Abstract
Trace element supplementation to the freshwater environment can influence the plankton density and species diversity, contributing to the nutrition of aquaculture species, especially during the juvenile stage. An experiment was conducted under laboratory conditions to evaluate the effects of supplementing different mixtures of manganese, silica and phosphorus on the plankton density and species diversity and their impact on cultured juvenile marron (Cherax cainii, Austin and Ryan, 2002). Manganese, silica and phosphorus in concentrations of 0.0024, 0.41, 0.05 mg*L−1; 0.0041, 0.82, 0.12 mg*L−1; and 0.0058, 1.26, 0.25 mg*L−1 respectively termed as low, medium and high were supplemented to tank water containing a phytoplankton density of 3.77 ± 0.16 × 106 cells*L−1 and 292.9 ± 17.6 individuals*L−1 of zooplankton, and plankton growth was observed every 24 h for 6 days. Afterwards, a 3-month trial was conducted studying the effects of these trace element concentrations and resulting plankton densities on marron growth, survival, moulting, gut microbiota and health indices. Silica supplementation at high concentration increased the diatom abundance, silica and phosphorus supplementation at higher concentration that resulted in a significant increase in plankton density and species diversity, leading to improved marron health indices than the control and the tanks receiving a low concentration. Marron-specific growth rate, weight gain and dissolved copper concentration in haemolymph were significantly higher in tanks with higher supplementation and higher plankton density. Marron survival, moult interval and total haemocyte count were not affected by the supplementation. Marron gut microbiota at higher trace element concentration supplementation showed a significant increase in abundance of phosphate solubilizing bacteria.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Among the various aquaculture species farmed in Western Australia (WA), marron (Cherax cainii, Austin and Ryan, 2002) is a commercially important freshwater crayfish due to its high value, distinct taste and disease-free status [1]. Marron can consume any available food including plant and animal matter within a ponds system [2]. The effects of trace element supplementation on fish production have been studied previously [3,4,5,6]. To increase the marron production through plankton productivity, marron farmers use different organic including fermented barley straws and inorganic fertilizers, a common practice in aquaculture [7, 8]. Several studies have shown that manganese (Mn), silica (Si) and phosphorus (P) are vital for plankton productivity in freshwater ecosystems [9,10,11,12,13,14,15]. Their deficiency can diminish the phytoplankton growth [10], and their supplementation can increase plankton biomass initiating a modulation of community structure [10, 12].
Phosphate fertilizers are widely used [8] to increase the plankton productivity and in turn to improve the growth performance of cultured aquatic animals [6, 16, 17]. Under controlled laboratory conditions and in the absence of planktons, juvenile signal crayfish (Pacifastacus leniusculus) culture remains largely unsuccessful, mainly due to the low survival and growth rates during the early life stages [18, 19], whereas a higher survival and growth rates of juveniles signal crayfish were obtained with the supply of live zooplankton [19].
Although Mn, Si and P are essential trace elements for the growth of plankton and crustaceans, limited research has investigated their effect on the plankton density and species diversity as well as on the growth, survival, health and gut microbiota of freshwater crayfish [20], a study by Sierp [20] where authors observed the effect of adult marron (C. tennuimanus) on plankton and nutrient dynamics and the response of phytoplankton to phosphorus fertilization in ponds having high hardness. The main focus of related studies has been to analyze different concentrations of Mn and Si and their toxic effects on the decapods [21,22,23,24,25]. Trace elements have the ability to reshape the gut microbiota of guppy fish (Poecilia reticulata) for improved digestion, immunity and adaptation [26]. No research has been reported investigating the effects of different concentrations of Mn, Si and P supplementation on water quality, crayfish growth, survival, health indices and gut microbiota mediated through plankton density or species diversity. We hypothesized that trace element supplementation will positively influence the plankton density and community structure and the plankton densities will improve marron growth, survival, health indices and gut microbiota. To test this hypothesis, we conducted an indoor laboratory experiment for 96 days under controlled conditions.
Materials and Methods
Experimental Design
The experiment was designed based on the outcomes of our previous field trial in commercial earthen marron ponds [14], wherein 12 pre-selected trace elements were measured, of which manganese (Mn), silica (Si) and phosphorus (P) were found to be strongly correlated with plankton density and species diversity over the seasons. The mean dissolved concentrations of Mn, Si and P of 28 ponds over four seasons were used to determine low 0.0024, 0.41 and 0.05 mg*L−1, medium 0.0041, 0.82 and 0.12 mg*L−1 and high 0.0058, 1.26 and 0.25 mg*L−1 concentrations, respectively. These concentrations were used to evaluate the influence of the trace elements on plankton density and diversity.
Plankton Collection and Preparation for Stock Culture
Planktons were collected from the commercial marron farm in Manjimup (34° 18′ 75″ S, 116° 06′ 61″ E) WA. Pond water was filtered through a phytoplankton net to obtain 20 L of phytoplankton sample, and zooplankton net was used to obtain 20 L of zooplankton sample separately. Collected planktons were cultured as a stock culture for the experiment in outdoor conditions at the Curtin Aquatic Research Laboratory (CARL), using 300-L water capacity plastic tanks, filled with 200 L of freshwater, with a supply of vigorous aeration and direct sunlight. A continuous culture system was used to culture the plankton and Aquasol® by Yates Pty Ltd was added to the tanks to boost and maintain the phytoplankton density at 11.45 × 106 cells*L−1. Daily observations of tank conditions and plankton were made and the planktons were counted three times a week. The phytoplankton were fed to zooplankton to maintain the density.
