Abstract
Zinc is an essential element affecting immune responses in aquatic organisms. In the present research, the immunomodulating effect of zinc oxide nanoparticles (ZnO NPs) was studied in Nile tilapia (Oreochromis niloticus). The minimum inhibitory concentration of zinc oxide nanoparticles (ZnO NPs) for Aeromonas hydrophila was estimated at 60 µg/mL. To evaluate the efficacy of ZnO NPs for improving disease resistance against A. hydrophila, three hundred fish were divided into 5 groups. Fish in the group T1 maintained on the control feed, T2 and T3 feed on ZnO at 60 and 30 µg/g, while T4 and T5 received ZnO NPs at 60 and 30 µg/g, respectively for 8 weeks. Immune responses were evaluated by determining the phagocytic activity, serum antibacterial activity, lysozymes, respiratory burst activity, and also gene expression of immunoglobin M-2, tumor necrosis factor-α, interleukin (IL)-1β, heat shock proteins, IL-10, insulin growth factor 1, transforming growth factor-β2, superoxide dismutase enzyme, and catalase enzyme genes. Results indicated that groups that received ZnO NPs have exaggerated immune response and upregulation in the most of expressed immune-related genes. After the feeding trial, all groups were experimentally infected with A. hydrophila, and the mortality rate was monitored. Among all the treated groups, a higher survival rate and disease resistance were observed for fish that received ZnO NPs at 30 and 60 µg/g. The inclusion of ZnO NPs in O. niloticus feed improves both fish immune response and disease resistance against A. hydrophila.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aquaculture is an important sector in the agriculture economy, as it participated in 42.2% of the global fish production in 2016. Aquatic animals and their products are the keys to meeting the nutritional protein needs of humans in Egypt [1]. The nutritional requirements of farmed fish should be fulfilled in their feed to positively affect reproductive performance and optimize production in the aquatic sector [2,3,4].
Zinc (Zn) is an essential micronutrient for fish, as it plays a vital role in biological activities [5, 6]. As a microelement, Zn participates in several enzymatic processes of animal metabolism such as growth, immune response, and enzyme function [7, 8]. Zn restrains the generation of reactive oxygen species (ROS) such as superoxide anion radical, hydroxyl radical, and hydrogen peroxide [9]. Zinc cannot be stored in the body, necessitating regular dietary intake [10, 11]. There are several Zn sources, including Zn carbonate, chloride, oxide, and sulfate; however, the primary mineral element content is higher in Zn than in other sources and represents 803.4 mg/g [6]. The bioavailability of Zn in fish feed is greatly affected by the tricalcium phosphate content [12]. Higher levels and continuous Zn supplementation should be included in feeds to compensate for the reduced bioavailability, so the continued search for additional techniques to enhance Zn bioavailability in fish is important.
In 2015, about 30,000 metric tons of zinc oxide nanoparticles (ZnO NPs) was produced and used that reflect the importance of this mineral form. Different suggestions have been offered to explain the effect of ZnO NPs on bacterial cells, for example, ZnO NPs can alter the function of the bacterial cell wall by establishing hydrogen bonds and ligands, changing its protein structure [13]. In addition, by binding to the cytoplasmic bacterial membrane disrupting its integrity, ZnO NPs subsequently interrupt the fundamental role of electron transport phosphorylation and energy transduction processes [14].
This work aimed to investigate the protective role of ZnO NPs against bacterial infection in Oreochromis niloticus by studying its influence on fish immunity and antioxidant activity.
Materials and Methods
Preparation of Zn NPs
Zinc sulfate (ZnSO4·7H2O) and zinc oxide (ZnO), El-Nasr Co., Giza, Egypt, were purchased from the local market. Nanocrystalline ZnO was prepared using the precipitation method of Daneshvar et al. [15]. Briefly, zinc sulfate was mixed with sodium hydroxide, then the mixture was continuously stirred for 12 h and then the produced zinc hydroxide precipitate was washed with deionized water. The precipitant was dried then calcined in air at a specific temperature to produce nanosized ZnO. The particle size of ZnO NPs was determined by a transmission electron microscope.
Fish Feed Preparation
ZnO NPs were ultrasonically distributed in water according to the procedure developed by Sherif et al. [16]. Briefly, 30 and 60 mg of ZnO NPs were sonicated in 100 mL of Milli-Q water and the same procedure was done for ZnO. One kg of pelleted fish feed was sprayed with 100 mL of the prepared ZnO NP solution to achieve a concentration of 30 and 60 µg/g. The prepared ration was dried at 50 °C for 1 h to remove the excess moisture. The physical and chemical composition of the experimental basal diet is presented in Table 1.
Determination of Zn Concentration in Fish Tissues
Zn concentrations in the fish tissues were analyzed following the method designated by Li et al. [17]. Briefly, the whole body of three randomly selected fish from each group was killed by an overdose of MS222 anesthetic, and 2 g of the dorsal musculature of each fish was taken and labeled. Each fish sample was minced then dried and finely ground and each muscle sample was ground in a clean mortar after that whole fish tissue and muscle samples were dried in a drying oven at 90 °C for 1 day. One hundred and fifty milligrams of dried sample was added to 15 mL of 65% nitric acid and 2 mL of 70% perchloric acid in Kjeldahl flasks after complete digestion, and 33 mL of deionized water was added. The sample was assayed in atomic absorption spectrophotometer equipped with a graphite furnace (Model AA-240Z; Varian, Australia).
