Abstract
In the past, diatoms were considered the primary food source for copepods in both wild and aquaculture settings. However, recent studies have found that diatoms have defense mechanisms against predators, making them unsuitable as copepod feed. This study assessed the impact of addition of 100 μg L−1 of silicate to an inorganic nutrition formula containing 700 μg L−1 nitrogen, 100 μg L−1 phosphorus, and 100 μg L−1 iron. Our objective was to assess the impact of increased diatom abundance on copepod production. The experiment was carried out in 1000 L outdoor tanks over a period of 20 days, with adult Pseudodiaptomus annandalei copepods, introduced into each tank on the second day at an initial density of 10 ind. L−1. The results indicated that adding silicate reduced the prevalence of Chlorophyta, replaced by a higher proportion of Dinophyta, and eventually dominated by diatoms. While silicate had a positive effect on diatom culture, it had a negative effect on copepod production. Adding silicate to the inorganic fertilization method resulted in increased costs, leading to a significant increase in the unit production cost of copepods.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Diatoms contribute significantly to marine phytoplankton, accounting for 40% of primary productivity in the world's oceans (Falkowski et al. 1998). They are a vital food source for many zooplankton species (Lebour 1922; Sommer et al. 2002), especially copepods (Irigoien et al. 2000a, b, 2002), which possess specialized teeth capable of breaking diatom shells (Michels and Gorb 2015a, b; Miller et al. 1990). Traditionally, diatoms such as Chaetoceros spp. (Ohs et al. 2010), Thalassiosira spp. (Rahman et al. 2022), Nitzschia spp. (Pinto et al. 2001), and Melosira spp. (Rasdi and Qin 2018) have been used as feed for culturing copepods in aquaculture. However, other studies have challenged the idea that diatoms are the primary food for copepods (Ianora et al. 2003; Jones and Flynn 2005; Vargas et al. 2006). It has been discovered that some diatoms produce chemicals such as polyunsaturated aldehydes (PUAs) (Ianora et al. 2004; Pohnert 2005), non-volatile oxylipins (NVOs) (Ianora et al. 2015), and domoic acid (Lundholm et al. 2018; Tammilehto et al. 2015) as defense mechanisms against predators. The negative effects of these chemicals on copepod fertility (Asai et al. 2020) and nauplii development (Brugnano et al. 2016) hinder the copepod population after consuming diatoms.
Diatoms are unique among phytoplankton in their heavy reliance on silicon as the primary component of their shells. Silicon serves multiple critical roles in diatom biology, including facilitating nutrient absorption (Mitchell et al. 2013), enhancing light capture (Romann et al. 2015), protecting against UV radiation (Aguirre et al. 2018), and providing defense against predators (Hamm et al. 2003). Diatoms have specialized proteins (Lechner and Becker 2015) and metabolic mechanisms (Martin-Jézéquel et al. 2000) for silicon uptake and transportation. Silicon also regulates diatom division cycles (Vaulot et al. 1987) and is a limiting factor for their growth in natural environments (Dortch and Whitledge 1992; Yin et al. 2000).
Despite the importance of silicon for diatom growth, few studies have examined the relationship between adding silicate as an inorganic nutrient, large-scale diatom cultivation, and the mass production of copepods. Inorganic media such as F/2 (Camus et al. 2009; Guillard and Ryther 1962; Liu and Xu 2010; Nogueira et al. 2018) and Walne medium (Andersen 2005) highlight the need for silicate in diatom culture, with concentrations of 2970 μg L−1 and 6510 μg L−1, respectively. However, due to the high cost associated with the high concentration of elemental composition in these media, they are practical only for laboratory-scale production of copepods.