Trace Element Supplementation and Plankton Culture
Under controlled laboratory conditions, sixteen 300-L water capacity tanks were filled with 150 L of water and were stocked with phytoplankton and zooplankton at a rate of 3.77 ± 0.16 × 106 cells*L−1 and 292.9 ± 17.6 individuals*L−1, respectively. On the same day, Mn in the form of manganese (II) chloride tetrahydrate (MnCl2.4H2O), silica-sodium metasilicate nonahydrate (Na2SiO3.9H2O) and phosphorus in the form of potassium hydrogen phosphate (K2HPO4) in three different concentrations as described in Table 1 were prepared by dissolving in distilled water in 100-mL beakers and were supplemented to the twelve treatment tanks. Mn, Si and P were supplemented only once at the start of the experiment. The experimental design included four treatments with four replicates: (1) only plankton (control; CTL); (2) plankton + low trace element supplementation (LTS); (3) plankton + medium trace element supplementation (MTS) and (4) plankton + high trace element supplementation (HTS). Continuous aeration and light were provided to grow the plankton. After trace element supplementation, the planktons were counted at 24, 48, 72, 96, 120 and 144 h to evaluate the plankton density and species diversity (Supplementary data #1). Trace element supplementation was conducted in the absence of marron in order to avoid any inadvertent phosphorous addition through feed.
The Table 1 shows the total weight of MnCl2.4H2O, Na2SiO3.9H2O and K2HPO4 used to achieve the required concentration of Mn, Si and P (mg*L−1).
Introducing Marron to Tanks Under Laboratory Conditions
Marron were collected from the commercial marron farm in Manjimup. A total of 190 juvenile marron (weighing average initial weight 6.13 ± 0.23 g, average orbital-carapace length (OCL) 2.95 ± 0.07 cm and total length of 6.26 ± 0.11 cm) were collected and transported to the CARL. Marron were stocked in 300-L water capacity tanks to acclimate to the laboratory conditions at the CARL for 15 days. After 6 days (144 h) of trace element supplementation and on plankton counting, all tanks were stocked with nine marron per tank in individual holding cages made up of plastic containers and top covered with mesh, to avoid cannibalism and escape of marron. The cages had a volume of 2000cm3 (170 mm × 115 mm × 135 mm) with four gaps of 4–5 mm on each sides to allow the water exchange directly from the tank water into the cage. Marron were fed at 2% of their body weight, once a day in the evening. The uneaten feed and faeces were removed, 1 h after the feeding.
Water Quality Analysis
All water parameters were kept in an optimum range for the growth of marron [27]. The water parameters including temperature, dissolved oxygen (DO) and pH were checked daily. An Oxyguard® digital DO meter (Handy Polaris 2, Norway) was used for DO and temperature measurements, and an Ecoscan pH 5 meter (Eutech instruments, Singapore) was used to record pH. A DR/890 portable colorimeter with Permachem reagents (Hach, USA) was used to analyze the total ammonia nitrogen (TAN), nitrite (NO2-N), nitrate (NO3-N) and reactive phosphate (PO4) once a week. The experiment was static, i.e. no water exchange was made. Tank water level was maintained at 150 L throughout the experiment by adding water to compensate for losses due to the evaporation.
Plankton and Trace Element Analysis
Throughout the experiment, plankton density was analyzed three times a week and was maintained at the same density as recorded after 144 h by either addition of plankton or removing by filtering plankton out, using respective plankton nets. For phytoplankton analysis, 2 L of tank water was filtered to obtain 100 mL of sample. For zooplankton analysis, 5 L of tank water was filtered to obtain 100 mL of sample. The filtered water was re-stocked into the same tank. The plankton species were identified to the lowest possible taxonomic level using keys from a manual by Ingram [28] and a book by Canter-Lund [29]. The plankton density (cells*L−1) was calculated by using the equations from Ingram [28] and Tulsankar [30]. The dissolved trace element concentration in tank water at initial, after supplementation, at the end of the experiment and in marron haemolymph were analyzed at Murdoch University, Perth, WA. The water samples were collected in 100-mL plastic containers directly from the tanks and were filtered through 0.45 μm Millipore filters to eliminate suspended particles. The haemolymph samples were collected using 1-mL syringe containing 0.2 mL of sodium citrate anticoagulant (100 mM glucose, 30 mM trisodium citrate, 26 mM citric acid, 15.5 mM NaCl and 10 mM EDTA) inserted in between the third and fourth pair of pereopod and were kept on ice during the sampling and transportation. Inductively, coupled plasma optical emission spectrometry (Agilent, ICP-OES, spike recovery limit 80–120%) with the standard methods described in APHA [31] was used to analyze the dissolved concentrations with the detection limits of Mn (<0.0002 mg*L−1); P (<0.02 mg*L−1) and Si (<0.02 mg*L−1) in water and Ca (<5 mg*L−1); Cu (<0.1 mg*L−1); Fe (<0.5 mg*L−1); Mn (<0.05 mg*L−1) and P (<5 mg*L−1) in haemolymph. The results of dissolved trace elements in haemolymph were calculated based on the dilution factor [32].
Marron Growth Analysis
Marron growth data were recorded fortnightly, and mortality was recorded daily. Marron-specific growth rate (SGR; g %/day), weight gain percentage (WG; g %) and survival rate (SR; %) were calculated by using the following equations
where Wt is final weight (g), Wi is initial weight (g), nt is the number of marron alive at (T) days and n0 is the number of marron stocked initially.
Marron moulting data such as dates and times were recorded daily. Moult interval (Tm; days) was measured on the basis of days required to moult, between two successive moults using the following equation:
where Tn = date of n moult, Tn + 1 = date of n + 1 moult.
Marron Health Indices
Marron health indices were analyzed at the end of the experiment, by testing haemolymph for total haemocyte count (THC) and differential haemocyte count (DHC), hepatopancreas wet and dry weight indices moisture content (HM %), wet weight (Hiw), dry weight (Hid), tail muscle moisture content (TM %), wet weight (TMiw) and dry weight (TMid) indices. Haemolymph samples were collected from one randomly selected marron per tank, and the haemolymph was drawn by using a 1-mL syringe inserted in between the third and fourth pair of pereopods. THC and DHC were analyzed according to [33]. One marron per tank was collected randomly, and hepatopancreas and tail muscle from each individual were collected and weighed. To obtain the dry weight, the samples were dried in crucibles at 105 °C in the oven for 24 h. The health indices were calculated as described by [34].