The Minimum Inhibitory Concentration of ZnO NPs for Aeromonas hydrophila
The minimum inhibitory concentration (MIC) was calculated following the recommendations described by Ravikumar et al. [18]. Briefly, the overnight Aeromonas hydrophila broth culture was adjusted to 1 × 106 CFU, then diluted to 1:200 with sterile broth. One hundred and ninety microliters from the diluted broth was added to each well of a 96-well microtiter plate. Ten microliters of ZnO NP solution was added to each well to achieve a final concentration of 100, 90, 80, 70, 60, 50, 40, 30, 20, and 10 μg/mL then the plate surface was sealed with cello tape to avoid dehydration. The whole setup was performed in triplicates and culture microplates were incubated at 37 °C for 48 h. MIC is determined as the lowest concentration of ZnO NPs that completely inhibited bacterial growth.
Experimental Design and Fish Accommodations
A total number of 320 apparently healthy O. niloticus (28 ± 3 g) were collected from a private fish farm in Kafrelsheikh, and fish were stocked in the wet laboratory of the Animal Health Research Institute, Egypt. Fish were acclimated in a 3-m3 fiberglass tank for 2 weeks, after that 20 fish were examined for clinical signs and postmortem following recommendations of Austin and Austin [19] to ensure that the experimental fish is free from any disease. Experimental fish were randomly divided into 5 groups (T1–T5) in triplicates (20 fish/replicate). Fish in the first group (T1) were maintained as a control and received a basal diet without any additives. The second and third treatment groups (T2 and T3) received ZnO at 60 and 30 µg/g feed while the fourth and fifth treatment groups (T4 and T5) were received ZnO NPs at 60 and 30 µg/g feed, respectively. Each replicate was maintained in 250-L glass aquaria (110 × 50 × 50 cm). During the experimental work, the water temperature was adjusted at 28 °C ± 1.5 °C, dissolved oxygen more than 5.5 ± 0.5 mg/L, pH 7.8, and salinity around 0.3 g/L. In addition, one-third of the water was exchanged daily with the removal of fish excreta.
Innate Immunity Responses
Blood Sampling
Blood samples were collected from 5 fishes in each group on the last day of the feeding trial, about 2 mL of blood was collected from the caudal vessels of each fish. Serum samples were separated by centrifugation at 2000 rpm for 10 min, and leukocytes were isolated from each blood sample as described by Faulmann et al. [20].
Phagocytic Activity
Phagocytic activity (PA) and the phagocytic index (PI) were calculated according to Kawahara et al. [21]. Twenty-four-hour Candida albicans culture was counted with hemocytometer then adjusted to 1 × 106 cells/mL, and the leukocytes of each sample were adjusted to 2.5 × 106 cells/mL. One millliter of adjusted leukocytes was added to 1 mL of C. albicans suspension, and the mixture was incubated in a CO2 incubator at 27 °C for 1 h. One smear was prepared from each sample then stained with Giemsa then one-hundred leukocytes were counted under the oil immersion lens, and the number of engulfed yeast cells was also determined. The PA and PI were calculated according to the following equations:
Serum Bactericidal Activity Percentage
Serum bactericidal activity (SBA) was determined by mixing equal volume (100 μL) of fish serum and A. hydrophila bacterial suspension containing 1 × 106 CFU/mL. The mixture was incubated for 1 h at 25 °C, and the blank sample was prepared by replacing fish serum with sterile phosphate-buffered saline (PBS). A series of tenfold dilutions were prepared from each mixture and streaked on blood agar, and plates were incubated for 24 h at 37 °C then the number of viable bacterial colonies was counted [22].
Analysis of Lysozyme Activity
Lysozyme (LYZ) activity was assayed as described by Helal and Melzig [23]. Initially, 10 μL of fish serum was added to 100 µL of Micrococcus lysodeikticus suspended in PBS (360 µg/mL, pH:6.24). The absorbance of samples was measured at 450 nm after incubation at 37 °C for 5 min.
Respiratory Burst Activity
Respiratory burst activity (RBA) was estimated for isolated leukocytes using nitro blue tetrazolium (NBT) assay via the method reported by Jang et al. [24]. Fifty from previously prepared leukocyte suspension was loaded in 96-well plate and incubated at 30◦C for 1 h. The wells were rinsed with PBS (pH 7.2) and set aside for 1 h at 30◦C after adding NBT (50 μL). Methanol (30%) was added to each well to fix the cells and left undisturbed for 5 min. The air-dried wells were filled with 60 μL of 2 mM potassium hydroxide and 70 μL of dimethyl sulfoxide. Absorbance was ultimately measured on a plate reader at 540 nm.
Gene Expression of Cytokines and Antioxidant Enzyme Genes in O. niloticus
Total RNA was extracted from the head kidney tissues that were collected from three fish in each group on the last day of the feeding trial using the Trizol reagent (iNtRON Biotechnology Inc., Korea) following the manufacturer’s procedure. The quantity and quality of extracted RNA were assessed by Nanodrop D-1000 spectrophotometer (NanoDrop Technologies Inc., USA). The complementary DNA (cDNA) was synthesized by the reverse transcription-polymerase chain reaction using SensiFAST cDNA synthesis kit (Bioline, USA) following the manufacturer’s protocol. For studying the gene expression, the resulting cDNA was used as a template in the quantitative real-time PCR using Nile tilapia-specific primer sets (Table 2) for immunoglobin (Ig) M-2, tumor necrosis factor (TNF)-α, interleukin (IL)-1β, heat shock protein (IL-10), insulin growth factor (IGF) 1, and transforming growth factor (TGF)-β2, as well as superoxide dismutase enzyme (SOD) and catalase (CAT) enzyme, while the gene encoding β-actin was used as the housekeeping gene, owing to its constitutive expression. After the efficiency of PCR was confirmed to be around 100%, the data of gene expression were calculated using the Eq. 2−ΔΔCT method according to the procedure of Livak and Schmittgen [25].
