Abstract
This study evaluated the effectiveness of dietary Ziziphus mauritiana leaf powder (ZLP) to control Aeromonas hydrophila infection in Nile tilapia and reduce damage to vital immune organs. Four experimental groups were fed a diet supplemented with ZLP at concentrations of 0, 5, 10, and 20 g/kg (w/w) for 6 weeks. At the end of the feeding trial, all groups were intraperitoneally injected with pathogenic A. hydrophila. It was found that Z. mauritiana significantly (P < 0.05) upregulated (lysozyme, interleukin 1 beta) and superoxide dismutase gene expressions as well as improved the activity of serum lysozyme and liver antioxidant enzymes. The fish that were fed a ZLP-supplemented diet also exhibited significantly higher survival rates after A. hydrophila challenge than those that were fed a ZLP-free diet (P < 0.05). Supplementation of 10 g/kg ZLP most effectively reduced the histopathological alterations caused by A. hydrophila challenge in the liver, spleen, kidney, and muscle of the fish. In conclusion, ZLP can be effective in controlling A. hydrophila infection in Nile tilapia (particularly at a concentration of 10 g/kg) through enhancement of its immune and antioxidant status.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aquaculture is an important sector that serves as a valuable and essential protein source for human consumption (Murray and Munro 2018). However, the intensification and extension of this industry have resulted in susceptibility to disease outbreaks (Dawood et al. 2018a). Antimicrobial substances were extensively used in aquaculture as prophylactic measures and metaphylactic treatments (Smith 2012). However, controlling the outbreak of diseases in aquaculture using antimicrobial substances has led to the emergence of drug-resistant pathogens. Recent microbiological and clinical evidence has revealed that antimicrobial resistance genes and bacteria can be transferred from livestock and aquatic animals to humans (Dawood et al. 2018b). Consequently, there is ongoing research on the effectiveness of alternatives such as antibiotics and other chemotherapeutics. The use of medicinal plants is one of the most promising means for the prevention and/or treatment of such diseases in aquaculture (Awad et al. 2015; Van Hai 2015). Medicinal plants are proven to be cost-effective, biodegradable, and safe options for the control of such diseases and widely used in the aquaculture industry (Abd El-Gawad et al. 2020; Sarhadi et al. 2020). Additionally, they provide longer protection periods than synthetic drugs, which have shorter recovery rates (Yostawonkul et al. 2019). It is well-documented that many types of medicinal plants have antioxidant properties that can delay or stop oxidative damage and thereby play an important role in disease prevention (Awad et al. 2019).
The leaves of Ziziphus mauritiana (common names: Ber, Chinese apple, and Indian jujube) have long been considered to have health-promoting effects (Abdallah et al. 2016; Abdulameer et al. 2017). Its leaves contain many bioactive compounds such as polyphenols, nitrogenous compounds, vitamins, inorganic elements, carbohydrates, lipids, proteins, free sugars, and mucilage (Dkhil et al. 2018). Previous studies have demonstrated the beneficial impacts of Ziziphus-supplemented diets for the treatment of digestive disorders, fever, diabetes, diarrhea, insomnia, and bronchitis in both humans and animals (AL-Marzooq 2014; Preeti and Shalini 2014). In the aquaculture sector, Z. mauritiana leave powder (ZLP) has effectively increased the growth of Nile tilapia fingerlings (Amin et al. 2019). However, limited information is available on the immune stimulatory effects of Z. mauritiana. To the best of the authors’ knowledge, only one relevant study has reported about the positive effects of Ziziphus on common carp (Cyprinus carpio) immunity (Hoseinifar et al. 2018). Generally, its immune-modulatory perspective remains unclear.
Nile tilapia remains one of the most commonly cultured fish species worldwide due to their easy breeding, tolerance to varied environments and diseases, fast growth, and high market demand (Dawood et al. 2019a, b). However, tilapia farms face challenges due to the infections of Streptococcus spp., Vibrio spp., Aeromonas hydrophila, and Flavobacterium spp. A. hydrophila, which threaten the health of fish by causing Motile Aeromonas Septicemia, a serious threat to cultured freshwater fishes including tilapia (Cipriano et al. 1984). A. hydrophila has developed into the most damaging impediment to the expansion of the tilapia industry worldwide.
Accordingly, the present study was conducted to assess the effectiveness of diet supplementation with different concentrations of Z. mauritiana leaf powder in reducing the deleterious effects of A. hydrophila on vital immune organs, as well as protective and immune-modulatory effects in Nile tilapia.
Materials and methods
Ethical statement
The experimental design was approved by the Central Laboratory for Aquaculture Research (CLAR), Abassa, Egypt (Approval no. 43429), and all procedures followed the guidelines for the care and use of fish.
Fish and experimental diet
A total of 260 healthy Nile tilapia (Oreochromis niloticus) with a mean weight of 9.63 ± 0.05 g were obtained from the Central Laboratory for Aquaculture Research (CLAR) hatchery, Abassa, Abu-Hammad, Sharkia, Egypt. The fish were acclimated to the lab conditions in 1000-L fiberglass tanks for 14 days. Next, 240 fish were randomly distributed in 12 glass aquaria (75 × 40 × 50 cm3) filled with dechlorinated water (120 L/aquarium) (4 groups, triplicates, 20 fish/aquarium). The remaining 20 fish were stocked in a separate aquarium and fed the control diet (negative control group) for the pathogenic challenge experiment.
The experimental diet (30% crude protein and 8.5% lipid) (Table 1) was prepared to add Z. mauritiana. The plant was collected from El Arish, North Sinai, Egypt, and identified by plant taxonomists of the Faculty of Environmental Agricultural Sciences, Suez University. Finely ground ZLP was added to the experimental diet at concentrations of 0 (control), 5, 10, and 20 g/kg diet (G1, G2, G3, and G4, respectively). The experimental diet was prepared following the methods adopted at the Fish Nutrition Laboratory, CLAR. The nutritional value of the experimental diet was analyzed following standard methods (AOAC 1990).