Marron Hindgut Microbiota Analysis
At the end of the experiment, a total of 24 marron, six per treatment were randomly selected. Gut content collection and separation of hindgut was performed inside a biosafety cabinet and the gut contents with mucosa were immediately lysed using Tissue Lyser II (Qiagen, Hilden, Germany). A subsequent pool of two marron gut contents from each respective tank was created by homogenization and transferred into 1.5-mL Eppendorf tube. Bacterial DNA was extracted using Blood and Tissue Kit (Qiagen, Hilden, Germany). DNA concentration was measured in Nanodrop Spectrophotometer (Thermo Fisher Scientific, USA) and diluted into 50 ng/μL for PCR. Fifty microliters of PCR master mix was prepared for each sample that contained 25 μL Hot Start 2X Master Mix (New England BioLabs Inc., Ipswich, MA, USA), 2 μL of bacterial DNA, 1 μL of each forward and reverse primers (V3-V4) and 21 μL of DEPC treated water. Forty cycles of PCR amplification were completed in a thermal cycler (BioRad S100, Bio-Rad Laboratories, Inc., Foster City, CA, USA). PCR products clean-up and amplicon barcoding was performed with a secondary PCR according to the Illumina standard protocol (Part # 15044223 Rev. B). Samples were then sequenced on Illumina MiSeq platforms (Illumina Inc., San Diego, CA, USA) using a v3 kit (600 cycles).
Bioinformatics and Statistical Analysis
Raw sequences were checked for initial quality in FastQC pipeline [35], trimmed for quality reads (parameters: -q 20 –l 200) in Sickle [36] and merged in MeFiT program [37]. MICCA pipelines used for filtering, open reference clustering and picking of OTUs at 97% similarity threshold [38]. SILVA 1.32 release used for phylogenetic assignment of operational taxonomic units (OTUs) at different taxa levels [39]. Multiple sequence alignment, FastTree (version 2.1.8) GTR+CAT phylogenetic tree was performed and constructed in PASTA algorithms [40, 41]. Rarefaction depth value was set to 32,996 bp and alpha-beta diversity was calculated using QIIME (v1.9.1) and R packages. Alpha diversity was calculated in terms of observed species, Shannon and Chao1 measurements. Beta ordination was calculated as Bray-Curtis dissimilarity of weighted UniFrac while permutational multivariate ANOVA (PERMNOVA) and non-metric multidimensional scaling (NMDS) analysis were performed to calculate and visualize the clustering of samples. Non-parametric statistical distance metric was calculated using ANOSIM with 1000 permutations. Relative and differential abundance of bacterial communities was calculated using phyloseq [42] and LEfSe [43], respectively.
Data Analysis
All the numerical data were analyzed using R software (v3.5.1) and are presented as mean ± standard error (SE). One-way analysis of variance (ANOVA) with Turkey’s HSD post hoc tests was used to determine the significant differences between treatments. Paired t-test was used to determine the dissolved trace element concentrations in water before and after supplementation. All tests were considered statistically significant at p < 0.05.
Results
Water Quality and Plankton Density
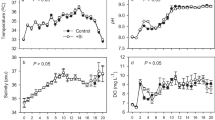
Temperature, DO and pH were observed at the constant level throughout the experiment (Table 2). On trace element supplementation, plankton density was significantly (p < 0.005) highest in HTS tanks.
Plankton Community
The plankton stock culture included Chlorophyceae consisting Cladymonas spp., Scenedesmus spp., Haematococcus spp., Eudorina spp., Selenastrum spp., Scenedesmus spp. and Volvox spp.; Trebouxiophyceae: Chlorella spp.; Zygnematophyceae consisting Closterium spp. and Bacillariophyceae consisted of Navicula spp., Fragilaria spp., Pinnularia spp., Nitzschia spp., Gyrosigma spp., Cymbella spp. and Gomphonema spp., Keratella quadrata, Keratella cochlearis, Daphnia spp., Copepoda adults and nauplii were also observed in juvenile marron tanks.
The ammonia, nitrite and nitrate fluctuated within acceptable range for marron (mean ± S. E.) as shown in Fig. 1A, B, C and D. Nitrate-nitrogen (NO3-N) concentration exponentially increased over the study time, whereas reactive phosphate (PO4) concentration was highest in HTS tanks (n = 4).
Water quality parameters of four different treatments. (A) TAN, (B) NO3-N, (C) NO2-N and (D) PO4. Different letters a, b and c indicate significant differences between treatments (p < 0.05). Abbreviations: CTL, control tanks; LTS, low trace element supplementation tanks; MTS, medium trace element supplementation tanks; HTS, high trace element supplementation tanks
Trace Element Concentrations in Water
Before supplementing trace elements, the mean concentration of pre-selected trace elements in tank water was Mn 0.0006 ± 0.0001, P 0.03 ± 0.00 and Si at 2.05 ± 0.07 mg*L−1. On supplementation and throughout the experiment, the dissolved concentration of some trace elements increased, for example Mn in MTS, where others either decreased or increased as depicted in Table 3. All of the trace elements that were supplemented showed an increase in concentration except Si under LTS. At the end of the experiment, the P concentration showed a progressive increase in concentration, whereas Si decreased, and Mn did not change.
Marron Growth, Survival and Moulting Days
Individual marron was weighed every fortnight, where total weight (g), OCL (cm) and total length (cm) were recorded for each marron. There were no significant differences in survival rate among the treatments. Marron SGR and WG were highest for HTS tanks (Table 4).
