Abstract
Growing evidence indicates that metal exposure is associated with metabolic syndrome (MetS); however, mixed results have been reported. The aim of this study was to clarify associations of exposure to essential and non-essential metals with body composition and risks of obesity and MetS. Anthropometry and blood biochemistry of metabolic parameters were obtained from 150 middle-aged Taiwanese adults. Plasma metals were assessed using inductively coupled plasma mass spectrometry, and body compositions were measured by a bioelectrical impedance analysis (BIA). The essential metals of copper (Cu), manganese (Mn), and chromium (Cr) were positively correlated with the body fat mass but inversely correlated with the skeletal muscle mass (all p < 0.05). An adjusted logistic regression showed that Mn [odds ratio (OR) = 1.624 (95% confidence interval 1.072, 2.462), p = 0.02] and, to a lesser extent, Cu [OR = 1.501 (0.985, 2.292), p = 0.059] predicted abdominal obesity, while plasma Cu [OR = 2.211 (1.146, 4.266), p = 0.02] and zinc (Zn) [OR = 2.228 (1.048, 4.736) p = 0.04] predicted MetS. Significant correlations between dyslipidemia and lithium [OR = 1.716 (1.080, 2.726)], Cu [OR = 2.210 (1.415, 3.454)], Mn [OR = 2.200 (1.320, 3.666)], molybdenum [OR = 1.853 (1.160, 2.958)], and Zn [OR = 1.993 (1.186, 3.349)], and between boron [OR = 2.583 (1.137, 5.868)] and hyperglycemia were observed (all p < 0.05). Exposure to essential metals may affect the body composition and metabolic profiles, exacerbating the risk of MetS.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
A worldwide survey in 2015 showed that obesity affects 107.7 million children and 603.7 million adults, with the highest adult obesity (35.3%) observed in Egypt and the highest childhood obesity (12.7%) in the USA [1]. Obesity is the strongest risk factor for metabolic syndrome (MetS). MetS refers to a cluster of metabolic disturbances including abdominal obesity, dyslipidemia, hypertension, and hyperglycemia. MetS is a non-communicable disease with major public health consequences. For example, cardiovascular disease (CVD) (70%) was estimated to be the major cause of death among obese persons [1].
Metals widely exist in the environment, including in the food chain, water, dust, and soils. However, they are also derived from environmental pollutants from domestic, industrial, and medical sources, and technology devices. Although there is yet no consensus on the classification of metals, metals can be broadly categorized as essential [e.g., iron (Fe), copper (Cu), chromium (Cr), manganese (Mn), magnesium (Mg), molybdenum (Mo), and zinc (Zn)], probable essentials [e.g., boron (B) and nickel (Ni)], and non-essentials [e.g., arsenic (As), cadmium (Cd), mercury (Hg), lead (Pb), lithium (Li), and strontium (Sr)] [2]. By acting as cofactors of metalloenzymes, essential metals exert diverse biological functions and are essential for proper functioning of the human body. Metals with high density (> 5 g/cm3) are often referred as “heavy metals,” and they can be either essential or non-essential. Toxic heavy metals are often found in the environment and bioaccumulate in plants, wildlife, and people. They are of major public health concern because of their high toxicity to the human body even at low concentrations at the ppm level. For example, a 2014 World Health Organization (WHO) report emphasizes that Hg is among the top 10 chemicals of major public health concern, and there is a need for policies to reduce Hg exposure particularly in countries with high intake of fish and seafood [3].
Emerging evidence suggests that metal exposure may be associated with risks of obesity [4,5,6] and MetS [7,8,9]; however, mixed results have been reported [4, 10, 11]. A recent systematic review and meta-analysis showed that individuals with MetS had a 1.16-fold [pooled effect size (ES) = 1.16, 95% confidence interval (CI) = 1.09, 1.23] higher risk of mixed exposure to toxic heavy metals (As, Cd, Hg, and Pb) compared to those without MetS. However, study heterogeneity was high (I2 = 75.6%), and a subgroup analysis only found significant relationships between MetS and Hg (pooled ES = 1.26, 95% CI = 1.06, 1.48; I2 = 67.7%) and Pb (pooled ES = 1.21, 95% CI = 1.00, 1.48; I2 = 82.9%). Another study evaluated 37 epidemiological studies investigating environmental toxic metal exposure (As, Cd, Hg, Pb, and Cu) and risk of CVD, and the authors concluded that except for Hg exposure, environmental pollutants were associated with an increased risk of CVD [4]. Several factors may contribute to the high study heterogeneity and discrepancies among studies, including study designs (e.g., cross-sectional vs. prospective follow-up study), country and region, participants’ age or gender, single vs. multiple metal exposure, and types of metals (non-essential vs. essential). Country and geographic location affect the degree of exposure to environmental pollutants (e.g., type of contaminants and concentrations) particularly nation and race [7]. For example, a population-based study in South Korea showed that Hg exposure predicted risks of obesity and MetS [5, 12, 13], but this relationship was inconclusive in the USA [14, 15]. Seafood has high Hg contaminant levels, and seafood consumption may explain regional differences in Hg exposure and risks of obesity or MetS [16]. Other possibilities include exposure to multiple environmental pollutants [11, 14] or accumulation of less-toxic essential metals, such as Cu, Fe, Zn [17,18,19], Cr, Mg, and Mn [6, 20, 21]. Overall, relationships between metal exposure and MetS are complicated, and multifactorial mechanisms may be involved.