Fish were fed for 6 weeks at a rate of 5% of their body weight per day. Water temperature was adjusted to 28 °C ± 2 °C using heaters. Oxygen and total ammonia levels were measured daily and maintained at acceptable levels, i.e., 6.25 ± 0.53 and 0.02 mg/l, respectively (pH ~ 7.5). The fish were exposed to 12-h light/dark cycles using artificial lighting. Daily partial water exchange was performed to remove fecal matter and excess feed. Special care was taken during this exchange to avoid stressful consequences on the fish.
Pathogenic challenge
A. hydrophila (verified virulent strain kindly provided by the Department of Aquatic Animals Diseases and Management, Faculty of Veterinary Medicine, Benha University) was grown in tryptic soy broth at 28 °C for 18 h. Bacterial cells were collected by centrifugation at 2550g for 20 min at 4 °C and harvested in sterile physiological saline. Bacterial concentration was adjusted to 9 × 108 cells/ml following the method reported by El Asely et al. (2014). Ten fish from each replicate (30 fish/group) were transferred to adjusted aquaria and maintained at 26 °C with 6 mg/l dissolved oxygen. The day before infection, fish were intraperitoneally (IP) injected with 0.2 ml of the bacterial suspension. The negative control group was IP injected with 0.2 ml of 0.9% saline. Fish were observed daily for any clinical signs or mortalities, and the number of dead fish was recorded for 10 successive days. Daily relative percentage of survival was calculated using the Kaplan–Meier equation.
Sampling
Fish were sedated with 150 mg/l MS222 (Argent Laboratories, Redmond, Washington) following the procedure reported by Barreto et al. (2007), and whole-blood samples were collected from the caudal vessels (n = 5 fish/aquarium) using a syringe without anticoagulant for serum separation. Clotted blood was centrifuged (3000 rpm, 15 min, 4 °C) and stored at − 20 °C for measurement of serum lysozyme activity. After bleeding, liver and spleen tissues were immediately collected, stored in RNAlater (Bioshop, Germany), and preserved at − 80 °C until RNA extraction and gene expression analysis. From the liver, another 50 mg of samples was excised (n = 5 fish/aquarium) and kept in cold phosphate-buffered saline to measure alkaline phosphatase (ALP) and antioxidant enzyme activity and stored at − 20 °C until determination.
Immune gene expression
Liver and spleen tissues (50–100 mg) were used for RNA isolation using Trizol Reagent (Invitrogen, USA) following the manufacturer’s instructions. The integrity and quantity of total RNA were determined by passing samples through formamide gel and using a spectrophotometer, respectively. The primer sequences of lysozyme, superoxide dismutase (SOD), and interleukin 1β (IL-1β) genes were specifically designed using Primer3 (Table 2). cDNA synthesis was conducted using a first-strand cDNA synthesis kit (Roche Diagnostic GmbH, Germany) in a thermal cycler (Prime Pro48, Techne, UK) under the following parameters: reverse transcription (RT) at 45 °C for 45 min and denaturation at 94 °C for 10 min. Each round of quantitative RT polymerase chain reaction (qRT-PCR) was performed in duplicate. Two housekeeping genes, β-actin and elongation factor 1 alpha, obtained using the primer designed by Gröner et al. (2015). Relative mRNA expressions were calculated using the 2−ΔΔCt method to determine fold difference.
Serum lysozyme activity
Serum lysozyme activity was determined following the protocol reported by Milla et al. (2010) using Micrococcus lysodeikticus (Sigma Chemical Co, USA). Absorbance was determined using a spectrophotometer at a wavelength of 450 nm after 0.5 and 4.5 min.
ALP and antioxidant enzyme activity
Liver tissue homogenates were centrifuged at 4000 rpm for 15 min at 4 °C. Supernatants were used for the determination of ALP activity using commercial kits (Abcam, UK) at a wavelength of 405 nm. Total SOD and reduced glutathione (GSH) levels were determined using commercially available kits (Biodiagnostic Lab, Egypt) at wavelengths of 560 and 405 nm, respectively, according to the manufacturer’s instructions.
Histopathological analysis
Liver, kidney, spleen, and muscle samples from all sacrificed fish in all groups (challenged and unchallenged fish) were excised and fixed in 10% neutral buffered formalin. All histopathological procedures were performed following the methods described by Bancroft et al. (1996). Hematoxylin–eosin-stained sections were examined under a light microscope for the evaluation of pathological changes. Severity of microscopic lesions was classified according to the classification reported by Bernet et al. (1999): degree 1, mild pathological changes such as circulatory disturbance, congestion, hemorrhage, and edema )with mild degenerative changes); degree 2, moderate pathological alterations including degenerative changes, in association with cell deposits with mild inflammatory cell infiltration; and degree 3, severe alterations such as necrotic areas with leukocyte aggregation.
Statistical analysis
All data were subjected to one-way ANOVA. Difference in means was tested at a 5% probability level using Tukey’s post hoc test. All statistical analyses were conducted using SPSS V.16 (SPSS, Richmond, USA).
Results
Experimental infection
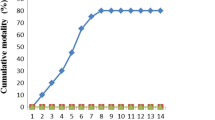
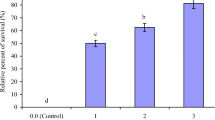
No mortalities were recorded in the negative control group or unchallenged ZLP-fed groups (data not shown). After IP injection of A. hydrophila, fish mortality began at post-infection 2 days in G1 and at post-infection 3 days in G2–G4 (Fig. 1). The cumulative survival rate at post-infection 10 days was the lowest in G1 (24.14%), whereas it was the highest (80%) in G3.