Pseudodiaptomus annandalei, a type of calanoid copepod commonly found in the Indo-Pacific region's subtropical to tropical coastal or brackish water ecosystems (Beyrend-Dur et al. 2011; Walter et al. 2006), including Taiwan (Blanda et al. 2015; Liao et al. 2001; Rayner et al. 2015), has been demonstrated to be suitable for mass production in artificial environments (Golez et al. 2004; GrØnning et al. 2019; Low et al. 2018) and used as a live feed for coral reef fish larvae (Chen et al. 2006; Lee et al. 2010). The mass production method currently in use, relying on organic matter fertilization, may be inconsistent and lead to copepod outputs that do not meet market demand (Hong and Tew 2022). In contrast, Hong and Tew (2022) proposed that inorganic fertilization is a more reliable method for producing copepods, offering benefits such as smaller size, greater purity, lower production costs, and no pathogens. Adding iron (Fe) to the inorganic fertilization method was further optimized by Hong et al. (2023), resulting in increased copepod production and nearly doubling net income. This study assesses the effects of adding silicate (+ Si) to the inorganic fertilization method on phytoplankton culture and mass-produced copepods, based on the potential of inorganic fertilization for copepod mass production and the silicate-diatom-copepod trophic pyramid. The objective is to develop strategies that enhance copepod production, achieving higher yields in a shorter timeframe, thereby benefiting the aquaculture industry.
Materials and methods
Experimental design
The experiment spanned 20 days and took place at the National Museum of Marine Biology and Aquarium (NMMBA), Taiwan. Ten outdoor tanks with a capacity of 1000 L each were utilized for the experiment. These tanks were filled with unfiltered natural seawater and equipped with air pumps to ensure oxygen supply and maintain water homogeneity. The approximate times of sunrise and sunset were 0550 h and 1800 h, respectively. Additionally, the light intensity at noon reached approximately 2900 μE m−2 s−1.
The control group and the + Si group both maintained the same fertilization conditions: N: 700 μg L−1, P: 100 μg L−1 (daily maintenance), and Fe: 10 μg L−1 (added daily). In addition, the + Si group received an extra daily maintenance of 100 μg L−1 silicate. Each group consisted of 5 replicates. The nitrogen (N) source was NH4NO3, the phosphorus (P) source was H3PO4 (Sigma-Aldrich, St. Louis, Missouri, USA), the iron (Fe) source was FeSO4•7H2O (J.T.Baker, Radnor, PA, USA) (Hong et al. 2023; Oliveira et al. 1999), and the silicon (Si) source was Na2SiO3•9H2O (Hayashi Pure Chemical Ind., Ltd., Chuo-ku, Osaka, Japan) (Nurhidayati et al. 2023), respectively.
The copepods (P. annandalei) used in this study were obtained from seawater pumped by the NMMBA and maintained as a purified population. To ensure their purity and abundance, copepod stocks were cultured in five 1000 L FRP tanks filled with freshly cultured phytoplankton. On day 2 of the experiment, adult copepods were collected from the stock tanks using a 25 μm mesh and introduced into each experimental tank at a density of 10 ind. L−1. This was done once the phytoplankton chl a concentration had reached a sufficient level, ensuring an adequate food supply for the copepods (Hong and Tew 2022; Hong et al. 2023).
Physicochemical analyses
During the experiment, water temperature, salinity, dissolved oxygen (DO), and pH were measured daily in situ using a YSI Professional Plus handheld multiparameter water quality meter (YSI, Yellow Springs, OH, USA) between 11:00 a.m. and 1:00 p.m. To analyze the concentrations of dissolved inorganic nitrogen (NH3-N, NO2-N, and NO3-N), phosphorus (PO4-P), total iron, and silica in all tanks, HACH products including ammonia kit (salicylate method 8155), nitrite kit (diazotization method 8507), phosphorus kit (ascorbic acid method 8048), total iron kit (USEPA FerroVer® method 8008), and silica kit (heteropoly blue method 8186) were used. Prior to analysis, NO3-N was converted to NO2-N using the method described by Pai and Riley (1994). Water samples were filtered through 0.45 μm pore size filter papers (Advantec, Tokyo, Japan) to remove impurities and then chemically colored using HACH products. The absorbance values were measured using a spectrophotometer (Synergy H4 Hybrid Reader, BioTek instruments, VT, USA) to convert the concentrations of these inorganic nutrients (Hong and Tew 2022).
Chl a analysis
Water samples (200 mL) were collected daily from each tank and filtered through 0.45 μm pore size filter papers to obtain phytoplankton. The filter papers were then extracted with 90% acetone and left overnight, following the method described by Parsons et al. (1984a, b). The chl a concentration in the extract was measured using a spectrophotometer (Hitachi U-5100, Hitachi, Tokyo, Japan).