Currently, few studies have investigated relationships between metal exposure and body composition. Since MetS is intertwined with obesity, the aim of this study was to clarify relationships of metal exposure and body composition with risks of obesity and MetS in 150 middle-aged Taiwanese adults. Specifically, we aimed to investigate (1) relationships of essential and non-essential metals with body composition and abdominal obesity, and (2) the predictive effects on MetS and its individual components.
Materials and Methods
Participants and Sample Inclusion and Exclusion
This study used non-probability volunteering sampling as the sampling method. Informed written consent was collected by face-to-face interview from 150 Taiwanese adults (100 men and 50 women) aged 20–64 years before the enrollment to the study. Inclusion criteria were (1) Taiwanese citizen; (2) speak fluent Chinese; and (3) adults. Subjects were excluded if they were (1) aged < 20 or ≥ 65 years; (2) pregnant or lactating; (3) anemia (men: Hb < 13 g/dL and women Hb < 12 g/dL); (4) disease history of cancer, hepatitis, nephritis, and dialysis; and (5) excessive alcohol intake (alcohol intake < 20 g/week for women or < 30 g/week for men). A total of 150 (100 men and 50 women) were entered for the analysis.
Anthropometric Parameters and Body Composition
Anthropometric parameters such as body weight, body height, waist circumference (WC), hip circumference (HC), and the waist/hip (W/H) ratio of each participant were measured by trained staff. The WC was measured around the midpoint between the lower margin of the last rib and the top of the iliac crest. The W/H ratio was calculated as the WC divided by the HC. The body mass index (BMI) was calculated as weight (in kg) / (height squared in m2). The body composition was measured using a bioelectrical impedance analysis (BIA) (X-SCAN Plus-II analyzer, Jawon, Korea) to record the total body fat mass (BFM) (%), skeletal muscle mass (SMM) (%), visceral fat (%), subcutaneous fat (%), and ratio of BFM/SMM.
Blood Biochemistry
Fasting blood samples were collected from overnight-fasted participants. Blood analyses included a complete blood cell count, blood glucose, insulin and lipid profiles [total cholesterol (TC), low- (LDL-C) and high-density lipoprotein cholesterol (HDL-C), and triglycerides (TGs)], which were performed in a certified medical laboratory (Le-Zen Clinical Laboratory, Taipei, Taiwan). Plasma metal levels were assessed in Tri-Service Hospital (Taipei, Taiwan) using inductively coupled plasma mass spectrometry (ICP-MS) with a NexION 300D (PerkinElmer, Shelton, CT, USA) equipped with an ESI SC-2 DX4 autosampler (Elemental Scientific, Omaha, NE, USA). Blood plasma were diluted (1:20 v:v) with a diluent consisting of 0.05% Triton X-100 (Sigma-Aldrich, Co., MO, USA), and 1% HNO3 (ULTREX® II Ultrapure Reagent, J.T.Baker, Co., Canada) in 18.2 MΩ cm distilled deionized water. Analysis of essential, probably essential, and non-essential metal contents was performed using the NexION 300D (PerkinElmer) equipped with an ESI SC-2 DX4 autosampler (Elemental Scientific). A standard 0, 10, 20, 30, 40, and 50 μg/L solution of each metals from Universal Data Acquisition Standards Kit (PerkinElmer) was employed to calibrate the system. Certipur® Certified Reference Material (Merck, Germany) was used in this study as a quality control.
Definitions
Dyslipidemia was classified if an individual had at least one of the following criteria: (1) total C of ≥ 240 mg/dL; (2) LDL-C of ≥ 160 mg/dL; (3) HDL-C of < 40 mg/dL; (4) TC/HDL-C ratio of ≥ 5, and (5) TGs of ≥ 200 mg/dL [22]. The homeostatic model assessment of insulin resistance (HOMA-IR) index was calculated as fasting insulin (µU/L) × fasting glucose (nmol/L)/22.5. Diabetes was defined as glycated hemoglobin (HbA1C) of ≥ 6.5% or fasting plasma glucose of ≥ 126 mg/dL. Hypertension was defined as systolic blood pressure of ≥ 130 mm/Hg and diastolic blood pressure of ≥ 85 mm/Hg. The criteria of MetS were based on the modified National Cholesterol Education Program Adult Treatment Panel III for the Asia Pacific [23]. MetS was confirmed if individuals had three or more of the following criteria: (1) fasting plasma glucose of ≥ 100 mg/dL; (2) fasting TGs of ≥ 150 mg/dL; (3) HDL-C of < 40 mg/dL; (4) systolic blood pressure of ≥ 130 mmHg or diastolic blood pressure of ≥ 85 mmHg; and (5) a WC in males of ≥ 90 cm and in females of ≥ 80 cm, which was also defined as abdominal obesity or central obesity. Toxicity of non-essential elements was defined according to the Agency for Toxic Substances and Disease Registry (ATSDR) as: As > 1 μg/L and Cd > 0.315 μg/L in blood.