Marron Health Indices and Trace Element Concentrations in Haemolymph
The percentage of granular cells was significantly lower in LTS tank marron. HTS tank marron had significantly improved haemolymph indices compared to CTL and LTS (Table 5). Total concentrations of Fe and Mn in marron haemolymph were below the detectable level, whereas the Cu concentration was significantly higher in HTS tank marron compared to other treatments. Hepatopancreas moisture content and dry weight indices showed that the marron in HTC tanks were healthy.
Sequence Quality and Alpha-Beta Diversity of the Gut Microbiota
After quality trimming, an average of 98,486 sequences and 358 OTUs per samples were obtained, ranging from 78,786 to 126,446 reads and 288 to 448 OTUs from 12 samples, respectively. Phylogeny assignment of quality reads obtained 15 phyla, 78 families and 126 genera. Near about saturation rarefaction plot revealed that each sample was sequenced at enough depth to capture most of the microbial diversity (Fig. 2A). Among the alpha diversity indices (observed species, Shannon, Chao1), Chao1 diversity was found significantly higher in LTS group, compared to MTS and HTS. Bray-Curtis beta-dispersion on basis of relative abundance (weighted UniFrac) revealed distinct clustering of samples; PERMANOVA identified significant (P = 0.0233) impacts of trace element on gut microbiota.
Alpha-beta diversity of microbial communities in the marron hindgut at the conclusion of the experiment. (A) Rarefaction curve in terms of sequence depth; (B) observed species; (C) Shannon diversity; (D) Chao1 diversity; (E) Beta ordination plot showing clustering of samples based on relative abundance of OTUs. Abbreviations: CTL, control tanks; LTS, low trace elements; MTS, medium trace elements; HTS- High trace element supplementation
Relative and Significantly Abundant Bacterial Communities
At phylum level, the overall relative abundance was almost similar for all treatment groups and 12 samples with same dominant phyla, Proteobacteria. Higher abundance of Tenericutes was only observed for the LTS group, compared to all other treatments (Fig. 3A). At genus level, Vibrio was the most abundant bacteria in all samples, followed by Aeromonas, Candidatus bacilloplasma and C. hepatoplasma (Fig. 3B). The number of shared and unshared genus was found higher in LTS group, followed by HTS, MTS and CTL, respectively (Fig. 3C). Differential abundance at 0.05 level of significance revealed that Vibrio, C. Bacilloplasma and Aeromonas in CTL, LTS and MTS were replaced by Citrobacter, Acinetobacter and Pseudomonas in the HTS treatment (Fig. 3D).
Relative and differential abundance of microbial communities in four different groups of marron at the conclusion of the experiment. (A) Relative abundance at phylum level; (B) relative abundance at genus level; (C) number of shared and unshared genus in different treatments; (D) statistically significant bacteria at various taxa level in four different treatments. Abbreviations: CTL, control tanks; LTS, low trace elements; MTS, medium trace elements; HTS- high trace element supplementation
Discussion
This is the first study that analyzes the effects of trace element supplementation on plankton and growth, health indices and intestinal flora of marron cultured in the same body of water under laboratory conditions. Maintaining favourable water quality conditions allowed for optimum plankton growth in the tanks, similar to autumn season [14]. Also, maintaining plankton density at a static density throughout the experiment by regular harvesting and addition prevented the results from being influenced by various phases of phytoplankton growth or nutrient influx due to plankton crashes. The constant input of nitrogen and P from marron feed, waste and senescence of phytoplankton would have helped to promote growth of phytoplankton, as nitrogen and P can both frequently be limiting to primary productivity [45]. However, fluctuations in TAN are common in aquaculture systems [46], as nutrient concentrations build up until bacterial populations can be established to oxidize ammonia and nitrite.
Phytoplankton require P to build biomolecules such as proteins and nucleic acids [13], which are essential for their growth. Addition of P resulted in an increase in reactive phosphate in the HTS treatment, which yielded the highest density of plankton [11, 47]. Similarly, higher fertilizer inputs resulted in increased primary productivity and tilapia (Oreochromis niloticus) production in ponds [48]. In our study, enhanced phytoplankton growth was triggered by the medium and high concentration supplementation of trace elements. The HTS tanks had the highest plankton density, due to higher phosphate and Si levels, and thus providing greater quantities of sustenance for the zooplankton community. Potassium, sodium and chloride were also added as part of chemical compounds of Mn, Si and P, which are essential nutrients for plankton growth, and may have improved marron growth and health mediated through planktons in HTS tanks [9, 49,50,51]. The added P may have been utilized by the phytoplankton, periphyton grown on tank edges and eventually by zooplankton or be returned as detritus on the tank bottom [5]. The quantity of P and Si added in the HTS treatment tanks was high enough to trigger plankton density, while this response was much reduced in the LTS and MTS tanks. On P addition in three different concentrations (0.2, 0.1 and 0.05 g*m−3), Shrestha [47] observed no significant effect of different P levels of chlorophyll-α in tilapia ponds and also higher P addition did not increase the tilapia yield.
The phosphate concentration increased over the experiment time, likely due to the marron feed, plankton senescence and marron waste. The plankton biomass and species composition are regulated by the availability of nutrients, while most planktons are limited by P and nitrogen, and diatoms are often limited by silica [5, 13]. The diatom presence was more abundant only in HTS (supplementary data #1), and similar results of increase in diatoms on Si enrichment was observed by Nwankwegu [12]. Addition of trace elements, such as Mn, to water may enrich phytoplankton, rotifers and other zooplankton that feed on them, and in turn improve the diet of cultured animals such as marron [52].