Bacterial Challenge
After the end of the feeding trial, two replicates in each group (40 fish/group) were experimentally infected with A. hydrophila (AHRAS22) strain accession number in NCBI (MW092007), in which one replicate was injected intraperitoneally (I/P) with the LD50 that equals 2.4 × 105 CFU/fish and the other replicate infected through the addition of the infective agent in aquarium water (cohabitation) to simulate the natural infection conditions in fish farms.
Daily mortality was recorded for 14 successive days and the cumulative mortality rate (CMR) was calculated using the following equation:
The relative level of protection (RLP) was verified among the challenged fish according to Ruangpan et al. [27] as follows:
A. hydrophila were re-isolated from dead and moribund fish according to the methods described by Austin and Austin [19]. For bacteriological examination, a loopful from the kidney, liver, spleen, and heart was added to brain heart infusion broth tube, then tubes were incubated at 28 °C for 24 h [28]. A loopful from the broth was streaked on Aeromonas selective agar base with ampicillin supplement then plates were incubated at 28 °C for 24 h, and after that, single pure colony from each plate was identified biochemically using API 20E strips. Phenotypic characterization of the bacterial isolates was confirmed according to the published instructions of Madigan and Martinko [29].
Biosafety Considerations
This study followed the biosafety measures outlined on the pathogen safety data sheets entitled infectious substances — A. hydrophila, Pathogen Regulation Directorate [30].
Statistical Analyses
Data were statistically analyzed for variance (ANOVA) with SPSS software, SPSS Inc., Chicago, IL, USA. Duncan’s multiple range test [31] was used to determine differences among treatments at a significance level of 0.05.
Results
Characterization of Zn NPs
Synthesized ZnO NPs used in this work were approximately 45 nm (99.7% purity). Scanning electron microscopy results show the different sizes of produced ZnO NPs as represented in Fig. 1.
Zn Residue in Fish Tissues
High Zn level was significantly retained in the whole body of O. niloticus that received 60 µg/g of ZnO NPs compared to other treatments while there was a non-significant difference between groups received 30 µg/g (T3 = 26.17 and T5 = 28.6 µg/g) regardless of the form of ZnO. The residue of Zn in fish muscles followed the same whole-body pattern, except in groups that received ZnO NPs (T4 = 22.2 and T5 = 17.4 µg/g) in which zinc levels were higher than other groups received ZnO as represented in Table 3.
The Minimum Inhibitory Concentration of ZnO NPs for A. hydrophila
The estimated MIC of the synthesized ZnO NPs for A. hydrophila was 60 µg/mL.
Effect of In-feed Supplementation with ZnO NPs on the Innate Immunity
The incorporation of ZnO NPs in the fish diet has a significant booster effect on innate immunity as represented in Table 4. In addition, the higher concentration of ZnO regardless of its form has improved innate immunity for groups T2 and T4 than other groups.
Level of Cytokine Gene Expression
Feeding dietary ZnO modulated the gene expression of IgM-2, TNF-α, IL-1β, IL-10, IGF 1, and TGF-β2 in the head kidney tissue of O. niloticus as shown in Figs. 2 and 3. IgM-2 and anti-inflammatory cytokines (IL-10 and TGF-β2) were significantly increased in fish that received ZnO NPs at 60 µg/g in their feed in comparison with the control group and representing 8.73-, 16.8-, and 6.6-fold, respectively; this finding indicates the enhancement of fish immune status.
Gene Expression of Antioxidant Enzymes
The gene expression levels of SOD and CAT had a significant upsurge in the head kidney of O. niloticus that received dietary ZnO NPs at both 30 and 60 µg/g. There was 8.83- and 3.2-fold increase for SOD also there was 2.27- and 2.5-fold increase for CAT in comparison with the control group, as represented in Fig. 4. Group received ZnO also showed 3.8-fold increase in SOD expression compared with the control group.
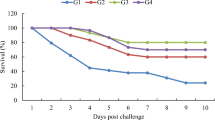
Results of Challenge Test with A. hydrophila Bacteria
In Table 5, fish that received dietary ZnO NPs in T4 and T5 showed a lower cumulative mortality rate (40%), and this was lower than the cumulative mortality in the other groups. Fish that received ZnO NPs at 30 and 60 µg/g also showed a higher relative level of protection estimated by 33.33% and 60%, respectively. While ZnO could not protect challenged fish against bacterial infection, it decreased the mortality in cohabitant fish compared to I/P-infected fish.
Discussion
Farmed fish are mainly fed diets of plant origin in which Zn absorption and bioavailability are low due to the presence of dietary phytate and tricalcium phosphate [32, 33].