Statistical Analysis
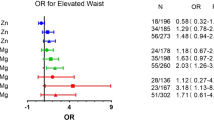
Analyses were carried out using IBM® SPSS® 21, SAS vers. 9.4, GraphPad Prism 5 and R software (version 1.4.1717). A normality test was carried out to test the distribution of each variable. Variables that were not normally distributed were log-transformed to meet the normality assumption for the analysis. Categorical data are presented as the number [percentage (%)], and continuous data are presented as the mean ± standard deviation (SD). Plasma metal concentrations were divided into tertiles (T) using SPSS by assigning T1 to the smallest value. Mann–Whitney test was used to identify the difference of metals concentration between two groups. A general linear model was used to analyze the p-trend between variables for continuous data, and chi-squared was used for categorical data. A multivariate linear regression analysis adjusted for age, sex, and smoking status was employed to evaluate relationships between metal exposure and body composition (W/H ratio, BMI, total body fat mass, subcutaneous fat mass, visceral fat mass, skeletal muscle mass, and ratio of body fat mass/skeletal muscle mass) as well as metabolic profiles (e.g., fasting plasma glucose, HOMA-IR, HDL, LDL, and TC). A multivariate logistic regression adjusted for age, sex, smoking, and BMI was conducted to estimate the odds ratio (OR) and 95% CI of MetS, and its components (dyslipidemia, diabetes, and hypertension). In Fig. 1, cut-off points for tertile (T) groups of each metal were As: < 1.0, 1.0–2.8 µg/L, > 2.8; Hg: < 0.9, 1.0–1.4, > 1.4 µg/L; Li: < 1.0, 1.0–1.4, > 1.4 µg/L; Sr: < 31.50, 31.50–43.10, > 43.10 µg/L; B: < 12.0, 12.0–28.40, > 28.40 µg/L; Cr: < 0.7, 0.7–1.2, > 1.2 µg/L; Cu: < 886.3, 886.3–1061.6, > 1061.6 µg/L; Ni: < 0.4, 0.4–1.6, > 1.6 µg/L; Mg: < 21,781, 21,781–25,894 µg/L, > 25,894; Mn: < 1.4, 1.4–1.7, > 1.7 µg/L; Mo: < 0.9, 0.9–1.2, > 1.2 µg/L, and Zn: < 764.5, 764.5–951.9, > 951.9 µg/L. Differences were considered significant at p ≤ 0.05.
Adjusted odds ratio (ORs) and 95% confidence intervals (CI) of essential and probably essential metals (A) and non-essential metals (B) for predicting metabolic syndrome (MetS) according to total and tertile (T) groups of plasma metals (n = 150). The multivariate model was adjusted for age, gender, body mass index, and smoking. Black, filled circles represent total predictive effects of each metal. Unfilled circles indicate the reference (Ref, T1) and black, filled circles represent T2 and T3 groups, respectively. *p < 0.05
Results
Baseline Characteristics
Table 1 shows the general characteristics of study participants. In total, 150 participants were included in the analysis. The mean age was 42.6 ± 12.4 year and mean BMI was 24.8 ± 4.4 kg/m2. The majority of study participants were male (70.7%), and 18.7% had a smoking history. Almost half of participants had abdominal obesity (45.3%) and dyslipidemia (55.3%), and one-quarter had hypertension (22.0%). Individuals with MetS had lower skeleton muscle mass but higher levels of fat mass, non-essential metals (Cd, Li, and Sr), and essential metals (B, Cu, Ni, Mg, Zn) compared to those without MetS (all p < 0.05) (Table 1).
MetS was confirmed if individuals had three or more of the following criteria: (1) fasting plasma glucose ≥ 100 mg/dL; (2) fasting TGs ≥ 150 mg/dL; (3) HDL-C < 40 mg/dL; (4) systolic blood pressure ≥ 130 mmHg or diastolic blood pressure ≥ 85 mmHg; and (5) a WC in males ≥ 90 cm and in females ≥ 80 cm.
Associations Between Plasma Metals and Body Composition
We first investigated relationships between plasma metals and the most common anthropometric indices used to screen for abodominal obesity (the waist (W)/hip (H) circumference ratio) or obesity (BMI). Table 2 shows no significant relationship between plasma metals and the W/H ratio or BMI after adjusting for age, gender, and smoking, except between plasma B and BMI (ß = 0.834 (0.017, 1.650), p < 0.05). We next investigated relationships between plasma metal exposure and body composition. For non-essential metals, a significant positive correlation was found between plasma Li and the body fat mass (%) (ß = 1.454 (0.005, 2.902), p < 0.05) after adjusting for covariates (Table 2, non-essential metals). Cr, Cu, and Mn essential metals were significantly positively correlated with the body fat mass (%) but inversely correlated with the skeletal muscle mass (%) (Table 2, essential metals) (all p < 0.05). Positive trends between plasma levels of B and Cu and the ratio of body fat mass/skeletal muscle mass were also observed (both p < 0.05).