Improved growth rate and health indices of marron in HTS can be associated to the higher plankton density; the plankton may have provided a food source in a fresh form or in the form of detritus, improving the marron nutrition. Natural productivity plays an important role in the production of white leg shrimp (Litopenaeus vannamei) and yabbies (C. albidus) [17, 53]. A study on red claw (C. quadricarinatus) juveniles showed the greatest increase in weight (%) and highest harvest mean weight in tanks with the use of zooplankton [54]. Comparatively, in a diet of zooplankton, unidentified bacteria and the macrophyte fed to juvenile red swamp crayfish (Procambarus clarkii), the higher growth rate was achieved with zooplankton [55]. It is likely that the marron grown in HTS obtained added nutrition from the high plankton density therein, although it is unclear whether they obtained nutrition from the phytoplankton, zooplankton or detritus. Past research has shown that zooplankton are an important food source for juvenile crayfish, while plant matter from phytoplankton and macrophytes may be less important [55]. However, plant material may provide nutritional elements not available in animal matter such as carotenoids [17]. Copepods, cladocerans and rotifers present in the tank water may have improved the health and growth of marron, as formulated feed often lacks important nutrients that the natural feeds contain [56]. Juvenile marron is thought to be poor filter feeders, so any phytoplankton probably would have been of value after settling on the cage bottom. Adult copepods or cladocerans may have been actively caught by the marron with their chelae or picked up off the cage bottom, while smaller zooplankton such as rotifers and copepod nauplii may have only been ingested as part of the detritus.
The SR and THC remained similar between treatments; however, these parameters are less associated with nutrition, and the results showed that all the treatment tanks had similar tank conditions. The HM (%) was significantly lower in MTS and HTS while Hid was significantly higher in HTS which suggests that marron from tanks MTS and HTS had good health condition as compared to CTL and LTS treatment tanks. The hepatopancreas is an important digestive gland and is used for storage of energy and nutrients [56] and maybe a good indicator of crayfish condition [57]. Higher plankton density in MTS and HTS may have increased the amount of nutrients and energy stored in the hepatopancreas reflecting an improvement in overall health of the juveniles.
The trace elements’ presence in tank water was not similar to their supplemented concentrations; the aquatic environmental dynamics are complex; the presence of plankton or bacteria may have absorbed or converted the trace elements. Also the supplementation of Mn and P and their presence in tank water did not affect their concentrations in marron haemolymph; Cu concentration was higher in marron from treatment HTS. Freshwater crustaceans accumulate and store the trace elements such as Ca and Cu in haemolymph [58]. More than 50% of the whole body Cu load is stored in haemolymph [59]. It is an essential micronutrient and is an integral part of the respiratory pigment haemocyanin [60]. Haemocyanin maintenance requires the accumulation of Cu in relatively large quantities than trace levels, for its transport and storage within the body [61]. The Cu accumulation in decapod crustaceans is regulated only up to the physiological threshold levels [60].
The gut bacterial communities have been reported to play a key role in digestion and immunity of aquatic animals, although the effects of dietary supplementation including probiotics, feed additives and protein sources on gut microbial communities of crayfish have been investigated [62, 63]. Different P levels had no effects on the alpha diversity measurements; however, the inclusion of different P levels on water influenced the gut microbial communities. Particularly, higher P level induced the growth of Acinetobacter, Pseudomonas and Citrobacter; the genera identified previously from marron gut [64] and widely reported as phosphate solubilizing bacteria in water [65, 66]. Bacteria can be transmitted from water to aquatic animals through feeding and the symbiotic correlation between water and gut microbiota of crayfish has been established in some studies [64, 67]. The phosphate solubilizing bacteria in the gut of marron in our study were likely sourced from the water in the culture tank. P inclusion also reduced the abundance for Vibrio, a genus commonly regarded as pathogenic for crayfish [68]. Though marron is a disease-free species [69], yet environmental pollution and habitat change constantly increase the chance of infections by emerging pathogen like Vibrio. Overall, different trace elements and plankton densities influenced marron growth, health and gut microbiota.
Conclusion
Trace elements influenced plankton growth with the addition of Si resulting in an increased diatom abundance. Increased plankton density was associated with improved juvenile marron growth, health indices and a more diverse gut microbiota. The individual roles of phytoplankton or zooplankton on marron growth and health cannot be isolated at this point. Feeding trials with phytoplankton or zooplankton separately may provide more insight on their overall effect on marron.
Data Availability
The experimental data will be provided on request and the raw data for marron gut microbiota in FASTQ files has been deposited to National Centre for Biotechnology Information (NCBI) BioProject under the accession number PRJNA682157.
Code Availability
Not applicable.