Immune gene and SOD expressions
Compared with G1, G3 showed significantly higher upregulation (P < 0.05) of lysozyme gene expression in the liver, followed by G4 (Fig. 2a). Similarly, Z. mauritiana supplementation significantly (P < 0.05) increased IL-1β expression in fish, with the highest values observed in G3 (Fig. 2b). In the same respect, SOD expression was significantly upregulated (P < 0.05) in G3, followed by in G2 and G4 compared with in G1 (Fig. 2c).
Immune-related genes alysozyme, bIL-1β, and c antioxidant related gene SOD expression of Nile tilapia fed with control 0 g (G1), 5 g (G2), 10 g (G3), and 20 g (G4) of ZLP per kg diet for 6 weeks and challenged with A. hydrophila. Data are expressed as mean ± SE, bars with different letters indicate significance at P < 0.05
Serum lysozyme activity
Feeding of Nile tilapia fingerlings with ZLP-supplemented diet significantly (P < 0.05) increased serum lysozyme activity in all groups, with higher activity in G3 than in G1 (Fig. 3).
ALP and antioxidant activity
No significant effects of dietary ZLP supplementation (P > 0.05) were observed on ALP activity in the liver in G2–G4 compared with in G1 (Fig. 4).
As shown in Fig. 5a, GSH level significantly increased (P < 0.05) in G3 compared with that in G1. There was a significant increase (P < 0.05) in SOD activity in G2–G4 compared with that in G1 (Fig. 5b).
Histopathological findings
Histopathological examination of the liver, kidney, spleen, and muscle indicated normal morphological characteristics in all groups (data not shown). The main characteristic lesions in the liver samples obtained from G1 revealed dilatation with congestion of hepatic sinusoids and hepatic portal vessels. Perivascular infiltration of mononuclear leukocytes, mainly lymphocytes and macrophages, and diffuse hemorrhage admixed with mononuclear inflammatory cells were observed in the hepatic parenchyma. Further, scattered degenerative changes were seen in hepatocytes as extensive hydropic degeneration as well as hepatic steatosis characterized by clear cytoplasmic vacuolation of hepatocytes with pushing of their nucleus to the periphery (Fig. 6a). Additionally, focal areas of coagulative necrotic hepatic cells with pyknotic nuclei associated with the loss of structural integrity were seen. Distributed areas of lytic necrosis characterized by replacement of hepatocytes by homogenous eosinophilic substances infiltrated by inflammatory cells (Fig. 6b) and eosinophilic necrotic debris admixed with mononuclear inflammatory cells were also observed (Fig. 6c). Moreover, extensive necrosis of pancreatic acinar cells with peri-pancreatic hemorrhage that replaced the hepatic parenchyma was detected (Fig. 6d). Vacuolation of most pancreatic acinar cells with peri-pancreatic infiltration of mononuclear leukocytes was also observed. Generally, the mean lesion score was degree 3 in the challenged fish.
H&E stained sections of liver (a–d) of Nile tilapia infected with A. hydrophila, e–g fish infected with A. hydrophila and treated with different concentrations of ZLP, G2 (e, f), G3 (g), and G4 (h). a Clear vacuolation of most hepatocytes with squeezing of the nucleus to the periphery (arrow, × 200), b scattered areas of lytic necrosis characterized by disappearance of hepatocytes and replaced by homogenous eosinophilic substances infiltrated by inflammatory cells (arrow, × 200), c diffuse areas of lytic necrosis characterized by disappearance of hepatocytes and replaced by eosinophilic necrotic debris admixed with mononuclear inflammatory cells (arrow) with necrosis of pancreatic acinar cells (asterisk, × 200), d extensive necrosis of pancreatic acinar cell (asterisk) with peri-pancreatic hemorrhage that replace to the hepatic parenchyma (arrow, × 200), e vacuolation and necrosis of pancreatic acinar cells with deposition of hemosiderin pigment in some pancreatic acini (arrow), notice also degenerative changes in hepatocytes (zigzag arrow, × 200), f focal area of coagulative necrosis in hepatic parenchyma (arrow, × 200), g mild degenerative changes in hepatocytes (× 200), h degenerative changes in the wall of hepatic blood vessels with few perivascular mononuclear leukocytic cellular infiltrations (arrow, × 200)
Severity of the mentioned histopathological changes that were recorded for liver samples in G1 was alleviated to varying degrees in ZLP-fed groups. Mild improvement in altered liver tissues was demonstrated in G2, with lesion score varying between degree 3 in two fish and degree 2 in the remaining fish. The main pathological changes detected in this group were marked dilatation with congestion of hepatic sinusoids and hepatic portal vessels, degenerative changes such as mild cytoplasmic vacuolation of hepatocytes, vacuolation and necrosis of pancreatic acinar cells with deposition of hemosiderin pigment in some pancreatic acinar cells (Fig. 6e), as well as small focal areas of coagulative necrosis in the hepatic parenchyma characterized by hypereosinophilic cytoplasm with pyknotic nucleus (Fig. 6f).
In contrast, marked improvement in the hepatocellular architecture with more regular and less altered hepatocytes was noticed in liver tissues obtained from G3 compared with in G1. The mean lesion score of this group was degree 1, with the most pronounced pathological alterations being only mild congestion of hepatic sinusoids and hepatic portal vessels with mild degenerative changes in hepatocellular architecture in some examined fish (Fig. 6g).
The lesion score of G4 was degree 2 based on congestion of hepatic sinusoids and hepatic portal vessels, degenerative changes in the wall of hepatic blood vessels with mild perivascular infiltration of mononuclear leukocytes including lymphocytes and macrophages, as well as mononuclear leukocyte infiltration (Fig. 6h) with degeneration of pancreatic acinar cells.