Associations Between Plasma Metals and Metabolic Components
Table 3 shows that no significant correlations between non-essential metals and metabolic components (total C, HDL, LDL, HOMA-IR, fasting plasma glucose) were observed (Table 3, non-essential metals), except for plasma Li and fasting plsma glucose levels (ß = 3.810 (0.972, 8.592), p < 0.05). In contrast, significant positive correlations were found between TC and the essential metals Cu [ß = 32.928 (6.259, 59.598)], Mn [ß = 45.308 (10.771, 79.846)], and Zn [ß = 31.814 (3.389, 60.239)], and between LDL-C and Cu [ß = 31.642 (7.850, 55.433)] and Mg [ß = 34.383 (3.285, 65.481)] (all p < 0.05). Significant positive relationships between fasting plasma glucose levels and plasma B [ß = 3.918 (0.661, 7.175)] and Mo [ß = 9.790 (2.615, 16.964)], as well as HOMA-IR and plasma Mo [ß = 0.633 (0.001, 1.266)], were found (Table 3, essential metals) (all p < 0.05).
Predictive Effects of Metal Exposure on MetS and Its Individual Components
We next investigated predictive effects of metal exposure on the risks of MetS and its individual components. After adjusting for age, gender, BMI, and smoking, plasma Cu (OR = 2.211 (1.146, 4.266), p = 0.02) and Zn (OR = 2.228 (1.048, 4.736) p = 0.04) independently predicted MetS, and to a lesser extent, so did Cd (OR = 2.807 (0.887, 8.878), p = 0.08), B (OR = 1.874 (0.931, 3.773), p = 0.08), and Mg (OR = 1.868 (0.959, 3.639), p = 0.07) (Table 4, Fig. 1). Plasma Mn independently predicted abdominal obesity (OR = 1.624 (1.072–2.462), p = 0.02), and Cd (OR = 2.269 (0.918–5.604), p = 0.08) and Cu (OR = 1.501 (0.985–2.288), p = 0.06) had borderline predictive effects on abdominal obesity. Five metals exerted strong predictive effects on dyslipidemia: plasma Li (OR = 1.883 (1.184, 2.993), p = 0.007), Cu (OR = 2.108 (1.361, 3.266), p = 0.001), Mg (OR = 1.940 (1.179, 3.193), p = 0.009), Mo (OR = 1.794 (1.131, 2.844), p = 0.01), and Zn (OR = 1.993 (1.186, 3.349), p = 0.009). Plasma B levels (OR = 2.606 (1.162, 5.842), p = 0.02) and, to a lesser extent, Mo (OR = 2.021 (0.955, 4.280), p = 0.07) were positively correlated with diabetes (Table 4).
Discussion
To our knowledge, this is one of the few studies to have investigated relationships between exposure to 13 (five non-essential and eight essential/probably essential) metals and body composition. The current study indicates that for middle-aged adults, plasma metal levels were not correlated with common anthropometric indices used to screen for abdominal obesity (W/H ratio) or obesity (BMI). In contrast, body fat mass (Cr, Cu, and Mn), skeletal muscle mass (Cr, Cu, and Mn), and the ratio of body fat mass/skeletal muscle mass (B and Cu) were sensitive to exposure to essential metals. Compared to non-essential xenobiotic metals (e.g., As, Cd, Hg, and Sr), essential metals independently predicted dyslipidemia (Cu, Mg, Mn, and Zn), hyperglycemia (B, Cr, Cu, Mn, and Mo), and MetS (Cu and Zn), and these effects were independent of the BMI. By contrast, no significant correlation between toxic non-essential heavy metals (As, Cd, Hg, Li, Sr) and MetS was found. According to the ATSDR ToxGuide, normal human blood levels for As and Cd should be < 1 μg/L and < 0.315 µg/L, respectively. In the current study, the mean plasma levels of Cd is 0.02 ± 0.05 μg/L [MetS( −): 0.02 ± 0.51, MetS( −): 0.03 ± 0.05, p = 0.035], which is within the normal reference range. However, the mean plasma levels of As in both MetS( −) and MetS( +) groups all exceeded the acceptable range [mean: 2.74 ± 3.32 [MetS( −): 2.70 ± 3.32, MetS( −): 2.86 ± 3.73, p = 0.868]]. Our finding is in agreement with a previous study, which showed that blood level of As in healthy Taiwanese adults is above the tolerable range (mean blood As: 7.41 ± 4.70 μg/L) [24]. Hence, it is less likely that no correlation between non-essential metals and MetS observed in the current study is simply due to the exposure dosage. However, since blood levels of metals are indicative of recent exposure rather than whole-body burdens, a longitudinal follow-up study is needed to clarify the causal relationship of non-essential xenobiotic metals exposure and risk of MetS. Overall, the current results provide evidence that exposure to essential metals may promote a risk of MetS, possibly acting through alterations of the body composition and disturbances of blood glucose and lipid homeostasis.