References
Machin D, Dearden M, Lacey P (2008) Marron aquaculture strategic extension campaign- an interim report. The Regional institure online publishing. http://www.regional.org.au/au/apen/2003/non_refereed/080machind.htm. Accessed 24 March 2020
Alonso AD (2009) Marron farming and environmental sustainability: Western Australia’s case. Environmentalist 29:388–397. https://doi.org/10.1007/s10669-008-9211-3
Abdel-Tawwab M, Abdelghany AE, El-Ayouty YM, El-Essawy A-FA (2002) Effect of different doses of inorganic fertilizer on water quality, primary productivity and production of Nile tilapia (Oreochromis niloticus) in earthen ponds. Qatar Univ Sci J 22:81–95 https://hdl.handle.net/10576/10328
Adhikari S (2003) Fertilization, soil and water quality management in small-scale ponds. Gher Revolution 8:1–52
Drenner RW, Threlkeld ST, Smith JD, Mummert JR, Cantrell PA (1989) Interdependence of phosphorus, fish, and site effects on phytoplankton biomass and zooplankton. Limnol Oceanogr 34:1315–1321
Tew KS, Conroy JD, Culver DA (2006) Effects of lowered inorganic phosphorus fertilization rates on pond production of percid fingerlings. Aquaculture 255:436–446. https://doi.org/10.1016/j.aquaculture.2006.01.003
Azim M, Little D (2006) Intensifying aquaculture production through new approaches to manipulating natural food. CAB Rev Perspect Agric Vet Sci Nutr Nat Resour 1:1–23. https://doi.org/10.1079/PAVSNNR20061062
Boyd CE (2018) Aquaculture pond fertilization. CAB Rev 13:1–12. https://doi.org/10.1079/PAVSNNR201813002
Boyd CE (2014) Silicon, diatoms in aquaculture. Glob Aquac Advocate 17:38–39
Goldman C (2010) Micronutrient elements (Co, Mo, Mn, Zn, Cu). In: Likens GE (ed) Biogeochemistry of inland waters. Academic Press, San Diego, pp 378–382
Li Y, Wang HZ, Liang XM, Yu Q, Xiao XC, Shao JC, Wang HJ (2017) Total phytoplankton abundance is determined by phosphorus input: evidence from an 18-month fertilization experiment in four subtropical ponds. Can J Fish Aquat Sci 74:1454–1461. https://doi.org/10.1139/cjfas-2016-0057
Nwankwegu AS, Li Y, Huang Y, Wei J, Norgbey E, Lai Q, Sarpong L, Wang K, Ji D, Yang Z (2020) Nutrient addition bioassay and phytoplankton community structure monitored during autumn in xiangxi bay of three gorges reservoir, china. Chemosphere 247:1–11. https://doi.org/10.1016/j.chemosphere.2020.125960
Pace ML, Lovett G (2013) Primary production: the foundation of ecosystems. In: Weathers KC, Strayer DL, Likens GE (eds) Fundamentals of ecosystem science, 2nd edn. Elsevier Academic Press, London, pp 27–51
Tulsankar SS, Cole AJ, Gagnon MM, Fotedar R (2020) Effects of seasonal variations and pond age on trace elements and their correlations with plankton productivity in commercial freshwater crayfish (Cherax cainii austin, 2002) earthen ponds. Aquac Res 51:1913–1922. https://doi.org/10.1111/are.14542
Wetzel RG (2001) Limnology: lake and river ecosystems, 3rd edn. Academic Press, San Diego, pp 239–328
Duffy RE, Godwin I, Nolan J, Purvis I (2011) The contribution of naturally occurring food items to the diet of Cherax destructor when fed formulated diets of differing protein levels. Aquaculture 313:107–114. https://doi.org/10.1016/j.aquaculture.2010.11.040
Jones P, Austin C, Mitchell B (1995) Growth and survival of juvenile Cherax albidus Clark cultured intensively on natural and formulated diets. Freshw Crayfish 10:480–493
Gonzalez A, Celada JD, Carral JM, Saez-Royuela M, Garcia V, Gonzalez R (2012) Effects of live artemia nauplii supplementation for different periods on survival and growth of juvenile signal crayfish Pacifastacus leniusculus in the first six months of intensive culture. N Am J Aquac 74:34–38. https://doi.org/10.1080/15222055.2011.649392
Sáez-Royuela M, Carral J, Celada J, Pérez J, González A (2007) Live feed as supplement from the onset of external feeding of juvenile signal crayfish (Pacifastacus leniusculus Dana. Astacidae) under controlled conditions. Aquaculture 269:321–327. https://doi.org/10.1016/j.aquaculture.2007.04.053
Sierp MT, Qin JG (2001) Effects of fertiliser and crayfish on plankton and nutrient dynamics in hardwater ponds. Hydrobiologia 462:1–7. https://doi.org/10.1023/A:1013184502119
Lambert CW (2019) Long-term effects of elevated manganese on Procambarus clarkii behavior. Dissertation, Marshall University
Jussila J, Henttonen P, Huner JV (1995) Calcium, magnesium and manganese content of noble crayfish (Astacus astacus (L.)) branchial carapace and its relationship to water and sediment mineral content of two ponds and one lake in Central Finland. Freshw Crayfish 10:230–238
Hossain MM, Huang H, Yuan Y, Wan T, Jiang C, Dai Z, Xiong S, Cao M, Tu S (2021) Silicone stressed response of crayfish (Procambarus clarkii) in antioxidant enzyme activity and related gene expression. Environ Pollut 274:1–11. https://doi.org/10.1016/j.envpol.2020.115836
Ackefors H (1996) The development of crayfish culture in Sweden during the last decade. Freshw Crayfish 11:627–654
Oweson CA, Baden SP, Hernroth BE (2006) Manganese induced apoptosis in haematopoietic cells of Nephrops norvegicus (L.). Aquat Toxicol 77:322–328. https://doi.org/10.1016/j.aquatox.2006.01.008
Kayath CA, Ibala Zamba A, Goma-Tchimbakala J, Mamonékéné V, Mombo Makanga GM, Lebonguy AA, Nguimbi E (2019) Microbiota landscape of gut system of guppy fish (Poecilia reticulata) plays an outstanding role in adaptation mechanisms. Int J Microbiol 2019:1–10. https://doi.org/10.1155/2019/3590584
Morrissy N (1990) Optimum and favourable temperatures for growth of Cherax tenuimanus (Smith 1912)(Decapoda: Parastoacidae). Mar Freshw Res 41:735–746
Ingram BA, Shiel RJ, Hawking JH (1997) Aquatic life in freshwater ponds: a guide to the identification and ecology of life in aquaculture ponds and farm dams in south eastern Australia. Co-operative Research Centre for Freshwater Ecology Albury, NSW, Australia
Canter-Lund H, Lund J (1995) Freshwater algae: their microscopic world explored. Biopress Ltd., Bristol, pp 1–360
Tulsankar SS, Cole AJ, Gagnon MM, Fotedar R (2021) Temporal variations and pond age effect on plankton communities in semi-intensive freshwater marron (Cherax cainii, Austin and Ryan, 2002) earthen aquaculture ponds in Western Australia. Saudi J Biol Sci 28:1392–1400. https://doi.org/10.1016/j.sjbs.2020.11.075
APHA (2012) (American Public Health Association) Standard methods for the examination of water and wastewater.