Microscopic examination of renal samples obtained from G1 fish challenged with A. hydrophila showed extensive congestion of renal blood vessels and perivascular edema admixed with erythrocytes and few leukocytes (Fig. 7a); this was associated with clear interstitial hemorrhage with degeneration of renal tubules (Fig. 7b). Additionally, hemosiderin deposits were observed in the lumen of degenerated renal tubules. Further, extensive cytoplasmic vacuolation and necrotic pyknosis of the nuclei of the renal tubular epithelial cells as well as shrinkage and hyalinization of the glomerular tuft were seen (Fig. 7c). Additionally, eosinophilic hyaline casts were detected in the lumen of some renal tubules. Multifocally, small areas of lytic necrosis that were characterized by replacement of some renal tubules by mononuclear leukocytes were also noticed (Fig. 7d). Collectively, a lesion score of degree 3 was observed in most challenged G1 fish.
Light micrograph of kidney (a–d) of Nile tilapia infected with A. hydrophila, e–g fish infected with A. hydrophila and treated with different concentratins of ZLP, G2 (e), G3 (f), and G4 (g), showing a, marked congestion of renal blood vessels and perivascular edema admixed with erythrocytes and few leukocytes (arrow, × 200). b Clear interstitial hemorrhage (H) with disconnection of renal tubules (×200). c Severe degenerative (arrow) and necrotic (zigzag arrow) changes of the lining epithelium of renal tubules with necrosis (N) and hyalinization (H) of the glomerular tuft (× 200). d Small areas of lytic necrosis of the renal tissues with infiltration of mononuclear cells (arrow, × 200). e Vacuolar degeneration of the renal tubular epithelium with necrosis and shrinkage of the glomerular tuft (× 200). f Mild vacuolar degeneration in lining epithelium of renal (× 200). g Vacuolation (arrow) of the lining epithelium of renal tubules with eosinophilic hyaline casts in their lumen (zigzag arrow, × 200) (H&E stain)
The severity of the pathological alterations induced by A. hydrophila reduced to a certain extent in ZLP-fed fish. However, most lesions detected in G2 were of degrees 2 and 3 based on congestion of renal blood vessels, mild perivascular hemorrhage, vacuolar degeneration, necrosis of renal tubular epithelial cells, as well as necrosis and shrinkage of the glomerular tuft (Fig. 7e) as the most common pathological changes observed during microscopic examination.
Overall, renal samples obtained from G3 displayed distinct histological improvement compared with those obtained from G1. Mild congestion of renal blood vessels and mild vacuolar degeneration of renal tubular epithelial cells (degree 1) were observed (Fig. 7f). Vacuolation of renal tubular epithelial cells with eosinophilic hyaline casts in their lumen (degree 2) was recorded in G4 (Fig. 7g).
Spleen samples of A. hydrophila-challenged fish showed severe pathological changes of degree 3 based on marked congestion of splenic blood vessels with diffuse splenic hemorrhage (Fig. 8a) and reduced lymphocyte population, resulting in severe lymphoid depletion with eosinophilic mesh free of lymphocytes (Fig. 8b). Moreover, the accumulation of hemosiderin with reduced number of melanomacrophage centers (MMCs) was demonstrated (Fig. 8c), where the density of pigmented granules and cell density in MMCs reduced. Generally, mild improvement in the splenic architecture was demonstrated (degree 2) because congestion of splenic blood vessels, mild hemorrhage in the red pulp (Fig. 8d) with mild lymphoid depletion, and mild reduction in the number of MMCs in some examined fish were observed. Interestingly, most detected lesions in the splenic tissues of G3 were of degree 0 or 1 because considerable increase was observed in the lymphocyte population with well-developed MMCs but, to a certain extent, mild reduction was observed in some examined fish (Fig. 8e). Meanwhile, there was an increase in the lymphocyte population and mild hemorrhage in the red pulp with mild activation of MMCs in challenged G4 fish (Fig. 8f).
H&E-stained sections of spleen (a–c) of Nile tilapia infected with A. hydrophila. d–f Fish infected with A. hydrophila and treated with different concentrations of ZLP, G2 (d), G3 (e), and G4 (f), showing a, diffuse splenic hemorrhage (× 200). b Severe lymphoid depletion (arrow, × 200). c Accumulation of hemosiderin with inhibition of melanomacrophage center (MMC, arrow, × 200). d Mild hemorrhage in the red pulp (arrow, × 200). e Extensive improvement in the lymphocytic population with well-developed melanomacrophage center (arrow, × 200). f Hemorrhage in red pulp with mild activation in melanomacrophage center (× 200)
Extensive intermuscular edema admixed with mononuclear leukocyte infiltration, mainly of lymphocytes and macrophages, was the most common pathological alterations in the muscular tissues obtained from G1 (Fig. 9a). Hyalinization of muscle fiber was observed based on muscle sarcoplasm appearing more eosinophilic with pyknotic nuclei (Fig. 9a). Additionally, extensive myomalacia with complete vacuolation of muscle sarcoplasm was detected (Fig. 9b).
H&E-stained sections (a, b) of muscular tissue of Nile tilapia infected with A. hydrophila. c–e Fish infected with A. hydrophila and treated with different concentrations of ZLP G2 (c), G3 (d), and G4 (e), showing a inter-muscular edema admixed with mononuclear leukocytic cellular infiltrations (arrow) with hyalinization of muscle fiber (asterisk, × 200). b Extensive myomalaciain which entire vacuolation of sarcoplasm (asterisk, × 200). c Mild intermuscular hemorrhage (arrow, × 200). d Muscle fiber appeared nearly similar to negative control group (× 200). e Small focal areas of intermuscular edema admixed with leukocytic cellular infiltration (× 200)
The most common pathological alterations in muscle samples obtained from G2 were mild intermuscular hemorrhage and degenerative changes in muscle sarcoplasm (Fig. 9c). Its muscle fiber appeared similar to that in the negative control group, except mild degenerative changes in muscle sarcoplasm (Fig. 9d). However, small focal areas of intermuscular edema admixed with leukocyte infiltration, mainly of lymphocytes and macrophages, were observed in muscle samples obtained from G4 (Fig. 9e).