Zooplankton sampling
By stirring the water layer, the zooplankton was evenly distributed, and then 1 L of water samples was filtered through a mesh with a pore size of 25 μm to collect the zooplankton. The collected zooplankton was preserved in a 5% formalin solution. Each day, non-copepod zooplankton, as well as copepod larvae and adults, were identified, classified, and counted. In this experiment, copepodites of P. annandalei were considered as adults and included in the calculations.
Copepod analysis
The dry weight of copepods in the 1000 L tank was estimated on a daily basis during the experiment. The calculation process involved several steps. Firstly, the daily average body lengths of copepod larvae and adults (n = 50) were used to determine the carbon weight using the geometric mean regression of body length and carbon weight as described in Rayner et al. (2015). Then, the relative ratio between carbon weight and dry weight, as described in Blanda et al. (2015), was applied to convert the carbon weight to dry weight. Finally, the calculated average individual dry weight was multiplied by the number of copepods present on that specific day to obtain the total dry weight of copepods in the tank.
The production cost of copepods was determined by dividing the total cost (in NT$) of the inorganic fertilizers (NH4NO3, H3PO4, FeSO4•7H2O, and Na2SiO3•9H2O) used until day 11 of the experiment by the maximum total dry weight of copepods reached in both groups on that day.
Phytoplankton analysis
To preserve the phytoplankton, 100 mL of water samples were treated with Lugol's solution (Sigma-Aldrich, St. Louis, Missouri, USA). The taxonomical composition of the preserved phytoplankton was subsequently determined by counting and identifying the different species using an optical microscope (Hong et al. 2023; Tew et al. 2016).
Statistical analysis
A one-way repeated measures analysis of variance (RM-ANOVA) was employed to assess the impact of various fertilization treatments on physicochemical and biological parameters, using the sampling date as the repeated factor. The unit production cost of copepods under different treatments was compared using a t-test. In cases where it was necessary to meet the assumptions of normality and homogeneity of variance, data were ln-transformed. All statistical analyses were performed using SigmaPlot 12.5, with a significance level of α < 0.05 considered statistically significant.
Results
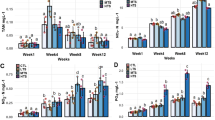
During the course of the experiment, the water temperature in all outdoor tanks was above 32 °C, with the highest recorded temperature reaching 36 °C. Despite being statistically significant, the mean temperature between the two groups remained comparable. The initial pH value in both groups was around 8.0, gradually increasing to approximately 9.5 by the end of the experiment. The salinity and dissolved oxygen levels exhibited similar patterns between the two groups, with no significant differences observed (p > 0.05) (Fig. 1).
The variations in temperature (a), salinity (b), pH (c), and dissolved oxygen (DO) (d) for both the control (n = 5) and + Si (n = 5) groups throughout the experiment. The values are presented as the mean ± SD. The P-value on each panel indicates the significance level (α = 0.05) of the different treatment effect based on repeated measure ANOVA
The initial concentrations of inorganic nitrogen and phosphorus were relatively low in both the control and + Si groups. The average concentrations of NH3-N, NO2-N, NO3-N, and PO4-P did not show any significant differences between the two groups (p > 0.05) as depicted in Fig. 2A, B, C, D. Subsequently, the concentrations of NH3-N, PO4-P, and SiO2-Si sharply decreased to nearly 0 μg L−1 on day 3 in both groups (Fig. 2A, D, E). The natural seawater used in this study had an initial silicate concentration of approximately 75 ± 3 μg L−1 (Fig. 2E). In the + Si group, the silicate concentration gradually accumulated and reached 653 ± 85 μg L−1 by the end of the experiment, while the control group maintained an average concentration of approximately 28 μg L−1 throughout the study period (Fig. 2E). With iron fertilization at a concentration of 10 μg L−1, the iron concentration in both groups fluctuated around 10 μg L−1 throughout the 20-day experiment, which was close to the detection limit.