The present study found that plasma Zn and Cu independently predicted MetS, and that there were direct relationships between plasma essential metals (Cu, Mg, Mo, and Zn) and dyslipidemia. These results are in agreement with recent studies which showed that exposure to Cu [25,26,27], Zn [17, 18], Mg [20], and Mn [21] predicted risks of MetS or its individual components. The pathological effects of non-essential metals on MetS have long been recognized and are well investigated [10]; in contrast, relationships between essential metals and metabolic function are inconsistent [18]. Essential metals play key roles in regulating body metabolism; however, a deficiency or excess may both promote a risk of MetS. For example, a recent meta-analysis showed that compared to those with the lowest dietary intake, individuals with the highest intake of dietary Zn had a 13% decreased risk of type 2 diabetes (OR = 0.87 (95% CI = 0.78, 0.98)); in contrast, plasma/serum Zn levels predicted diabetic risk (OR = 1.64, 95% CI = 1.25, 2.14) [18]. Zn is not only an essential trace element but is also a heavy metal, which means that the human body only requires a small amount. However, like most essential trace elements, Zn has a high dietary absorption rate and can enter the body through a Zn-contaminated soil–water-food-cooking container cycle. Minerals are inorganic elements, which cannot be destroyed by cooking processes (e.g., heat and acid), and tend to accumulate in the body once absorbed. Metallic elements generally exist in the environment and in food. Ingestion of metal-contaminated foodstuffs is the most common route of heavy metal exposure. When exposed to large amounts, they may disturb energy metabolism, possibly through interfering with enzymatic activities and inducing free radical-induced oxidative stress leading to endocrine disorders [26, 28]. Nonetheless, future studies are warranted to investigate consumption of heavy metal-contaminated foods (e.g., type of crops and consumption dosage) and health risks of MetS.
Obesity is a significant public health issue in Taiwan, affecting almost one in two adults (45.3%) in this study. Few studies have investigated relationships between metal exposure and body composition. Interestingly, we found that plasma metal levels were not correlated with conventional anthropometric indices such as the W/H ratio or BMI. Instead, body composition seemed to be more sensitive to plasma levels of essential metals. This suggests that anthropometric indices are not sensitively enough to reflect the exposure of trace elements on the body fat mass, which may be due to the low abundance of trace elements in the human body. Specifically, we found that plasma Mn, and a borderline effect of Cu, independently predicted abdominal obesity after adjusting for covariates. Both Mn and Cu were significantly positively correlated with the total body fat mass but inversely associated with the skeletal muscle mass. These results were in agreement with findings in a nationwide nutrition and health survey in US children and adolescents, which showed that compared to those with the lowest, children with the highest blood levels of Cu (OR = 9.27, 95% CI = 5.43, 15.82) and Mn (OR = 2.29, 95% CI = 1.74, 3.02) had increased risks of obesity [29]. Subsequently, this relationhsip was confirmed in a meta-analysis (n = 21 related articles), and the authors showed that compared to controls, the serum Cu level was higher in obese children [standard mean difference (SMD): 0.74 (0.16, 1.32)] and adults [SMD: 0.39 (0.02, 0.76)] [27]. Direct relationships between essential metals and obesity were observed in studies in US women (Cu, Mn, and Mo) [14] and Chinese adults (Cu, Mn, Zn, Mo, and B) [6]. However, the clinical implication of the role of essential trace elements in the etiology of obesity and MetS remains difficult to interpret. This is due in part to the lack of cut-off point for the acceptable or toxic reference range of trace elements in a diverse specimen (e.g., blood, serum or plasma, nail, hair, and urine). Furthermore, a sensitive method, such as the ICP-MS, is required to accurately quantitate the concentrations of trace elements. The advantages of the ICP-MS method over the conventional methods include its accuracy, reliability, speed, and simplicity. However, the ICP-MS is expensive and required trained technicians to operate the machine. The lack of exposure data is also one of the limitations. It is believed that diet represents the most relevant exposure source of trace elements in general population. Future study is needed to clarify the role of diet in the relationship between essential trace elements and risk of MetS.
We also observed an inverse relationship between plasma Mo and the visceral fat mass (ß = − 1.622 (− 3.23, − 0.017), p < 0.05). This result supports the findings of Wang et al., who showed that urinary Mo levels were correlated with favorable profiles of adipokines (e.g., leptin, adiponectin, and soluble leptin receptor), while exposure to non-essential metals (Cd and Pb) was associated with adverse adipokine profiles in 1228 US women [30].
A possible relationship between exposure to Mo and B and a risk of hyperglycemia was found in this study. Our result agrees with findings of Flores et al., which showed that compared to non-diabetic healthy controls, diabetic patients had higher levels of serum Mo, and serum Mo concentrations further increased in patients with more-severe diabetic complications [31]. Mo is an essential trace element and has numerous biological functions, including purine synthesis, detoxification of aldehyde, and ATP synthesis. However, Mo is also a potent inhibitor of Cu, and high plasma Mo levels may disrupt enzymatic activity of Cu-containing enzymes (e.g., superoxide dismutase and ceruloplasmin). It was postulated that Mo overload may lead to a Cu deficiency resulting in disruption of Cu-carbohydrate interactions. Nonetheless, the exact mechanism linking Mo and hyperglycemia remains to be elucidated. Another interesting observation was a positive relationship between plasma B exposure and the risk of diabetes (OR = 3.92 (0.66, 7.18)). Currently, mechanisms linking B exposure to glucose homeostasis are not fully understood. B, a probably essential metal, is present in the human body and plants (as boric acid or borate forms as food preservatives). It was suggested that B in nutritional amounts may have beneficial health effects on bone metabolism and metabolic function [32]. The recommended daily intake of B is 0.16 mg/kg body weight according to the European Food Safety Agency, and daily intake of 500 mg boric acid (87 mg B) may cause appetite and digestive discomfort [32].