Discussion
The use of herbal medicine for improving the immune status of fish takes a great interest from researchers to prove its effectiveness in different fish species (Awad and Awaad 2017). However, limited information is available about the role of herbs in alleviating the damaging effect of pathogenic bacterial infection at the gene and cellular levels.
Although there is adequate research on the use of natural products for controlling Motile Aeromonas Septicemia in Nile tilapia (Ardo’ et al. 2008; El Asely et al. 2012; El Asely et al. 2014; Negm et al. 2016), the mortality rate associated with disease outbreaks remain a major problem in tilapia farming (Fernandes et al. 2019).
The present study demonstrated that G2–G4 exhibited tolerance to A. hydrophila challenge, which resulted in significantly reduced mortality compared with G1. Jiang et al. (2016) reported that A. hydrophila pathogenesis is highly correlated with oxidative stress. Based on this concept, the protective effect of Ziziphus may be associated with its richness in flavonoids and polyphenol, in addition to the high concentrations of ascorbic acid that neutralizes excessive free radicals and contributes to enhanced immune system (Abdallah et al. 2016; Elaloui et al. 2016). It is worth noting that Z. mauritiana was effective in the treatment of various human and animal diseases (Abdulameer et al. 2017).
In the present study, ZLP supplementation of Nile tilapia juvenile diet significantly upregulated lysozyme gene expression in G3, followed by in G4. Similarly, dietary ginger significantly increased lysozyme gene expression in zebrafish (Ahmadifar et al. 2019), and methanolic extract of marine macroalga significantly increased lysozyme gene expression in the spleen of Nile tilapia at post-infection 24 h (Yengkhom et al. 2019).
A significant increase was observed in IL-1β expression in ZLP-fed groups. Contrary to the present results, Z. spina-christi L. leaf extract significantly decreased IL-1β expression in the renal tissues of mice with induced sepsis (Dkhil et al. 2018). This may be due to the difference in plant species and animal subjects used in the present study. In the present study, ZLP supplementation significantly upregulated SOD expression. Similar results were reported by Hassaan et al. (2019) when they studied the effects of Silybum marianum seeds as feed additives on tilapia. In another study, 20 mg/kg dietary anthocyanin significantly increased SOD expression (Yilmaz 2019).
In the present study, serum lysozyme activity significantly increased in G2–G4 compared with in G1. Lie et al. (1989) linked the increase in lysozyme activity in fish spleen and kidney to the abundance of immune cells such as monocytes, macrophages, and polymorphonuclear granulocytes, which are the main sources of such proteolytic enzymes. Engstad et al. (1992) reported that an increase in lysozyme population in the blood of stimulated fish was associated with proliferating phagocytes or increased lysozyme population produced from lysosomes, rendering lysozyme activity to be one of the best markers for evaluating the bactericidal effect of feed additives. The increase in lysozyme activity observed in the present study could be due to flavonoids, which stimulate leucocytes and phagocytosis (Awad et al. 2015). Similar results were recorded in tilapia that were fed Withania sominefera (Zahran et al. 2018). The elevation in ALP level serves an alarm for the occurrence of cell damage (Kumar and Sharma 2012). In the present study, no changes were observed in ALP level between the treatment and control groups, confirming the safety of Z. mauritiana treatments.
SOD is an enzyme that acts as the first line of defense against reactive oxygen species (ROS) damage by converting ROS to less harmful H2O2 (Wang et al. 2018). In this context, the application of medicinal herbs can potentially increase endogenous antioxidants in addition to the natural antioxidant activity (Rajasekaran et al. 2005). Significant upregulation of SOD levels in liver homogenates was recorded in ZLP-fed groups. Similarly, higher SOD levels were reported in genetically improved farmed tilapia that were fed Aloe vera for 8 weeks (Gabriel et al. 2015) and in the serum of O. mossambicus that were fed Psidium guajavaleaf extract (Gobi et al. 2016).
GSH is one of the antioxidants that exert cellular defense against oxidative stress by scavenging H2O2, free radicals, and other peroxides (Srikanth et al. 2013). A significant increase was observed in GSH level in G3 compared with in the other groups.
Histopathological examination is widely performed to study the pathological alterations caused by chemicals or biological infectious agents (Camargo and Martinez 2007). In addition, the inclusion of immune cells within the head kidney, spleen, and liver is involved in fish immunity (Zapata et al. 2006). In the present study, the addition of Z. mauritania to the diet of Nile tilapia reduced the pathological changes associated with A. hydrophila infection in all examined organs, with an alleviating effect observed in G3. In the same context, Ostaszewska et al. (2008) reported structural improvement in hepatocytes in silver bream (Vimba vimba) fed natural feed additives. In line with the present findings, Owatari et al. (2018) proved that sylimarin significantly reduced the pathological alterations of the liver and spleen of Nile tilapia challenged with Streptococcus agalactiae.
The damaging effects of bacterial infection on host tissues are, in part, associated with induction of oxidative stress (Tkachenko et al. 2014). The protective mode of action of most flavonoid-containing plants originates from the ability of flavonoids to quench ROS and consequently relieve harmful effects (Brunetti et al. 2013).
MMCs are pigmented macrophage aggregations in the kidney and spleen that are associated with immunity through phagocytosis of foreign materials (Steinel and Bolnick 2017). Similar to the present findings, an increase in the number of MMCs in Nile tilapia that were fed an Echinacea purpurea-supplemented diet (El Asely et al. 2012) and in farmed sea bass that were fed polyphenol-enriched diet has been reported (Magrone et al. 2016).
The immune response of fish muscles to different stimuli, including infections, was studied by Chatterjee et al. (2016) and Valenzuela et al. (2017). Remarkably, the muscles of ZLP-fed groups in the present study exhibited mild degenerative changes in the muscle sarcoplasm.