In this study, whole body and muscle tissues of O. niloticus which received dietary ZnO NPs retained higher Zn levels than fish in the control group and both groups that received dietary ZnO. In all treatments, Zn concentration in the muscle showed higher values than the controls but was still within the range of the permissible limit (40 ppm) set by the Food and Agriculture Organization [34]. Accordingly, Cyprinus carpio fed ZnO NPs (30 and 100 µg/g) had a higher Zn level in their tissues than those provided similar concentrations of ZnSO4 and Zn-proteinate. These findings are due to the ability of ZnO NPs to pass through the intestine epithelium and accumulate in the tissues [35]. In addition, Zn concentrations in the whole body of O. niloticus were insignificantly affected (P > 0.05) by incorporation of a ZnO mix of zeolite and ZnO or ZnSO4 in their diets [36]. Supporting our results, inorganic Zn forms (ZnO, ZnSO4, and zinc carbonate [ZnCO3]) possess a low rate of absorption via fish intestine [37]. Different findings obtained with rainbow trout larvae [38] found that feeding the inorganic form of Zn (ZnSO4) resulted in a higher accumulation in fish tissues than ZnO NPs and organic Zn.
Based on our results, the MIC of ZnO NPs was determined to be 60 µg/mL for A. hydrophila. Accordingly, when the potential bacterial inhibition property of Murraya koenigii berry extract-based ZnO NPs was assessed by Yazhiniprabha et al. [39], a concentration of 25 μg/mL was the least quantity required to inhibit 1 × 106 cells/mL of A. hydrophila, whereas a concentration of 50 μg/mL could diminish approximately 70–80% of biofilm activity. The antibacterial properties of ZnO NPs could be due to their ability to penetrate the bacterial cell membrane by releasing ions to disrupt cellular metabolism [40] and/or to generate ROS within bacterial cells, causing damage to the cell envelope, cell membrane, cell structure, and biomolecules [41].
Respiratory burst activities are initiated when the immune system identified any microbial pathogen as a part of the innate immune response [42, 43]. The LYZ is a part of the innate immunity of fish that protects against diseases [44]. Therefore, the significant increase of PA, PI, SBA, LYZ, and RBA in the experimental fish fed dietary ZnO NPs showed that ZnO NPs could boost the innate immunity of O. niloticus. In a similar study, Gharaei et al. [45] found that the activity of LYZ is significantly increased in beluga (Huso huso) after feeding dietary chitosan–ZnO NP. In addition, the response of Oreochromis mossambicus resembled that of our study as the LYZ and myeloperoxidase activities were increased in the serum after ZnO NP-based β-glucan binding protein was added to the feed [46]. Many enzymes and proteins in freshwater fish could restrict the invasion and replication of pathogenic bacteria [47]. In this study, dietary ZnO NPs activated SBA in O. niloticus. Similarly, Yazhiniprabha et al. [39] found that ZnO NPs promote the innate immunity of O. mossambicus by stimulating serum antiprotease activity and natural complement activity, which in turn restricted the invasion and replication of pathogens in the serum.
The innate and adaptive immune response could be promoted by NPs [48] through activating toll-like receptors [49] and downregulating the pro-inflammatory cytokines [7, 43, 50]. Our results showed that dietary ZnO NPs (60 µg/g) could enhance the immunity of O. niloticus as the expression of some genes involved in immune responses had a significant upsurge, such as IgM-2 and anti-inflammatory cytokines (IL-10 and TGF-β2). High gene expression of cytokines TNF-α and IL6 is considered signs of an inflammatory response [51,52,53], so low levels of mRNA gene expression of IL6 and TNF-α indicated an enhanced health status of blunt snout bream (Megalobrama amblycephala) fed zinc-bearing palygorskite (Zn-Pal) [54, 55]. Accordingly, Song et al. [52] mentioned that pro-inflammatory cytokines are downregulated in young grass carp (Ctenopharyngodon idella) that received dietary Zn.
Similarly, the gene expression of IgM was upregulated in response to dietary ZnO NPs (30 mg/kg) in O. niloticus [56]. The sera globulin in C. carpio was also significantly upregulated [57]. Zn bioavailability affects the potential immune role. Soaudy et al. [58] reported that the gene expression of IgM-2 is enhanced by supporting Zn with K. This upregulation may be due to the continuous and slow release of Zn from the ZnO-K form compared with other Zn forms.
It is well known that Zn and ZnO NPs can stimulate antioxidant responses via activation of enzymatic antioxidants, glutathione peroxidase [59], and CAT [60, 61], as well as non-enzymatic cellular responses, eliminating the generation of ROS and protecting tissues from oxidation stress-based damages. In this study, regardless of the concentration, the gene expression of SOD and CAT was upregulated in O. niloticus fed dietary ZnO NPs. Similarly, with freshwater fish, the antioxidant status of O. niloticus fed dietary ZnO NPs was enhanced through improving the activities of SOD and CAT [56]. In addition, Dekani et al. [35] recorded a significant increase in the activity of SOD and minimum activity of CAT in C. carpio fed a diet containing 500 mg/kg of ZnO NPs compared with those that received the same concentration of ZnSO4. Gene expression of mRNA of CAT and glutathione S-transferase mu was increased and attained the peak in abalone (Haliotis discus hannai) fed a diet containing 33.8 mg/Zn/kg [62], supporting our findings. Different findings were obtained by Soaudy et al. [58], who noticed that enhancing the Zn availability of the ZnO-K form resulted in an increase in the expression of CAT and SOD genes in fish compared with those fed dietary ZnO NPs.