As the increasing widespread use of Li-containing batteries in electric cars and mobile devices continues, Li has emerged as an environmental contaminant. Li is also one of the well-known medicines for bipolar disorder, with potential hypoglycemic effects [33]. However, a meta-analysis showed that Li is associated with increased risks of a reduced glomerular filtration rate, hypothyroidism, and hyperparathyroidism [34]. In addition, patients receiving Li medication had a 1.89-fold (OR = 1.89, 1.27, 2.82, p = 0·002) higher risk of weight gain compared to those who did not [34]. This result agrees with the findings of the current study, in which a significant positive relationship between plasma Li levels and body fat mass was observed. Furthermore, we also observed positive relationships of plasma Li levels with fasting plasma glucose and dyslipidemia. Although an animal study showed that low doses of Li enhance glucose utilization [33], excess Li exposure can trigger chronic inflammaton and oxidative stress and may lead to endocrine disruption. Indeed, bipolar disorder patients who received Li medication had increased serum atherogenic lipid profiles including TC, TGs, and LDL-C [35].
There are several limitations to this study which should be taken into account to avoid over-interpreting the results. First, this study was a cross-sectional study, and there was a small sample size (n = 150) which limited our ability to determine causal relationships between metal exposure and obesity risks. Nonetheless, Niehoff and colleagues investigated the causal relationship of the BMI and metal exposure in US women in a 5.2-year follow-up period, and those authors concluded that metal exposure at the baseline predicted a risk of obesity [14]. Although we adjusted for the smoking history as a potential confounding factor, other residual confounding effects known to affect the BMI or metal exposure could not be fully controlled for in this study, such as total calories, food intake, sources of environmental pollutants, and exercise or physical activity. Another limitation includes a one-time measurement of plasma metal exposure and not cumulative levels. Although plasma metal concentrations indicate the fraction of circulating metals in the body, they reflect short-term exposure, rather than long-term external exposure to metals.
Conclusions
In summary, the current study demonstrated significant relationships between essential metals and body composition but not common anthropometric indices (e.g., BMI or W/H ratio). Our findings provide evidence that exposure to essential metals may also exert effects on abdominal obesity, dyslipidemia, and hyperglycemia, which might be mechanisms by which metal exposure leads to MetS risks. Future studies are needed to confirm these relationships and elucidate the mechanisms underlying the link between exposure to essential metals and MetS risk.
References
Collaborators GBDO, Afshin A, Forouzanfar MH, Reitsma MB, Sur P, Estep K, Lee A, Marczak L, Mokdad AH, Moradi-Lakeh M, Naghavi M, Salama JS, Vos T, Abate KH, Abbafati C, Ahmed MB, Al-Aly Z, Alkerwi A, Al-Raddadi R, Amare AT, Amberbir A, Amegah AK, Amini E, Amrock SM, Anjana RM, Arnlov J, Asayesh H, Banerjee A, Barac A, Baye E, Bennett DA, Beyene AS, Biadgilign S, Biryukov S, Bjertness E, Boneya DJ, Campos-Nonato I, Carrero JJ, Cecilio P, Cercy K, Ciobanu LG, Cornaby L, Damtew SA, Dandona L, Dandona R, Dharmaratne SD, Duncan BB, Eshrati B, Esteghamati A, Feigin VL, Fernandes JC, Furst T, Gebrehiwot TT, Gold A, Gona PN, Goto A, Habtewold TD, Hadush KT, Hafezi-Nejad N, Hay SI, Horino M, Islami F, Kamal R, Kasaeian A, Katikireddi SV, Kengne AP, Kesavachandran CN, Khader YS, Khang YH, Khubchandani J, Kim D, Kim YJ, Kinfu Y, Kosen S, Ku T, Defo BK, Kumar GA, Larson HJ, Leinsalu M, Liang X, Lim SS, Liu P, Lopez AD, Lozano R, Majeed A, Malekzadeh R, Malta DC, Mazidi M, McAlinden C, McGarvey ST, Mengistu DT, Mensah GA, Mensink GBM, Mezgebe HB, Mirrakhimov EM, Mueller UO, Noubiap JJ, Obermeyer CM, Ogbo FA, Owolabi MO, Patton GC, Pourmalek F, Qorbani M, Rafay A, Rai RK, Ranabhat CL, Reinig N, Safiri S, Salomon JA, Sanabria JR, Santos IS, Sartorius B, Sawhney M, Schmidhuber J, Schutte AE, Schmidt MI, Sepanlou SG, Shamsizadeh M, Sheikhbahaei S, Shin MJ, Shiri R, Shiue I, Roba HS, Silva DAS, Silverberg JI, Singh JA, Stranges S, Swaminathan S, Tabares-Seisdedos R, Tadese F, Tedla BA, Tegegne BS, Terkawi AS, Thakur JS, Tonelli M, Topor-Madry R, Tyrovolas S, Ukwaja KN, Uthman OA, Vaezghasemi M, Vasankari T, Vlassov VV, Vollset SE, Weiderpass E, Werdecker A, Wesana J, Westerman R, Yano Y, Yonemoto N, Yonga G, Zaidi Z, Zenebe ZM, Zipkin B, Murray CJL (2017) Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med 377(1):13–27
