, Mclean E (2008) Hyperaccumulation of selenium in hybrid striped bass: a functional food for aquaculture? Aquac Nutr 14(3):215–222. https://doi.org/10.1111/j.1365-2095.2007.00520.x
Satgurunathan T, Bhavan PS, Joy RDS (2019) Green synthesis of chromium nanoparticles and their effects on the growth of the prawn Macrobrachium rosenbergii Post-larvae. Biol Trace Elem Res 187(2):543–552. https://doi.org/10.1007/s12011-018-1407-x
Droge W (2002) Free radicals in the physiological control of cell function. Physiol Rev 82:47–95. https://doi.org/10.1152/physrev.00018.2001
Kong Y, Li S, Liu M, Yao C, Yang X, Zhao N, Li M (2019) Effect of dietary organic selenium on survival, growth, antioxidation, immunity and gene expressions of selenoproteins in abalone Haliotis discus hannai. Aquac Res 50(3):847–855. https://doi.org/10.1111/are.13956
Abdel-Aziem SH, Mahrous KF, Abd El-Hafez MAM, Abdel Mordy M (2018) Genetic variability of myostatin and prolactin genes in popular goat breeds in Egypt J Genet Eng. Biotechnol 16(1):89–97. https://doi.org/10.1016/j.jgeb.2017.10.005
Matsakas A, Patel K (2009) Intracellular signalling pathways regulating the adaptation of skeletal muscle to exercise and nutritional changes. Histol Histopathol 24:209–222. https://doi.org/10.14670/HH-24.209
Segev-Hadar A, Alupo G, Tal K, Nitzan T, Biran J (2020) Identification and characterization of a non-muscular myostatin in the Nile tilapia. Front Endocrinol (Lausanne) 11:94. https://doi.org/10.3389/fendo.2020.00094
Qian Z, Mi X, Wang X, He S, Liu Y, Hou F, Liu X (2013) cDNA cloning and expression analysis of myostatin/GDF11 in shrimp, Litopenaeus vannamei. Comp Biochem Physiol A: Mol Integr Physiol 165(1):30–39. https://doi.org/10.1016/j.cbpa.2013.02.001
Yan Y, Lu X, Kong J, Meng X, Luan S, Dai P, Luo K (2020) Molecular characterization of myostatin and its inhibitory function on myogenesis and muscle growth in Chinese shrimp, Fenneropenaeus chinensis. Gene 758:144–986. https://doi.org/10.1016/j.gene.2020.144986
McDonald AA, Chang ES, Mykles DL (2011) Cloning of a nitric oxide syntheses from green shore crab, Carcinus maenas: a comparative study of the effects of eyestalk ablation on expression in the molting glands (Y-organs) of C. maenas and blackback land crab, Gecarcinus lateralis. Comp Biochem Physiol A 158(1):150–162. https://doi.org/10.1016/j.cbpa.2010.10.013
Diarte-Plata G, Sainz-Hernandez JC, Aguinaga-Cruz JA, Fierro-Coronado JA, Polanco-Torres A, Puente-Palazuelos C (2012) Eyestalk ablation procedures to minimize pain in the freshwater prawn Macrobrachium americanum. Appl Anim Behav Sci 140:172–178. https://doi.org/10.1016/j.applanim.2012.06.002
Pamuru RR, Rosen O, Manor R, Chung JS, Zmora N, Glazera L (2012) Stimulation of molt by RNA interference of the molt inhibiting hormone in the crayfish Cherax quadricarinatus. Gen Comp Endocrinol 178(2):227–236. https://doi.org/10.1016/j.ygcen.2012.05.007
Jung H, Lyons RE, Hurwood DA, Mather PB (2013) Genes and growth performance in crustacean species: a review of relevant genomic studies in crustaceans and other taxa. Rev Aquac 5(2):77–110. https://doi.org/10.1111/raq.12005
Qiao H, Jiang F, Xiong Y, Jiang S, Fu H, Li F, Wu Y (2018) Characterization, expression patterns of molt-inhibiting hormone gene of Macrobrachium nipponense and its roles in molting and growth. PLoS ONE 13(6):e0198861. https://doi.org/10.1371/journal.pone.0198861
Xu Y, Peng G, Sun M, Li J, Yan W, Tang J, Xu Z (2019) Genomic organization of the molt-inhibiting hormone gene in the red swamp crayfish Procambarus clarkii and characterization of single-nucleotide polymorphisms associated with growth. Comp Biochem Physiol B: Biochem Mole Biol 237:110–334. https://doi.org/10.1016/j.cbpb.2019.110334
Acknowledgements
The authors would like to acknowledge Dr. R. Sathiskumar, and one of his Research Scholars, M. Saravanan, Plant Biotechnology Laboratory, Bharathiar University, Coimbatore, India for extending RT-PCR facility and data analysis.
Author information
Authors and Affiliations
Contributions
Thangavelu Satgurunathan has conducted the experiment and drafted the manuscript. Saravana Bhavan Periyakali has designed, supervised, and scrutinized the entire work. Ramasamy Kalpana has validated the references and constructed the tables, figures, and graphical abstract. Sheu Joen-Rong and Jayakumar Thanasekaran have evaluated the manuscript. Manubolu Manjunath has checked the manuscript overall.
Corresponding author
Ethics declarations
Conflict of Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Satgurunathan, T., Bhavan, P.S., Kalpana, R. et al. Influence of Garlic (Allium sativum) Clove-Based Selenium Nanoparticles on Status of Nutritional, Biochemical, Enzymological, and Gene Expressions in the Freshwater Prawn Macrobrachium rosenbergii (De Man, 1879). Biol Trace Elem Res 201, 2036–2057 (2023). https://doi.org/10.1007/s12011-022-03300-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-022-03300-9