From our results, O. niloticus fed dietary ZnO NPs and experimentally infected I/P with A. hydrophila and cohabitants achieved RPL of 33.33% and 60%, respectively. These results may be because ZnO NPs could enhance the innate immunity (cellular and humoral) and oxidative status of O. niloticus that could compete for the bacterial infection. Zhang et al. [63] stated that the intestinal digesta of blunt snout bream fed dietary Zn-Pal shows a decrease in Escherichia coli and A. hydrophila. Jiao et al. [64] claimed that a low bacterial number could be due to the released Zn ions from Zn-Pal. Moreover, it is well documented that Zn deficiency reduces immune responses and disease resistance in humans and animals [65]. The high SBA of fish is mediated by molecules capable of inhibiting bacterial growth [66]. LYZ and phagocytosis are non-specific humoral immune defenses against gram‐positive and gram-negative bacteria [67], activated in response to dietary ZnO NPs. Similarly, Yazhiniprabha et al. [39] found that O. mossambicus fed with Mb-ZnO NP-supplemented diet at different concentrations (0.5, 1, and 2 mg/kg) has improved immune responses and the ability to resist A. hydrophila infection. In freshwater fish Pangasius hypophthalmus, ZnO NPs enhanced immunological parameters such as total protein, albumin, globulin, and A/G ratio, as well as stress biomarkers such as blood glucose, cortisol, and HSP 70 that allow the fish to resist biotic (Aeromonas veronii biovar sobria) and abiotic multi-stressors as lead toxicity and water temperature of 34 °C [68]. Survival of freshwater prawn (Macrobrachium rosenbergii) and rainbow trout (Oncorhynchus mykiss) was significantly increased after dietary supplementation with the same concentration of ZnO NPs at 60 mg/kg [38, 69].
Conclusion
The study results indicate that ZnO can boost the immune status of O. niloticus and protect fish against bacterial infection. ZnO nanoparticles have a superior effect over the inorganic ZnO form in stimulating the immune system of fish through increasing the innate immune response together with upregulating the expression of immune-related, cytokines and antioxidant enzyme genes. ZnO nanoparticles also have a protective effect for O. niloticus against A. hydrophila infection when administrated infeed at 60 ppm.
Data Availability
Data are available on request from the authors.
Change history
05 May 2022
A Correction to this paper has been published: https://doi.org/10.1007/s12011-022-03268-6
References
FAO, Food and Agriculture Organization (2018) Global aquaculture production Rome, Italy. http://www.fao.org/fishery/statistics/global-aquaculture-production/query/en
Mabroke RS, Tahoun AM, El-Haroun ER, Suloma A (2012) Influence of dietary protein on growth, reproduction, seed chemical composition and larval survival rate of Nile tilapia (Oreochromis niloticus) broodstocks of different size groups under hapa-in-pond hatchery system. Araban Aquac Soc 7(2):203–220
Suloma A, Tahoun AM, Mabrok RS (2017) Development of brood-stock diets for Nile tilapia under hapa-in-pond hatchery system; optimal dietary vitamin C level for the optimum reproductive performance and fry survival. J Aquac Res Dev S2:010. https://doi.org/10.4172/2155-9546.S2-010
Sherif AH, Gouda MY, Naena NA, Ali AH (2020) Alternate weekly exchanges of feeding regime affect the diversity of intestinal microbiota and immune status of Nile tilapia Oreochromis niloticus. Aquac Res 51(10):4327–4339. https://doi.org/10.1111/are.14778
Antony N, Balachandran S, Mohanan PV (2016) Immobilization of diastase α-amylase on nano zinc oxide. Food Chem 211:624–630. https://doi.org/10.1016/j.foodchem.2016.05.049
NRC, National Research Council (2011) Nutrient requirements of fish and shrimp. National Academy Press, Washington, p 392
Tawfik M, Moustafa M, Abumourad IMK, El-Meliegy E, Refai M (2017) Evaluation of nano zinc oxide feed additive on tilapia growth and immunity. In 15th International Conference on Environmental Science and Technology, Rhodes, Greece (Vol. 1342, No. 1, pp. 1–9)
Uniyal S, Garg AK, Jadhav SE, Chaturvedi VK, Mohanta RK (2017) Comparative efficacy of zinc supplementation from different sources on nutrient digestibility, hemato-biochemistry and anti-oxidant activity in guinea pigs. Livest Sci 204:59–64. https://doi.org/10.1016/j.livsci.2017.08.009
Kumar N, Ambasankar K, Krishnani KK, Bhushan S, Minhas PS (2016) Dietary pyridoxine protects against stress and maintains immunohaematological status in Chanos chanos exposed to endosulfan. Basic Clin Pharmacol Toxicol 119(3):297–308. https://doi.org/10.1111/bcpt.12589