Conclusion
ZLP supplementation of tilapia feed, particularly at a concentration of 10 g/kg, reduced mortalities associated with A. hydrophila infection. The damaging effects of oxidative stress were reduced through the elevation of antioxidant enzyme activity associated with the infection, reducing histopathological alterations and improving fish immune status. Thus, ZLP supplementation of tilapia diet is recommended as an effective treatment for the avoidance of harmful impacts of A. hydrophila infection.
References
Abd El-Gawad EA, El Asely AM, Soror EI, Abbass AA, Austin B (2020) Effect of dietary Moringa oleifera leaf on the immune response and control of Aeromonas hydrophila infection in Nile tilapia (Oreochromis niloticus) fry. Aquac Int 28(1):389–402. https://doi.org/10.1007/s10499-019-00469-0
Abdallah EM, Elsharkawy ER, Ed-dra A (2016) Biological activities of methanolic leaf extract of Ziziphus mauritiana. Biosci Biotech Res Comm 9(4):605–614
Abdulameer YS, Husain F, Al-cekal SHA (2017) Efficacy of Ziziphus mauritiana leaves extract as antibiotic alternatives in broiler chicken. J Entomol Zool Studies 5(5):742–746
Ahmadifar E, Sheikhzadeh N, Roshanaei K, Dargahid N, Faggio C (2019) Can dietary ginger (Zingiber officinale) alter biochemical and immunological parameters and gene expression related to growth, immunity and antioxidant system in zebrafish (Danio rerio)? Aquaculture 507:341–348. https://doi.org/10.1016/j.aquaculture.2019.04.049
AL-Marzooq MA (2014) Phenolic compounds of Napek leave (Ziziphus spina-christi L.) as natural antioxidants. J Food Nutr Sci 2(5):207–214
Amin A, El Asely A, Abd El-Naby AS, Samir F, El-Ashram A, Sudhakaran R, Dawood MAO (2019) Growth performance, intestinal histomorphology and growth-related gene expression in response to dietary Ziziphus mauritiana in Nile tilapia (Oreochromis niloticus). Aquaculture 512:734301. https://doi.org/10.1016/j.aquaculture.2019.734301
AOAC (1990) The Official Methods of Analyses Association of Official Analytical Chemists International (15th edition), Association of Official Analytical Chemists, Arlington, VA, 2220, USA
Ardo’ L, Yin G, Xu P, Var’adi L, Szigeti G, Jeney Z, Jeney G (2008) Chinese herbs (Astragalus membranaceus and Lonicera japonica) and boron enhance the non-specific immune response of Nile tilapia (Oreochromis niloticus) and resistance against Aeromonas hydrophila. Aquaculture 275:26–33. https://doi.org/10.1016/j.aquaculture.2007.12.022
Awad E, Awaad AS (2017) Role of medicinal plants on growth performance and immune status in fish. Fish Shellfish Immunol 67:40–54. https://doi.org/10.1016/j.fsi.2017
Awad E, Awaad AS, Esteban MA (2015) Effects of dihydroquercetin obtained from deodar (Cedrus deodara) on immune status of gilthead seabream (Sparus aurata L.). Fish Shellfish Immunol 43:43–50. https://doi.org/10.1016/j.fsi.2014.12.009
Awad E, Austin D, Lyndon A, Awaad A (2019) Possible effect of hala extract (Pandanus tectorius) on immune status, anti-tumour and resistance to Yersinia ruckeri infection in rainbow trout (Oncorhynchus mykiss). Fish Shellfish Immun 87:620–626. https://doi.org/10.1016/j.fsi.2019.02.012
Bancroft J, Stevens A, Turner D (1996) Theory and practice of histological techniques, 4th edn. Churchill Living Stone, New York
Barreto RE, Gontijo AMMC, Alves-de-Lima RO, Raymundi VC, Pinhal D, Reyes et al (2007) MS222 does not induce primary DNA damage in fish. Aquac Int 15:163–168. https://doi.org/10.1007/s10499-007-9073-6
Bernet D, Schmidt H, Meier W, Burkhardt-Holm P, Wahli T (1999) Histopathology in fish: proposal for a protocol to assess aquatic pollution. J Fish Dis 1999:25–34
Brunetti C, Di Ferdinando M, Fini A, Pollastri S, Tattini M (2013) Flavonoids as antioxidants and developmental regulators: relative significance in plants and humans. Int J Mol Sci 14:3540–3555. https://doi.org/10.3390/ijms14023540
Camargo MMP, Martinez CBR (2007) Histopathology of gills, kidney and liver of a Neotropical fish caged in an urban stream. Neotropic Ichthyol 5:327–336. https://doi.org/10.1590/S1679-62252007000300013
Chatterjee A, Roy D, Patnaik E, Nongthomba U (2016) Muscles provide protection during microbial infection by activating innate immune response pathways in Drosophila and zebrafish. Dis Model Mech 9:697–705. https://doi.org/10.1242/dmm.022665
Cipriano RC, Bullock GL, Pyle SW (1984) Aeromonas hydrophila and motile Aeromonad septicemias of fish. United States Fish and Wildlife Service, Washington, DC
Dawood MA, Koshio S, Esteban MÁ (2018a) Beneficial roles of feed additives as immunostimulants in aquaculture: a review. Rev Aquac 10(4):950–974. https://doi.org/10.1111/raq.12209
Dawood MA, Koshio S, Abdel-Daim MM, Van Doan H (2018b) Probiotic application for sustainable aquaculture. Rev Aquac 11(3):907–924. https://doi.org/10.1111/raq.12272
Dawood MA, Magouz FI, Salem MF, Abdel-Daim HA (2019a) Modulation of digestive enzyme activity, blood health, oxidative responses and growth-related gene expression in GIFT by heat-killed Lactobacillus plantarum (L-137). Aquaculture 505:127–136. https://doi.org/10.1016/j.aquaculture.2019.02.053