Mehri A (2020) Trace elements in human nutrition (II)—an update. Int J Prev Med 11:2
Sheehan MC, Burke TA, Navas-Acien A, Breysse PN, McGready J, Fox MA (2014) Global methylmercury exposure from seafood consumption and risk of developmental neurotoxicity: a systematic review. Bull World Health Organ 92(4):254-269F
Chowdhury R, Ramond A, O’Keeffe LM, Shahzad S, Kunutsor SK, Muka T, Gregson J, Willeit P, Warnakula S, Khan H, Chowdhury S, Gobin R, Franco OH, Di Angelantonio E (2018) Environmental toxic metal contaminants and risk of cardiovascular disease: systematic review and meta-analysis. BMJ 362 k3310
Lee K (2018) Blood mercury concentration in relation to metabolic and weight phenotypes using the KNHANES 2011–2013 data. Int Arch Occup Environ Health 91(2):185–193
Zhu X, Fan Y, Sheng J, Gu L, Tao Q, Huang R, Liu K, Yang L, Chen G, Cao H, Li K, Tao F, Wang S (2021) Association between blood heavy metal concentrations and dyslipidemia in the elderly. Biol Trace Elem Res 199(4):1280–1290
Skalny AV, Chang JS, Bobrovnitsky IP, Kopylov PY, Skalnaya MG, Huang SY, Paoliello MMB, Ivanova ES, Wang W, Tinkov AA (2020) Relationship between elevated hair mercury levels, essential element status, and metabolic profile in overweight and obese adults. Biol Trace Elem Res
Tinkov AA, Aschner M, Ke T, Ferrer B, Zhou JC, Chang JS, Santamaria A, Chao JC, Aaseth J, Skalny AV (2021) Adipotropic effects of heavy metals and their potential role in obesity. Fac Rev 10:32
Ayoub N, Mantash H, Dhaini HR, Mourad A, Hneino M, Daher Z (2021) Serum cadmium levels and risk of metabolic syndrome: a cross-sectional study. Biol Trace Elem Res 199(10):3625–3633
Xu P, Liu A, Li F, Tinkov AA, Liu L, Zhou JC (2021) Associations between metabolic syndrome and four heavy metals: a systematic review and meta-analysis. Environ Pollut 273 116480
Zhong Q, Qin QR, Yang WJ, He JL, Zhu JL, Zhu ZY, Huang F (2021) Multiple metal exposure and obesity: a prospective cohort study of adults living along the Yangtze River. China, Environ Pollut 285 117150
Lee S, Yoon JH, Won JU, Lee W, Lee JH, Seok H, Kim YK, Kim CN, Roh J (2016) The association between blood mercury levels and risk for overweight in a general adult population: results from the Korean National Health and Nutrition Examination Survey. Biol Trace Elem Res 171(2):251–261
Shin YY, Ryu IK, Park MJ, Kim SH (2018) The association of total blood mercury levels and overweight among Korean adolescents: analysis of the Korean National Health and Nutrition Examination Survey (KNHANES) 2010–2013. Korean J Pediatr 61(4):121–128
Niehoff NM, Keil AP, O’Brien KM, Jackson BP, Karagas MR, Weinberg CR, White AJ (2020) Metals and trace elements in relation to body mass index in a prospective study of US women. Environ Res 184 109396
Rothenberg SE, Korrick SA, Fayad R (2015) The influence of obesity on blood mercury levels for U.S. non-pregnant adults and children: NHANES 2007–2010. Environ Res 138:173–80
Skalny AV, Skalnaya MG, Serebryansky EP, Zhegalova IV, Grabeklis AR, Skalnaya OA, Skalnaya AA, Huang PT, Wu CC, Bykov AT, Tinkov AA (2018) Comparative hair trace element profile in the population of Sakhalin and Taiwan Pacific Islands. Biol Trace Elem Res 184(2):308–316
Lu CW, Lee YC, Kuo CS, Chiang CH, Chang HH, Huang KC (2021) Association of serum levels of zinc, copper, and iron with risk of metabolic syndrome. Nutrients 13(2)
Fernandez-Cao JC, Warthon-Medina M, Arija HMVV, Doepking C, Serra-Majem L, Lowe NM (2019) Zinc intake and status and risk of type 2 diabetes mellitus: a systematic review and meta-analysis, Nutrients 11(5)
Cempaka AR, Tseng SH, Yuan KC, Bai CH, Tinkov AA, Skalny AV, Chang JS (2019) Dysregulated iron metabolism-associated dietary pattern predicts an altered body composition and metabolic syndrome. Nutrients 11(11)
Gaman MA, Dobrica EC, Cozma MA, Antonie NI, Stanescu AMA, Gaman AM, Diaconu CC (2021) Crosstalk of magnesium and serum lipids in dyslipidemia and associated disorders: a systematic review. Nutrients 13(5)
Lo K, Yang JL, Chen CL, Liu L, Huang YQ, Feng YQ, Yang AM (2021) Associations between blood and urinary manganese with metabolic syndrome and its components: cross-sectional analysis of National Health and Nutrition Examination Survey 2011–2016. Sci Total Environ 780 146527