The variations in Ammonium-nitrogen (NH3-N) (a), nitrite-nitrogen (NO2-N) (b), nitrate-nitrogen (NO3-N) (c)、phosphate (PO4-P) (d), and silicate (SiO2-Si) (e) for control (n = 5) and + Si (n = 5) groups throughout the experiment. The values are presented as the mean ± SD. The P-value on each panel indicates the significance level (α = 0.05) of the different treatment effect based on repeated measure ANOVA
During the initial two days of the experiment, both the + Si group and the control group exhibited low phytoplankton chl a concentrations (Fig. 3A). However, there was a rapid overnight increase followed by a subsequent decline. Although there was a statistically significant difference in chl a concentration between the + Si group and the control group (p < 0.05), the overall patterns of chl a performance were similar in both groups (Fig. 3A). The densities of non-copepod zooplankton did not significantly differ between the two groups (p > 0.05). They peaked on day 5 of the experiment and then gradually decreased to nearly 0 ind. L−1 (day 8 and beyond) (Fig. 3B).
The variations in phytoplankton chl a concentration (a) and non-copepod zooplankton density (b) for control (n = 5) and + Si (n = 5) groups throughout the experiment. The values are presented as the mean ± SD. The P-value on each panel indicates the significance level (α = 0.05) of the different treatment effect based on repeated measure ANOVA
The density of copepod nauplii exhibited no significant difference between the control and + Si groups (p > 0.05). Both groups showed a gradual increase in density after the start of the experiment, reaching a peak on day 13, followed by a decline, and began to increase again in day 20 (Fig. 4A). The adult density in tanks with + Si fertilization was significantly lower than in the control group (p < 0.05). After peaking on day 11, the density in both groups gradually declined and stabilized at approximately 709 ± 76 ind. L−1 in the control group and 666 ± 121 ind. L−1 in the + Si group (Fig. 4B). The pattern of total copepod dry weight mirrored that of adult density, with a significant decrease observed in the + Si group compared to the control (p < 0.05) (Fig. 4C). The unit cost of copepods produced by the + Si group was significantly higher than that of the control group (p < 0.05), at 1.22 ± 0.23 NT$ g−1 and 0.72 ± 0.08 NT$ g−1, respectively.
The variations in copepod nauplii (a), adult copepod (b), and total copepod dry weight (c) for control (n = 5) and + Si (n = 5) groups throughout the experiment. The values are presented as the mean ± SD. The P-value on each panel indicates the significance level (α = 0.05) of the different treatment effect based on repeated measure ANOVA
The analysis of phytoplankton species composition revealed that on day 2 in both groups, diatoms were the predominant group (> 99%), with Chaetoceros spp. accounting for over 99% of the total diatom abundance. The remaining diatoms consisted of Nitzschia spp., Skeletonema spp., Navicula spp., and Amphiprora spp. (Fig. 5). From day 4 onwards, the control group consistently exhibited a dominance of Chlorophyta (> 85%), with Chlamydomonas spp. being the most abundant species (> 99%) and Pandorina spp. present in smaller numbers (Fig. 5). However, on day 6, there was a noticeable increase in the proportion of Dinophyta observed in the control group (Fig. 5). In the + Si group, Chlorophyta was also among the main phytoplankton species observed. However, on days 4, 6, and 8 of the experiment, Dinophyta were more abundant compared to the control group. Particularly on day 6, Dinophyta accounted for over 96% of the total phytoplankton species, with Peridinium spp., Amphidinium spp., Protoperidinium spp., and Gymnodinium spp. being the dominant genera. Furthermore, starting from day 8, there was an increase in the proportion of diatoms, with Nitzschia spp. comprising over 91% of the total phytoplankton species in the last two days (Fig. 5). The control group exhibited a significantly higher percentage of Chlorophyta compared to the + Si group (Paired t-test, t = -3.254, P = 0.017). However, there were no significant differences observed in the percentages of diatoms, Dinophyta, and other algal groups between the treatments (P > 0.05).
Discussion
At present, there is a persistent shortage of copepods in aquaculture, emphasizing the importance of optimizing copepod culture techniques to enhance production (Hong and Tew 2022; Hong et al. 2023). The objective of this study is to develop strategies that enable higher copepod yields in a shorter period, which can have practical implications for the aquaculture industry. Adopting a one-time full-harvest approach and transitioning to the subsequent culture cycle may prove to be a more efficient method compared to the extensive approach. Moreover, this study did not focus on analyzing the specific qualities or nutritional composition of copepods.