Dawood MA, Eweedah NM, Moustafa Moustafa E, Shahin MG (2019b) Effects of feeding regimen of dietary Aspergillus oryzae on the growth performance, intestinal morphometry and blood profile of Nile tilapia (Oreochromis niloticus). Aquac Nutr 25:1063–1072. https://doi.org/10.1111/anu.12923
Dkhil MA, Kassab RB, Al-Quraishy S, Abdel-Daim MM, Zrieq R, Abdel Moneim AE (2018) Ziziphus spina-christi (L.) leaf extract alleviates myocardial and renal dysfunction associated with sepsis in mice. Biomed Pharmacother 102:64–75. https://doi.org/10.1016/j.biopha.2018.03.032
El Asely AM, Amin RA, El-Habashi NM (2012) Effect of dietary administration of Echinacea purpurea on immune responses, histopathological alteration and microbial safety in Nile tilapia (Oreochromis niloticus) infected with Aeromonas hydrophila. Proceedings of the 5th Global Fisheries and Aquaculture Research Conference. Giza: Faculty of Agriculture, Cairo University, 2012:100-114
El Asely AM, Abbass AA, Austin B (2014) Honey bee pollen improves growth, immunity and protection of Nile tilapia (Oreochromis niloticus) against infection with Aeromonas hydrophila. Fish Shellfish Immun 40(2):500–506. https://doi.org/10.1016/j.fsi.2014.07.017
Elaloui M, Laamouri A, Ennajah A, Cerny M, Mathieu C, Vilarem G, Chaar H, Hasnaoui B (2016) Phytoconstituents of leaf extracts of Ziziphus jujuba Mill. plants harvested in Tunisia. Ind Crop Prod 83:133–139. https://doi.org/10.1016/j.indcrop.2015.11.029
Engstad RE, Robertsen B, Frivold E (1992) Yeast glucan induces increase in lysozyme and complement-mediated haemolytic activity in Atlantic salmon blood. Fish Shellfish Immunol 2:287–297. https://doi.org/10.1016/S1050-4648(06)80033-1
Fernandes DC, Eto SF, Funnicelli MIG, Fernandes CC, Charlie-Silva I, Belo MAA, Pizauro JM (2019) Immunoglobulin Y in the diagnosis of Aeromonas hydrophila infection in Nile tilapia (Oreochromis niloticus). Aquaculture 500:576–585. https://doi.org/10.1016/j.aquaculture.2018.10.045
Gabriel NN, Qiang J, Ma XY, He J, Xu P, Liu K (2015) Dietary Aloe vera improves plasma lipid profile, antioxidant, and hepatoprotective enzyme activities in GIFT-tilapia (Oreochromis niloticus) after Streptococcus iniae challenge. Fish Physiol Biochem 41:1321–1332. https://doi.org/10.1007/s10695-015-0088-z
Gobi N, Ramya C, Vaseeharan, Malaikozhundan B, Vijayakumar SK, Murugan G, Benelli (2016) Oreochromis mossambicus diet supplementation with Psidium guajava leaf extracts enhance growth, immune, antioxidant response and resistance to Aeromonas hydrophila. Fish Shellfish Immunol 58:572–583. https://doi.org/10.1016/j.fsi.2016.09.062
Gröner F, Ziková A, Kloas W (2015) Effects of the pharmaceuticals diclofenac and metoprolol on gene expression levels of enzymes of biotransformation, excretion pathways and estrogenicity in primary hepatocytes of Nile tilapia (Oreochromis niloticus). Comp Biochem Physiol C 167:51–57. https://doi.org/10.1016/j.cbpc.2014.09.003
Hassaan MS, Mohammady EY, Soaudy MR, El-Garhy HAS, Moustafa MMA, Mohamed SA, El-Haroun ER (2019) Effect of Silybum marianum seeds as a feed additive on growth performance, serum biochemical indices, antioxidant status, and gene expression of Nile tilapia, Oreochromis niloticus (L.) fingerlings. Aquaculture 509:178–187
Hoseinifar SH, Khodadadian Zou H, Paknejad H, Ahmadifar E, Van Doan H (2018) Non-specific immune responses and intestinal immunity of common carp (Cyprinus carpio) fed Jujube (Ziziphus jujube) fruit extract. Aquac Res 49(9):2995–3003. https://doi.org/10.1111/are.13759
Jiang WD, Hu K, Liu Y, Jiang J, Wu P, Zhao J, Zhang YA, Zhou XQ, Feng L (2016) Dietary myo-inositol modulates immunity through antioxidant activity and the Nrf2 and E2F4/cyclin signalling factors in the head kidney and spleen following infection of juvenile fish with Aeromonas hydrophila. Fish Shellfish Immun 49:374–386
Kumar A, Sharma B, Pandey (2012) Assessment of stress in effect to pyrethroid insecticides, λcyhalothrin and cypermethrin, in a freshwater fish, channa punctatus (bloch). Cell Mol Biol 58(1):153–159
Lie Ø, Evensen Ø, SØrensen A, FrØysadal E (1989) Study on lysozyme activity in some fish species. Dis Aquat Org 6:1–5
Magrone T, Fontana S, Laforgia F, Dragone T, Jirillo E, Passantino L (2016) Administration of a polyphenol-enriched feed to farmed sea bass (Dicentrarchus labrax L.) modulates intestinal and spleen immune responses. Oxidative Med Cell Longev 2016:28–31. https://doi.org/10.1155/2016/2827567
Milla S, Mathieu C, Wang N, Lambert S, Nadzialek S, Massart S, Henrotte E, Douxfils J, Mélard C, Mandiki SN, Kestemont P (2010) Spleen immune status is affected after acute handling stress but not regulated by cortisol in Eurasian perch, Perca fluviatilis. Fish Shellfish Immunol 28(5–6):931–941. https://doi.org/10.1016/j.fsi.2010.02.012