Lin C-F, Chang Y-H, Chien S-C, Lin Y-H, Yeh H-Y (2018) Epidemiology of dyslipidemia in the Asia Pacific Region. Int J Gerontol
Tan C-E, Ma S, Wai D, Chew S-K, Tai E-S (2004) Can we apply the National Cholesterol Education Program Adult Treatment Panel definition of the metabolic syndrome to Asians? Diabetes Care 27(5):1182–1186
Liu T-Y, Hung Y-M, Huang W-C, Wu M-L, Lin S-L (2017) Do people from Taiwan have higher heavy metal levels than those from Western countries? Singapore Med J 58(5):267–271
Lian S, Zhang T, Yu Y, Zhang B (2021) Relationship of circulating copper level with gestational diabetes mellitus: a meta-analysis and systemic review. Biol Trace Elem Res
Chen J, Lan C, An H, Jin Y, Li Q, Ge S, Yu Y, Shen G, Pan B, Xu Y, Ye R, Li Z, Wang B (2021) Potential interference on the lipid metabolisms by serum copper in a women population: a repeated measurement study, Sci Total Environ 760 143375
Gu K, Li X, Xiang W, Jiang X (2020) The relationship between serum copper and overweight/obesity: a meta-analysis. Biol Trace Elem Res 194(2):336–347
Romero A, Ramos E, de Los Rios C, Egea J, Del Pino J, Reiter RJ (2021) A review of metal-catalyzed molecular damage: protection by melatonin. J Pineal Res 56(4):343–70
Fan Y, Zhang C, Bu J (2017) Relationship between selected serum metallic elements and obesity in children and adolescent in the U.S. Nutrients 9(2)
Wang X, Karvonen-Gutierrez CA, Mukherjee B, Herman WH, Park SK (2021) Urinary metals and adipokines in midlife women: the study of women’s health across the nation (SWAN). Environ Res 196 110426
Flores CR, Puga MP, Wrobel K, GaraySevilla ME, Wrobel K (2021) Trace elements status in diabetes mellitus type 2: possible role of the interaction between molybdenum and copper in the progress of typical complications. Diabetes Res Clin Pract 91(3):333–41
Nielsen FH, Eckhert CD (2020) Boron. Adv Nutr 11(2):461–462
Jung SR, Park SY, Koh JH, Kim JY (2021) Lithium enhances exercise-induced glycogen breakdown and insulin-induced AKT activation to facilitate glucose uptake in rodent skeletal muscle. Pflugers Arch 473(4):673–682
McKnight RF, Adida M, Budge K, Stockton S, Goodwin GM, Geddes JR (2012) Lithium toxicity profile: a systematic review and meta-analysis. Lancet 379(9817):721–728
Aliyazicioglu R, Kural B, Colak M, Karahan SC, Ayvaz S, Deger O (2007) Treatment with lithium, alone or in combination with olanzapine, relieves oxidative stress but increases atherogenic lipids in bipolar disorder. Tohoku J Exp Med 213(1):79–87
Acknowledgements
Authors would like to thank the technique assistances of Trace Element Research Center, Tri-Service General Hospital, Taipei, Taiwan, and Dr. Skalny Laboratory Asia-Pacific promotion center.
Funding
This work was supported by grants from the Ministry of Science and Technology,Taiwan (MOST 109–2923-B-038–001-MY3) and the Russian Foundation for Basic Research (No. 20–515-S52003). Dr. JS Chang was also supported by the grants from the Taipei Medical University Hospital (110TMU-TMUH-109) and the Ministry of Science and Technology (MOST 109–2923-B-038–001-MY3, MOST 110–2320-B-038–046). Российский Фонд Фундаментальных Исследований (РФФИ),No. 20–515-S52003,Anatoly V. Skalny,Ministry of Science and Technology,Taiwan,MOST 110–2320-B-038–046,Jung-Su Chang,MOST109-2923-B-038–001-MY3,Jung-Su Chang,Taipei Medical University Hospital,110TMU-TMUH-109,Jung-Su Chang
Author information
Authors and Affiliations
Contributions
Conceptualization, J.S.C., A.V.S., and A.A.T.; methodology, Y.K.C., C.F.C., and C.S.T.; validation, A.V.S. and A.A.T.; formal analysis, Y.J.N.; investigation, Y.J.N.; resources, Y.K.C. and C.F.C.; data curation, C.F.C.; writing—original draft preparation, J.S.C.; writing—review and editing, Y.K.C. and C.F.C.; supervision, C.S.T., A.V.S., and A.A.T.; project administration, C.S.T.; funding acquisition, J.S.C. and A.V.S. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board of Taipei Medical University Hospital (201,502,018).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ngu, Y.J., Skalny, A.V., Tinkov, A.A. et al. Association Between Essential and Non-essential Metals, Body Composition, and Metabolic Syndrome in Adults. Biol Trace Elem Res 200, 4903–4915 (2022). https://doi.org/10.1007/s12011-021-03077-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-021-03077-3