Applying inorganic nitrogen and inorganic phosphorus at concentrations of N: 700 μg L−1 and P: 100 μg L−1 to natural seawater has been demonstrated to establish favorable water quality conditions, including stable pH and dissolved oxygen levels, and stimulate the growth of planktonic organisms such as unicellular microalgae and copepods (Tew et al. 2013, 2016; Kuo et al. 2021; Hong and Tew 2022). Hong et al. (2023) further refined this inorganic fertilization method by introducing iron, resulting in increased phytoplankton production as a food source and doubling copepod production, even under challenging conditions such as high temperatures of 36 °C and a rise in pH to 9.5 due to direct sunlight exposure. In the present study, the control group successfully replicated the high copepod production observed in the + Fe group of Hong et al. (2023). However, the addition of silicate in the + Si group significantly inhibited copepod growth compared to the control group.
Begum et al. (2015) observed that the maximum silicate concentration during diatom blooms in seawater reached 158 μg L−1, and Kranzler et al. (2019) found that diatom abundance increased when the local bay's silicate concentration exceeded 128 μg L−1. Moreover, Kuo et al. (2021) and Tew et al. (2016) demonstrated that diatoms can dominate without additional silicate supplementation. Analyzing local seawater data, this experiment revealed that the typical silicate concentration ranged from 30–300 μg L−1, indicating that natural seawater already contained sufficient silicate to support diatom growth. The experiment maintained a target concentration of 100 μg L−1 of silicate daily in the + Si group, resulting in diatoms becoming the dominant species in more than half of the experimental period. However, the rapid depletion of silicate by a large population of diatoms can lead to the replacement of diatoms by other phytoplankton species (Wang et al. 2018; Zhang et al. 2020). The study observed a pattern similar to the field environment, where diatoms exhibited strong competitiveness and rapidly expanded their populations following initial fertilization. Among the diatoms, Chaetoceros spp. emerged as the dominant species, which is commonly found in marine algal blooms (Kiørboe et al. 1998; Leising et al. 2005; Shikata et al. 2009). As the experiment progressed, nutrient levels, specifically NH3-N, PO4-P, and SiO2-Si, were quickly depleted by the growing diatom populations. This depletion resulted in a decline in diatom populations, as they rely on these nutrients for their growth. Consequently, there was a shift in the community composition, with Chlorophyta and Dinophyta becoming the dominant groups. Chlorophyta are commonly found in aquaculture ponds and play a crucial role in transferring energy to zooplankton (Blanda et al. 2017; Tulsankar et al. 2021). Specifically, the dominant chlorophyte species in this study were mainly Chlamydomonas spp., which are small in size (approximately 5 μm) and meet the dietary requirements of copepods at various growth stages (Berggreen et al. 1988).
However, the addition of silicate had a significant impact on the phytoplankton community. The bloom of dinoflagellates in the + Si group was more intense and prolonged compared to the control group, indicating that copepods would consume more dinoflagellates as a result. In the natural environment, dinoflagellates are known to be one of the main food sources for copepods (Yeh et al. 2020). It has also been reported that copepods prefer to feed on dinoflagellates rather than diatoms when given a choice (Kleppel et al. 1991). However, ingesting excessive amounts of dinoflagellates, some of which are toxic marine phytoplankton, may have detrimental effects on copepods, such as reduced motility (Delgado and Alcaraz 1999; Han et al. 2021). Additionally, dinoflagellates can produce aldehydes that negatively affect copepod reproduction (Li et al. 2019). These findings may explain why copepods in the + Si group of this study experienced negative effects due to the increased consumption of dinoflagellates during the population growth stage, resulting in the maximum density of adult copepods not reaching the level observed in the control group.
The utilization of inorganic fertilization methods can support the cultivation of various non-copepod zooplankton species (Hong and Tew 2022; Hong et al. 2023; Kuo et al. 2021), including ciliates, which have been recognized as excellent live feed sources (Das et al. 2012; Lee et al. 2018). Certain species can thrive under favorable environmental conditions (Hong and Tew 2022; Wu et al. 2018). However, since these non-copepod zooplankton may compete with copepods for phytoplankton resources (Berk et al. 1977; Calbet and Saiz 2005), it is important to assess their population growth status. In this experiment, several similarities were observed in the non-copepod zooplankton between the two groups, including an earlier growth peak compared to copepods, similar density performance, and an inability to compete with copepods. Both groups were predominantly composed of Strombidium spp. and tintinnid ciliates. However, maintaining the population density of these non-copepod zooplankton proved challenging, and further development of mass production techniques is required (Mendes et al. 2016; Pandey and Yeragi 2004).