Murray AG, Munro LA (2018) The growth of Scottish salmon (Salmo salar) aquaculture 1979–2016 fits a simple two-phase logistic population model. Aquaculture 496:146–152. https://doi.org/10.1016/j.aquaculture.2018.07.023
Negm IM, EL Asely AM, Abbass AA (2016) Influence of dietary ginger (Zingiber officinale) on haemato-biochemical parameters, spleen histopathological changes and resistance of Oreochromis niloticus fingerlings to Aeromonas hydrophila infection. Egyptian J Aquacult 6(1):25–45
Ostaszewska T, Dabrowski K, Hliwa P, Gomółka P, Kwasek K (2008) Nutritional regulation of intestine morphology in larval cyprinid fish, silver bream (Vimba vimba). Aquac Res 39:1268–1278. https://doi.org/10.1111/j.1365-2109.2008.01989.x
Owatari MS, Alves Jesus GF, Brum A, Pereira SA, Lehmann NB, de Pádua PU et al (2018) Sylimarin as hepatic protector and immunomodulator in Nile tilapia during Streptococcus agalactiae infection. Fish Shellfish Immunol 8:565–572. https://doi.org/10.1016/j.fsi.2018.08.061
Preeti T, Shalini T (2014) Ziziphus jujuba: a phytopharmacological review. Int J Res Dev Pharm Life Sci 3:959–966
Rajasekaran S, Ravi K, Sivagnanam K, Subramanian S (2005) Antioxidant effect of Aloevera gel extract in streptozotocin-induced diabetes in rats. Pharmarcoll Report 57:90–96
Sarhadi I, Alizadeh E, Ahmadifar E, Adineh H, Dawood MA (2020) Skin mucosal, serum immunity and antioxidant capacity of common carp (Cyprinus carpio) fed artemisia (Artemisia annua). Ann Animal Sci
Smith P (2012) 7-Antibiotics in aquaculture: reducing their use and maintaining their efficacy. In: Austin B (ed) Infectious disease in aquaculture. Woodhead Publishing, pp 161–189. https://doi.org/10.1533/9780857095732.2.161
Srikanth K, Pereira E, Duarte AC, Ahmad I (2013) Glutathione and its dependent enzymes’ modulatory responses to toxic metals and metalloids in fish-a review. Environ Sci Pollut Res 20:2133–2149. https://doi.org/10.1007/s11356-012-1459-y
Steinel NC, Bolnick DI (2017) Melanomacrophage centers as a histological indicator of immune function in fish and other Poikilotherms. Front Immunol 8:827. https://doi.org/10.3389/fimmu.2017.00827
Tkachenko H, Kurhaluk N, Andriichuk A, Gasiuk E, Beschasniu S (2014) Oxidative stress biomarkers in liver of sea trout (Salmo trutta m. trutta L.) affected by ulcerative dermal necrosis syndrome. Turk J Fish Aquat Sci 14:391–402. https://doi.org/10.4194/1303-2712-v14_2_09
Valenzuela CA, Zuloaga R, Poblete-Morales M, Vera-Tobar T, Mercado L, Avendano-Herrera R, Valdes JA, Molina A (2017) Fish skeletal muscle tissue is an important focus of immune reactions during pathogen infection. Dev Comp Immunol 73:1–9. https://doi.org/10.1016/j.dci.2017.03.004
Van Hai N (2015) The use of medicinal plants as immunostimulants in aquaculture. Aquaculture 446:88–96. https://doi.org/10.1016/j.aquaculture.2015.03.014
Wang Y, Branicky R, Noë A, Hekimi S (2018) Superoxide dismutases: dual roles in controlling ROS damage and regulating ROS signaling. J Cell Biol 217(6):1915–1928. https://doi.org/10.1083/jcb.201708007
Yengkhom O, Shalini K, Subramani S, Subramani PA, Michael RD (2019) Stimulation of non-specific immunity, gene expression, and disease resistance in Nile Tilapia, Oreochromis niloticus (Linnaeus, 1758), by the methanolic extract of the marine macroalga, Caulerpa scalpelliformis. Vet World 12(2):271–276
Yilmaz E (2019) Effects of dietary anthocyanin on innate immune parameters, gene expression responses, and ammonia resistance of Nile tilapia (Oreochromis niloticus). Fish Shellfish Immune 93:694–702. https://doi.org/10.1016/j.fsi.2019.08.033
Yostawonkul J, Kitiyodom S, Kaewmalun S, Suktham K, Nittayasut N, Khongkow M, Namdee K, Ruktanonchai UR, Rodkhum C, Pirarat N, Surassmo S, Yata T (2019) Bifunctional clove oil nanoparticles for anesthesia and anti-bacterial activity in Nile tilapia (Oreochromis niloticus). Aquaculture 503:589–595. https://doi.org/10.1016/j.aquaculture.2018.12.058
Zahran E, Abd El-Gawad EA, Engy R (2018) Dietary Withania sominefera root confers protective and immunotherapeutic effects against Aeromonas hydrophila infection in Nile tilapia (Oreochromis niloticus). Fish Shellfish Immun 80:641–650. https://doi.org/10.1016/j.fsi.2018.06.009
Zapata A, Diez B, Cejalvo T, Gutierrez-de Frias C, Cortes A (2006) Ontogeny of the immune system of fish. Fish Shellfish Immunol 20:126–136. https://doi.org/10.1016/j.fsi.2004.09.005
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical statement
The experimental design was approved by the Central Laboratory for Aquaculture Research (CLAR), Abassa, Egypt (Approval no. 43429), and all procedures followed the guidelines for the care and use of fish.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
El Asely, A., Amin, A., Abd El-Naby, A.S. et al. Ziziphus mauritiana supplementation of Nile tilapia (Oreochromis niloticus) diet for improvement of immune response to Aeromonas hydrophila infection. Fish Physiol Biochem 46, 1561–1575 (2020). https://doi.org/10.1007/s10695-020-00812-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-020-00812-w