Baden SP, Neil DM (1998) Accumulation of manganese in the haemolymph, nerve and muscle tissue of Nephrops norvegicus (L.) and its effect on neuromuscular performance. Com Biochem Physiol Part A Mol Integr Physiol 119:351–359. https://doi.org/10.1016/S1095-6433(97)00437-6
Nugroho RA, Fotedar R (2013) Dietary organic selenium improves growth, survival and resistance to Vibrio mimicus in cultured marron, Cherax cainii (Austin, 2002). Fish Shellfish Immunol 35:79–85. https://doi.org/10.1016/j.fsi.2013.04.011
Fotedar R (1998) Nutrition of marron, Cherax tenuimanus (Smith) under different culture environments: a comparative study. Dissertation, Curtin University
Andrews S (2010) FastQC: a quality control tool for high throughput sequence data. https://www.bioinformatics.babraham.ac.uk/projects/fastqc/
Joshi N, Fass J (2011) Sickle-a windowed adaptive trimming tool for FASTQ files using quality. https://github.com/najoshi/sickle
Parikh HI, Koparde VN, Bradley SP, Buck GA, Sheth NU (2016) MeFiT: merging and filtering tool for illumina paired-end reads for 16S rRNA amplicon sequencing. BMC Bioinf 17:1–6. https://doi.org/10.1186/s12859-016-1358-1
Albanese D, Fontana P, De Filippo C, Cavalieri D, Donati C (2015) MICCA: a complete and accurate software for taxonomic profiling of metagenomic data. Sci Rep 5:1–7. https://doi.org/10.1038/srep09743
Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P, Peplies J, Glöckner FO (2012) The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res 41:590–596. https://doi.org/10.1093/nar/gks1219
Mirarab S, Nguyen N, Guo S, Wang L-S, Kim J, Warnow T (2015) PASTA: ultra-large multiple sequence alignment for nucleotide and amino-acid sequences. J Comput Biol 22:377–386. https://doi.org/10.1089/cmb.2014.0156
Price MN, Dehal PS, Arkin AP (2010) FastTree 2–approximately maximum-likelihood trees for large alignments. PLoS One 5:1–10. https://doi.org/10.1371/journal.pone.0009490
McMurdie PJ, Holmes S (2013) Phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PLoS One 8:1–11. https://doi.org/10.1371/journal.pone.0061217
Segata N, Izard J, Waldron L, Gevers D, Miropolsky L, Garrett WS, Huttenhower C (2011) Metagenomic biomarker discovery and explanation. Genome Biol 12:1–18. https://doi.org/10.1186/gb-2011-12-6-r60
Cole AJ, Tulsankar SS, Saunders BJ, Fotedar R (2019) Effects of pond age and a commercial substrate (the water cleanser™) on natural productivity, bacterial abundance, nutrient concentrations, and growth and survival of marron (Cherax cainii Austin, 2002) in semi-intensive pond culture. Aquaculture 502:242–249. https://doi.org/10.1016/j.aquaculture.2018.12.046
Downs TM, Schallenberg M, Burns CW (2008) Responses of lake phytoplankton to micronutrient enrichment: a study in two New Zealand lakes and an analysis of published data. Aquat Sci 70:347–360. https://doi.org/10.1007/s00027-008-8065-6
Thakur DP, Lin CK (2003) Water quality and nutrient budget in closed shrimp (Penaeus monodon) culture systems. Aquac Eng 27:159–176. https://doi.org/10.1016/S0144-8609(02)00055-9
Shrestha MK, Lin CK (1996) Phosphorus fertilization strategy in fish ponds based on sediment phosphorus saturation level. Aquaculture 142:207–219. https://doi.org/10.1016/0044-8486(95)01214-1
Diana JS, Dettweiler DJ, Lin CK (1991) Effect of Nile tilapia (Oreochromis niloticus) on the ecosystem of aquaculture ponds, and its significance to the trophic cascade hypothesis. Can J Fish Aquat Sci 48:183–190. https://doi.org/10.1139/f91-025
Padrão J, Mota DK, Nicolau A, Mota M (2016) Growth optimization of marine diatom Amphora sp. by tailoring silica and nitrate concentration. Front Mar Sci 5:1–12. https://doi.org/10.3389/conf.FMARS.2018.06.00100
Raven JA (2016) Chloride: essential micronutrient and multifunctional beneficial ion. J Exp Bot 68:359–367. https://doi.org/10.1093/jxb/erw421
Civitello DJ, Hite JL, Hall SR (2014) Potassium enrichment stimulates the growth and reproduction of a clone of Daphnia dentifera. Oecologia 175:773–780. https://doi.org/10.1007/s00442-014-2943-5
Nordgreen A, Penglase S, Hamre K (2013) Increasing the levels of the essential trace elements Se, Zn, Cu and Mn in rotifers (Brachionus plicatilis) used as live feed. Aquaculture 380:120–129. https://doi.org/10.1016/j.aquaculture.2012.11.032
Gamboa-Delgado J (2014) Nutritional role of natural productivity and formulated feed in semi-intensive shrimp farming as indicated by natural stable isotopes. Rev Aquac 6:36–47. https://doi.org/10.1111/raq.12023
Jones CM (1995) Production of juvenile redclaw crayfish, Cherax quadricarinatus (von Martens)(Decapoda, Parastacidae) III. Managed pond production trials. Aquaculture 138:247–255. https://doi.org/10.1016/0044-8486(95)00067-4
Brown PB, Wetzel JE, Spacie A, Konopka A (1992) Evaluation of naturally-occurring organisms as food for juvenile crayfish Procambarus clarkii. J World Aquacult Soc 23:211–216
Jussila J, Mannonen A (1997) Marron (Cherax tenuimanus) and noble crayfish (Astacus astacus) hepatopancreas energy and its relationship to moisture content. Aquaculture 149:157–161. https://doi.org/10.1016/S0044-8486(96)01425-1
Jussila J (1999) Comparison of selected condition indices between intermolt and post-molt marron, Cherax tenuimanus, of different feeding status raised under intensive culture conditions. J Appl Aquac 9:57–66. https://doi.org/10.1300/J028v09n03_05