Diatoms possess silica shells, which serve as a fundamental defense mechanism (Hamm et al. 2003) and are influenced by environmental silicate levels (Pančić et al. 2019). Changes in silicate concentration impact the mechanical strength of diatom shells, subsequently affecting copepod selectivity (Xu et al. 2021). Thicker diatom shells make them less appealing to copepods (Ryderheim et al. 2022), and diatoms can reinforce their shells in response to predation pressure (Pondaven et al. 2007). In the later stages of our experiment, intense predation pressure from copepods led to a near depletion of phytoplankton in both groups. As a result, diatoms gradually increased their dominance in the + Si group, comprising over 90% of the phytoplankton composition. This increase can be attributed to the promotion of diatom growth by added silicate and copepods’ preference for consuming chlorophytes and dinoflagellates as their primary food source. Consequently, copepods had a limited food selection and had to rely on diatoms with increasingly robust siliceous shells as their primary food source. As a consequence, the mean density of adult copepods was significantly lower in the + Si group compared to the control group where chlorophytes were dominant. Furthermore, the accumulation of high silicate concentration (> 600 μg L−1) observed at the end of the experiments in the + Si group was likely due to the release of silicate stored in diatoms into the water as they were gradually consumed by copepods and/or decayed.
The objective of this study was to assess the impact of incorporating silicate into an inorganic fertilization method on copepod mass production. The findings revealed that the addition of silicate altered the composition of phytoplankton, shifting from chlorophytes to diatoms, while unexpectedly increasing the density of dinoflagellates. Furthermore, the inclusion of silicate in the inorganic fertilization method resulted in a significant decline in adult copepod density and an increase in fertilizer expenses, consequently leading to a substantial rise in the unit production cost of copepods. Therefore, the inclusion of silicate in the inorganic fertilization method may not be necessary for achieving mass production of copepods. Moreover, the study's results support previous research indicating that diatoms possess effective defense mechanisms against their predators. As a result, enhancing growth of microscopic chlorophytes for large-scale copepod production may be a more viable approach, offering cost-effectiveness and reliability. The use of an inorganic fertilization method can be employed to support the cultivation of chlorophytes for this purpose.
Data availability
All data will be provided whenever requested.
References
Aguirre LE, Ouyang L, Elfwing A, Hedblom M, Wulff A, Inganäs O (2018) Diatom frustules protect DNA from ultraviolet light. Sci Rep 8:1–6. https://doi.org/10.1038/s41598-018-21810-2
Andersen RA (2005) Algal culturing techniques. Elsevier, Cambridge
Asai S, Sanges R, Lauritano C, Lindeque PK, Esposito F, Ianora A, Carotenuto Y (2020) De novo transcriptome assembly and gene expression profiling of the copepod Calanus helgolandicus feeding on the PUA-producing diatom Skeletonema marinoi. Mar Drugs 18:392. https://doi.org/10.3390/md18080392
Begum M, Sahu BK, Das AK, Vinithkumar NV, Kirubagaran R (2015) Extensive Chaetoceros curvisetus bloom in relation to water quality in Port Blair Bay, Andaman Islands. Environ Monit Assess 187:1–14. https://doi.org/10.1007/s10661-015-4461-2
Berggreen U, Hansen B, Kiørboe T (1988) Food size spectra, ingestion and growth of the copepod Acartia tonsa during development: Implications for determination of copepod production. Mar Biol 99:341–352. https://doi.org/10.1007/BF02112126