Case CL, Carlson MS (2002) Effect of feeding organic and inorganic sources of additional zinc on growth performance and zinc balance in nursery pigs. J Anim Sci 80(7):1917–1924. https://doi.org/10.2527/2002.8071917x
Zalewski PD, Truong-Tran AQ, Grosser D, Jayaram L, Murgia C, Ruffin RE (2005) Zinc metabolism in airway epithelium and airway inflammation: basic mechanisms and clinical targets. A review. Pharmacol Ther 105(2):127–149. https://doi.org/10.1016/j.pharmthera.2004.09.004
Satoh S, Poe WE, Wilson RP (1989) Effect of supplemental phytate and/or tricalcium phosphate on weight gain, feed efficiency and zinc content in vertebrae of channel catfish. Aquaculture 80(1–2):155–161
Jiang W, Yang K, Vachet RW, Xing B (2010) Interaction between oxide nanoparticles and biomolecules of the bacterial cell envelope as examined by infrared spectroscopy. Langmuir 26(23):18071–18077. https://doi.org/10.1021/la103738e
Lyon DY, Thill A, Rose J, Alvarez PJJ (2007) Environmental nanotechnology: applications and impacts of nanomaterials. In: Wiesner M, Bottero J-Y (Eds.). McGraw Hill Publishing, New York, 445–480
Daneshvar N, Aber S, Dorraji MS, Khataee AR, Rasoulifard MH (2007) Preparation and investigation of photocatalytic properties of ZnO nanocrystals: effect of operational parameters and kinetic study. Int J Nuc Quan Eng 1(5):62–67. https://doi.org/10.5281/zenodo.1072778
Sherif AH, El‐Sharawy MES, El‐Samannoudy SI, Adel Seida A, Sabry NM, Eldawoudy M, ..., Younis NA (2021) The deleterious impacts of dietary titanium dioxide nanoparticles on the intestinal microbiota, antioxidant enzymes, diseases resistances and immune response of Nile tilapia. Aquac Res 52(12): 6699-6707. https://doi.org/10.1111/are.15539
Li YLPXJ, Li YBHZQ, Qing-Song HLL (2006) Determination of seven trace elements in tea samples by atomic absorption spectrometry. Chin J Spectrosc Lab 5
Ravikumar S, Gokulakrishnan R, Selvanathan K, Selvam S (2011) Antibacterial activity of metal oxide nanoparticles against ophthalmic pathogens. Int J Pharm Res Dev 3(5):122–127
Austin B, Austin DA (2012) Bacterial fish pathogens: disease of farmed and wild fish, 3rd edition, 112–115
Faulmann E, Cuchens MA, Lobb CJ, Miller NW, Clem LW (1983) An effective culture system for studying in vitro mitogenic responses of channel catfish lymphocytes. Trans Am Fish Soc 112(5):673–679. https://doi.org/10.1577/1548-8659
Kawahara E, Ueda T, Nomura S (1991) In vitro phagocytic activity of white-spotted char blood cells after injection with Aeromonas salmonicida extracellular products. Fish Pathol 26(4):213–214
Kajita Y, Sakai M, Atsuta S, Kobayashi M (1990) The immunomodulatory effects of levamisole on rainbow trout, Oncorhynchus mykiss. Fish Pathol 25(2):93–98
Helal R, Melzig MF (2008) Determination of lysozyme activity by a fluorescence technique in comparison with the classical turbidity assay. Pharmazie 63(6):415–419
Jang SI, Hardie LJ, Secombes CJ (1995) Elevation of rainbow trout Oncorhynchus mykiss macrophage respiratory burst activity with macrophage-derived supernatants. J Leukoc Biol 57(6):943–947. https://doi.org/10.1002/jlb.57.6.943
Livak KJ, Schmittgen HD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25:402–408
Buonocore F, Randelli E, Bird S, Secombes CJ, Facchiano A, Costantini S, Scapigliati G (2007) Interleukin-10 expression byreal-time PCR and homology modelling analysis in the European sea bass (Dicentrarchus labrax L.). Aquaculture 270(1–4):512–522. https://doi.org/10.1016/j.aquaculture2007.05.040
Ruangpan L, Kitao T, Yoshida T (1986) Protective efficacy of Aeromonas hydrophila vaccines in Nile tilapia. Vet Immunol Immunopathol 12(1–4):345–350. https://doi.org/10.1016/0165-2427(86)90139-X
El-Bahar HM, Ali NG, Aboyadak IM, Khalil SA, Ibrahim MS (2019) Virulence genes contributing to Aeromonas hydrophila pathogenicity in Oreochromis niloticus. Int Microbiol. https://doi.org/10.1007/s10123-019-00075-3
Madigan MT, Martinko J (2005) Brock biology of microorganisms, 11th edn. Prentice Hall
Public Health Agency of Canada (2010) The Honourable Leona Aglukkaq, P.C., M.P. Minister of Health
Duncan DB (1955) Multiple range and multiple “F” test. Biometrics 11:10
Faiz H, Zuberi A, Nazir S, Rauf M, Younus N (2015) Zinc oxide, zinc sulfate and zinc oxide nanoparticles as source of dietary zinc: comparative effects on growth and hematological indices of juvenile grass carp (Ctenopharyngodon idella). Int J Agric Biol 17(3):568–574
Swain PS, Rao SB, Rajendran D, Dominic G, Selvaraju S (2016) Nano zinc, an alternative to conventional zinc as animal feed supplement: a review. Anim Nutr 2(3):134–141. https://doi.org/10.1016/j.aninu.2016.06.003
FAO, SIDA, (1983) Manual of methods in aquatic environment research. Part 9. Analyses of metals and organochlorines in fish. FAO Fish Tech Pap 212:21–33