Wilder MN, Jasmani S, Jayasankar V, Kaneko T, Aida K, Hatta T, Nemoto S, Wigginton A (2009) Hemolymph osmolality, ion concentrations and calcium in the structural organization of the cuticle of the giant freshwater prawn Macrobrachium rosenbergii: changes with the molt cycle. Aquaculture 292:104–110. https://doi.org/10.1016/j.aquaculture.2009.03.034
Depledge M, Bjerregaard P (1989) Haemolymph protein composition and copper levels in decapod crustaceans. Helgoländer Meeresun 43:207–223
Alcorlo P, Otero M, Crehuet M, Baltanás A, Montes C (2006) The use of the red swamp crayfish (Procambarus clarkii, Girard) as indicator of the bioavailability of heavy metals in environmental monitoring in the River Guadiamar (SW, Spain). Sci Total Environ 366:380–390. https://doi.org/10.1016/j.scitotenv.2006.02.023
Taylor H, Anstiss JM (1999) Copper and haemocyanin dynamics in aquatic invertebrates. Mar Freshw Res 50:907–931. https://doi.org/10.1071/MF99117
Foysal MJ, Fotedar R, Siddik MA, Tay A (2020) Lactobacillus acidophilus and L. plantarum improve health status, modulate gut microbiota and innate immune response of marron (Cherax cainii). Sci Rep 10:1–13. https://doi.org/10.1038/s41598-020-62655-y
Parrillo L, Coccia E, Volpe MG, Siano F, Pagliarulo C, Scioscia E, Varricchio E, Safari O, Eroldogan T, Paolucci M (2017) Olive mill wastewater-enriched diet positively affects growth, oxidative and immune status and intestinal microbiota in the crayfish, Astacus leptodactylus. Aquaculture 473:161–168. https://doi.org/10.1016/j.aquaculture.2017.02.013
Foysal MJ, Fotedar R, Tay C-Y, Gupta SK (2019) Dietary supplementation of black soldier fly (Hermetica illucens) meal modulates gut microbiota, innate immune response and health status of marron (Cherax cainii, Austin 2002) fed poultry-by-product and fishmeal based diets. PeerJ 7:1–24. https://doi.org/10.7717/peerj.6891
Qian Y, Shi J, Chen Y, Lou L, Cui X, Cao R, Li P, Tang J (2010) Characterization of phosphate solubilizing bacteria in sediments from a shallow eutrophic lake and a wetland: isolation, molecular identification and phosphorus release ability determination. Molecules 15:8518–8533. https://doi.org/10.3390/molecules15118518
Wan W, Qin Y, Wu H, Zuo W, He H, Tan J, Wang Y, He D (2020) Isolation and characterization of phosphorus solubilizing bacteria with multiple phosphorus sources utilizing capability and their potential for lead immobilization in soil. Front Microbiol 11:1–15. https://doi.org/10.3389/fmicb.2020.00752
Liu S, Qi C, Jia Y, Gu Z, Li E (2020) Growth and intestinal health of the red claw crayfish, Cherax quadricarinatus, reared under different salinities. Aquaculture 524:1–12. https://doi.org/10.1016/j.aquaculture.2020.735256
Bean N, Maloney E, Potter M, Korazemo P, Ray B, Taylor J, Seigler S, Snowden J (1998) Crayfish: a newly recognized vehicle for Vibrio infections. Epidemiol Infect 121:269–273. https://doi.org/10.1017/S0950268898001381
Ambas I, Suriawan A, Fotedar R (2013) Immunological responses of customised probiotics-fed marron, Cherax tenuimanus, (Smith 1912) when challenged with Vibrio mimicus. Fish Shellfish Immunol 35:262–270. https://doi.org/10.1016/j.fsi.2013.04.026
Acknowledgements
The authors are thankful to Mr. and Mrs. Hall for allowing the collection of plankton and marron from their farm.
Author information
Authors and Affiliations
Contributions
Smita Sadanand Tulsankar: conceptualization, designing and set up of the experiment, day to day feeding, data collection, data analysis and writing of the manuscript. Md. Javed Foysal: marron gut microbiota analysis, writing and reviewing the manuscript. Anthony J. Cole: plankton analysis, writing, reviewing and editing the manuscript. Monique Marthe Gagnon: designing experiment, supervision, reviewing and editing the manuscript. Ravi K. Fotedar: conceptualization, supervision, methodology validation, reviewing and editing of the manuscript.
Corresponding author
Ethics declarations
Ethics Approval
Animal ethics approval is not mandatory for the invertebrate animal studies at Curtin University, Australia. However, all the required protocols were followed while handling the animals, as per the guidelines of Animal Welfare Act, Western Australia and the Australian Code for the Care and Use of Animals for Scientific Purposes (NHMRC, 2013).
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Submission Declaration
The manuscript has not been published previously and accepted for publication elsewhere or it is not under consideration for publication elsewhere. The submitted manuscript has been approved by all authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 97 kb)
Rights and permissions
About this article
Cite this article
Tulsankar, S.S., Foysal, M.J., Cole, A.J. et al. A Mixture of Manganese, Silica and Phosphorus Supplementation Alters the Plankton Density, Species Diversity, Gut Microbiota and Improved the Health Status of Cultured Marron (Cherax cainii, Austin and Ryan, 2002). Biol Trace Elem Res 200, 1383–1394 (2022). https://doi.org/10.1007/s12011-021-02721-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-021-02721-2