Berk S, Brownlee D, Heinle D, Kling H, Colwell R (1977) Ciliates as a food source for marine planktonic copepods. Microb Ecol 4:27–40. https://doi.org/10.1007/BF02010427
Beyrend-Dur D, Kumar R, Rao TR, Souissi S, Cheng SH, Hwang JS (2011) Demographic parameters of adults of Pseudodiaptomus annandalei (Copepoda: Calanoida): temperature–salinity and generation effects. J Exp Mar Biol Ecol 404:1–14. https://doi.org/10.1016/j.jembe.2011.04.012
Blanda E, Drillet G, Huang CC, Hwang JS, Jakobsen HH, Rayner TA, Su HM, Wu CH, Hansen BW (2015) Trophic interactions and productivity of copepods as live feed from tropical Taiwanese outdoor aquaculture ponds. Aquaculture 445:11–21. https://doi.org/10.1016/j.aquaculture.2015.04.003
Blanda E, Drillet G, Huang CC, Hwang JS, Højgaard JK, Jakobsen HH, Rayner TA, Su HM, Hansen BW (2017) An analysis of how to improve production of copepods as live feed from tropical Taiwanese outdoor aquaculture ponds. Aquaculture 479:432–441. https://doi.org/10.1016/j.aquaculture.2017.06.018
Brugnano C, Granata A, Guglielmo L, Minutoli R, Zagami G, Ianora A (2016) The deleterious effect of diatoms on the biomass and growth of early stages of their copepod grazers. J Exp Mar Biol Ecol 476:41–49. https://doi.org/10.1016/j.jembe.2015.11.015
Calbet A, Saiz E (2005) The ciliate-copepod link in marine ecosystems. Aquat Microb Ecol 38:157–167. https://doi.org/10.3354/ame038157
Camus T, Zeng C, McKinnon AD (2009) Egg production, egg hatching success and population increase of the tropical paracalanid copepod, Bestiolina similis (Calanoida: Paracalanidae) fed different microalgal diets. Aquaculture 297:169–175. https://doi.org/10.1016/j.aquaculture.2009.09.018
Chen Q, Sheng J, Lin Q, Gao Y, Lv J (2006) Effect of salinity on reproduction and survival of the copepod Pseudodiaptomus annandalei Sewell, 1919. Aquaculture 258:575–582. https://doi.org/10.1016/j.aquaculture.2006.04.032
Das P, Mandal SC, Bhagabati SK, Akhtar MS, Singh SK (2012) Important live food organisms and their role in aquaculture. Front Aquac 5:69–86. https://doi.org/10.13140/RG.2.2.21105.07523
Delgado M, Alcaraz M (1999) Interactions between red tide microalgae and herbivorous zooplankton: the noxious effects of Gyrodinium corsicum (Dinophyceae) on Acartia grani (Copepoda: Calanoida). J Plankton Res 21:2361–2371. https://doi.org/10.1093/plankt/21.12.2361
Dortch Q, Whitledge TE (1992) Does nitrogen or silicon limit phytoplankton production in the Mississippi River plume and nearby regions? Cont Shelf Res 12:1293–1309. https://doi.org/10.1016/0278-4343(92)90065-R
Falkowski PG, Barber RT, Smetacek V (1998) Biogeochemical controls and feedbacks on ocean primary production. Science 281:200–206. https://doi.org/10.1126/science.281.5374.200
Golez MN, Takahashi T, Ishimarul T, Ohno A (2004) Post-embryonic development and reproduction of Pseudodiaptomus annandalei (Copepoda: Calanoida). Plankton Biology and Ecology 51:15–25
GrØnning J, Doan NX, Dinh NT, Dinh KV, Nielsen TG (2019) Ecology of Pseudodiaptomus annandalei in tropical aquaculture ponds with emphasis on the limitation of production. J Plankton Res 41:741–758. https://doi.org/10.1093/plankt/fbz053
Guillard RR, Ryther JH (1962) Studies of marine planktonic diatoms: I. Cyclotella nana Hustedt, and Detonula confervacea (Cleve) Gran. Can J Microbiol 8:229–239. https://doi.org/10.1139/m62-029
Hamm CE, Merkel R, Springer O, Jurkojc P, Maier C, Prechtel K, Smetacek V (2003) Architecture and material properties of diatom shells provide effective mechanical protection. Nature 421:841–843. https://doi.org/10.1038/nature01416