Dekani L, Johari SA, Joo HS (2019) Comparative toxicity of organic, inorganic and nanoparticulate zinc following dietary exposure to common carp (Cyprinus carpio). Sci Total Environ 656:1191–1198. https://doi.org/10.1016/j.scitotenv.2018.11.474
Hu CH, Xiao K, Jiao LF, Song J (2014) Effects of zinc oxide supported on zeolite on growth performance, intestinal barrier function and digestive enzyme activities of Nile tilapia. Aquac Nutr 20(5):486–493. https://doi.org/10.1111/anu.12101
Tan B, Mai K (2001) Zinc methionine and zinc sulfate as sources of dietary zinc for juvenile abalone, Haliotis discus hannai Ino. Aquaculture 192(1):67–84. https://doi.org/10.1016/S0044-8486(00)00435-X
Shahpar Z, Johari SA (2019) Effects of dietary organic, inorganic, and nanoparticulate zinc on rainbow trout, Oncorhynchus mykiss larvae. Biol Trace Elem Res 190(2):535–540
Yazhiniprabha M, Gopi N, Mahboob S, Al-Ghanim KA, Al-Misned F, Ahmed Z, ..., Vaseeharan B (2022) The dietary supplementation of zinc oxide and selenium nanoparticles enhance the immune response in freshwater fish Oreochromis mossambicus against aquatic pathogen Aeromonas hydrophila. J Trace Elem Med Biol 69: 126878. https://doi.org/10.1016/j.jtemb.2021.126878
Slavin YN, Asnis J, Häfeli UO, Bach H (2017) Metal nanoparticles: understanding the mechanisms behind antibacterial activity. J Nanobiotechnol 15(1):1–20. https://doi.org/10.1186/s12951-017-0308-z
Su HL, Chou CC, Hung DJ, Lin SH, Pao IC, Lin JH, ..., Lin JJ (2009) The disruption of bacterial membrane integrity through ROS generation induced by nanohybrids of silver and clay. Biomaterials 30(30): 5979-5987. https://doi.org/10.1016/j.biomaterials.2009.07.030
Biller-Takahashi JD, Takahashi LS, Saita MV, Gimbo RY, Urbinati EC (2013) Leukocytes respiratory burst activity as indicator of innate immunity of pacu Piaractus mesopotamicus. Braz J Biol 73:425–429. https://doi.org/10.1590/S1519-69842013000200026
Sherif AH, Gouda MY, Zommara MA, Abd El-Rahim AH, Mahrous K, Abd-El halim Salama SS, (2021) Inhibitory effect of nano selenium on the recurrence of Aeromonas hydrophila bacteria in Cyprinus carpio. Egypt EJABF 25(3):713–738. https://doi.org/10.21608/EJABF.2021.180901
Kaya H, Aydın F, Gürkan M, Yılmaz S, Ates M, Demir V, Arslan Z (2016) A comparative toxicity study between small and large size zinc oxide nanoparticles in tilapia (Oreochromis niloticus): organ pathologies, osmoregulatory responses and immunological parameters. Chemosphere 144:571–582. https://doi.org/10.1016/j.chemosphere.2015.09.024
Gharaei A, Khajeh M, Khosravanizadeh A, Mirdar J, Fadai R (2020) Fluctuation of biochemical, immunological, and antioxidant biomarkers in the blood of beluga (Huso huso) under effect of dietary ZnO and chitosan–ZnO NPs. Fish Physiol Biochem 46(2):547–561. https://doi.org/10.1007/s10695-019-00726-2
Anjugam M, Vaseeharan B, Iswarya A, Gobi N, Divya M, Thangaraj MP, Elumalai P (2018) Effect of β-1, 3 glucan binding protein-based zinc oxide nanoparticles supplemented diet on immune response and disease resistance in Oreochromis mossambicus against Aeromonas hydrophila. Fish Shellfish Immunol 76:247–259. https://doi.org/10.1016/j.fsi.2018.03.012
Borgia VF, Thatheyus AJ, Murugesan AG, Alexander SCP, Geetha I (2018) Effects of effluent from electroplating industry on the immune response in the freshwater fish, Cyprinus carpio. Fish Shellfish Immunol 79:86–92. https://doi.org/10.1016/j.fsi.2018.05.010
Luo YH, Chang LW, Lin P (2015) Metal-based nanoparticles and the immune system: activation, inflammation, and potential applications. Biomed Res Int 2015:Article 143720. https://doi.org/10.1155/2015/143720
Lucarelli M, Gatti AM, Savarino G, Quattroni P, Martinelli L, Monari E, Boraschi D (2004) Innate defence functions of macrophages can be biased by nano-sized ceramic and metallic particles. Eur Cytokine Netw 15(4):339–346
Sherif AH, Al-Sokary ET, Rizk WF, Mahfouz ME (2020) Immune status of Oreochromis niloticus subjected to long-term lead nitrate exposure and a Arthrospira platensis treatment trial. Environ Toxicol Pharmacol 76:103352. https://doi.org/10.1016/j.etap.2020.103352
Shi L, Feng L, Jiang WD, Liu Y, Jiang J, Wu P, Zhou XQ (2015) Folic acid deficiency impairs the gill health status associated with the NF-κB, MLCK and Nrf2 signaling pathways in the gills of young grass carp (Ctenopharyngodon idella). Fish Shellfish Immunol 47(1):289–301. https://doi.org/10.1016/j.fsi.2015.09.023
Song ZX, Jiang WD, Liu Y, Wu P, Jiang J, Zhou X Q, ..., Feng L (2017) Dietary zinc deficiency reduced growth performance, intestinal immune and physical barrier functions related to NF-κB, TOR, Nrf2, JNK and MLCK signalling pathway of young grass carp (Ctenopharyngodon idella). Fish Shellfish Immunol 66: 497-523. https://doi.org/10.1016/j.fsi.2017.05.048
Sherif AH, Alsokary ET, Esam HA (2019) Assessment of titanium dioxide nanoparticle as treatment of Aeromonas hydrophila infection in Oreochromis niloticus. J Hellenic Vet Med Soc 70(3):1697–1706. https://doi.org/10.12681/jhvms.21796
Kutyrev I, Cleveland B, Leeds T, Wiens GD (2016) Proinflammatory cytokine and cytokine receptor gene expression kinetics following challenge with Flavobacterium psychrophilum in resistant and susceptible lines of rainbow trout (Oncorhynchus mykiss). Fish Shellfish Immunol 58:542–553. https://doi.org/10.1016/j.fsi.2016.09.053
Wang T, Secombes CJ (2013) The cytokine networks of adaptive immunity in fish. Fish Shellfish Immunol 35(6):1703–1718. https://doi.org/10.1016/j.fsi.2013.08.030
Awad A, Zaglool AW, Ahmed SA, Khalil SR (2019) Transcriptomic profile change, immunological response and disease resistance of Oreochromis niloticus fed with conventional and nano-zinc oxide dietary supplements. Fish Shellfish Immunol 93:336–343. https://doi.org/10.1016/j.fsi.2019.07.067
Gopal V, Parvathy S, Balasubramanian PR (1997) Effect of heavy metals on the blood protein biochemistry of the fish Cyprinus carpio and its use as a bio-indicator of pollution stress. Environ Monit Assess 48(2):117–124. https://doi.org/10.1023/A:1005767517819
Soaudy MR, Mohammady EY, Ali MM, Elashry MA, Hassaan MS (2021) Potential effects of dietary ZnO supported on kaolinite (ZnO-K) to improve biological parameters, reproduction indices, lipid profile and antioxidant enzymes activities for broodstock of Nile tilapia (Oreochromis niloticus). Anim Feed Sci Technol 281:115117. https://doi.org/10.1016/j.anifeedsci.2021.115117
Rocha-Santos C, Bastos FF, Dantas RF, Hauser-Davis RA, Rodrigues LC, Bastos VC, Bastos JC (2018) Glutathione peroxidase and glutathione S-transferase in blood and liver from a hypoxia-tolerant fish under oxygen deprivation. Ecotoxicol Environ Saf 163:604–611
Vasylkiv OY, Kubrak OI, Storey KB, Lushchak VI (2011) Catalase activity as a potential vital biomarker of fish intoxication by the herbicide aminotriazole. Pestic Biochem Phys 101(1):1–5. https://doi.org/10.1016/j.pestbp.2011.05.005
Dehghan G, Rashtbari S, Yekta R, Sheibani N (2019) Synergistic inhibition of catalase activity by food colorants sunset yellow and curcumin: an experimental and MLSD simulation approach. Chem Biol Interact 311:108746. https://doi.org/10.1016/j.cbi.2019.108746
Wu C, Zhang W, Mai K, Xu W, Zhong X (2011) Effects of dietary zinc on gene expression of antioxidant enzymes and heat shock proteins in hepatopancreas of abalone Haliotis discus hannai. Comp Biochem Physiol Part C Toxicol Pharmacol 154(1):1–6. https://doi.org/10.1016/j.cbpc.2011.03.003
Zhang R, Wen C, Chen Y, Liu W, Jiang Y, Zhou Y (2020) Zinc-bearing palygorskite improves the intestinal development, antioxidant capability, cytokines expressions, and microflora in blunt snout bream (Megalobrama amblycephala). Aquac Rep 16:100269
Jiao L, Lin F, Cao S, Wang C, Wu H, Shu M, Hu C (2017) Preparation, characterization, antimicrobial and cytotoxicity studies of copper/zinc-loaded montmorillonite. J Anim Sci Biotechnol 8(1):1–7. https://doi.org/10.1186/s40104-017-0156-6
Chesters JK, O'Dell BL, Sunde RA (1997) Handbook of nutritionally essential mineral elements. Marcel Dekker Inc., New York, 185–230. https://doi.org/10.1201/9781482273106
Magnadottir B (2006) Innate immunity of fish (overview). Fish Shellfish Immunol 20(2):137–151
Saurabh S, Sahoo PK (2008) Lysozyme: an important defense molecule of fish innate immune system. Aquac Res 39(3):223–239. https://doi.org/10.1111/j.1365-2109.2007.01883.x
Kumar N, Krishnani KK, Singh NP (2018) Effect of dietary zinc-nanoparticles on growth performance, anti-oxidative and immunological status of fish reared under multiple stressors. Biol Trace Elem Res 186(1):267–278. https://doi.org/10.1007/s12011-018-1285-2
Thirunavukkarasu M, Periyakali SB, Subramanian R, Perumal S (2019) Influence of two different dietary zinc sources in freshwater prawn Macrobrachium rosenbergii post larvae. J Oceanol Limnol 37(1):290–299. https://doi.org/10.1007/s00343-018-7253-z
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical Approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed by the authors.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sherif, A.H., Abdelsalam, M., Ali, N.G. et al. Zinc Oxide Nanoparticles Boost the Immune Responses in Oreochromis niloticus and Improve Disease Resistance to Aeromonas hydrophila Infection. Biol Trace Elem Res 201, 927–936 (2023). https://doi.org/10.1007/s12011-022-03183-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-022-03183-w