Han J, Park JS, Park Y, Lee J, Shin HH, Lee KW (2021) Effects of paralytic shellfish poisoning toxin-producing dinoflagellate Gymnodinium catenatum on the marine copepod Tigriopus japonicus. Mar Pollut Bull 163:111937. https://doi.org/10.1016/j.marpolbul.2020.111937
Hong GK, Tew KS (2022) The advantages of inorganic fertilization for the mass production of copepods as food for fish larvae in aquaculture. Life 12:441. https://doi.org/10.3390/life12030441
Hong GK, Kuo J, Tew KS (2023) Iron fertilization can enhance the mass production of copepod, Pseudodiaptomus annandalei, for fish aquaculture. Life 13:529. https://doi.org/10.3390/life13020529
Ianora A, Poulet SA, Miralto A (2003) The effects of diatoms on copepod reproduction: a review. Phycologia 42:351–363. https://doi.org/10.2216/i0031-8884-42-4-351.1
Ianora A, Miralto A, Poulet SA, Carotenuto Y, Buttino I, Romano G, Casotti R, Pohnert G, Wichard T, Colucci-D’Amato L (2004) Aldehyde suppression of copepod recruitment in blooms of a ubiquitous planktonic diatom. Nature 429:403–407. https://doi.org/10.1038/nature02526
Ianora A, Bastianini M, Carotenuto Y, Casotti R, Roncalli V, Miralto A, Romano G, Gerecht A, Fontana A, Turner JT (2015) Non-volatile oxylipins can render some diatom blooms more toxic for copepod reproduction. Harmful Algae 44:1–7. https://doi.org/10.1016/j.hal.2015.02.003
Irigoien X, Harris R, Head R, Harbour D (2000a) The influence of diatom abundance on the egg production rate of Calanus helgolandicus in the English Channel. Limnol Oceanogr 45:1433–1439. https://doi.org/10.4319/lo.2000.45.6.1433
Irigoien X, Head R, Harris R, Cummings D, Harbour D, Meyer-Harms B (2000b) Feeding selectivity and egg production of Calanus helgolandicus in the English Channel. Limnol Oceanogr 45:44–54. https://doi.org/10.4319/lo.2000.45.1.0044
Irigoien X, Harris RP, Verheye HM, Joly P, Runge J, Starr M, Pond D, Campbell R, Shreeve R, Ward P (2002) Copepod hatching success in marine ecosystems with high diatom concentrations. Nature 419:387–389. https://doi.org/10.1038/nature01055
Jones RH, Flynn KJ (2005) Nutritional status and diet composition affect the value of diatoms as copepod prey. Science 307:1457–1459. https://doi.org/10.1126/science.1107767
Kiørboe T, Tiselius P, Mitchell-Innes B, Hansen JL, Visser AW, Mari X (1998) Intensive aggregate formation with low vertical flux during an upwelling-induced diatom bloom. Limnol Oceanogr 43:104–116. https://doi.org/10.4319/lo.1998.43.1.0104
Kleppel GS, Holliday D, Pieper R (1991) Trophic interactions between copepods and microplankton: a question about the role of diatoms. Limnol Oceanogr 36:172–178. https://doi.org/10.4319/lo.1991.36.1.0172
Kranzler CF, Krause JW, Brzezinski MA, Edwards BR, Biggs WP, Maniscalco M, McCrow JP, Van Mooy BA, Bidle KD, Allen AE (2019) Silicon limitation facilitates virus infection and mortality of marine diatoms. Nat Microbiol 4:1790–1797. https://doi.org/10.1038/s41564-019-0502-x
Kuo J, Chen CY, Han CC, Ju YM, Tew KS (2021) Analyses of diet preference of larval orange-spotted grouper (Epinephelus coioides) grown under inorganic fertilization method using next-generation sequencing. Aquaculture 531:735916. https://doi.org/10.1016/j.aquaculture.2020.735916
Lebour MV (1922) The food of plankton organisms. J Mar Biolog Assoc UK 12:644–677. https://doi.org/10.1017/S0025315400009681
Lechner CC, Becker CF (2015) Silaffins in silica biomineralization and biomimetic silica precipitation. Mar Drugs 13:5297–5333. https://doi.org/10.3390/md13085297
Lee CH, Dahms HU, Cheng SH, Souissi S, Schmitt FG, Kumar R, Hwang JS (2010) Predation of Pseudodiaptomus annandalei (Copepoda: Calanoida) by the grouper fish fry Epinephelus coioides under different hydrodynamic conditions. J Exp Mar Biol Ecol 393:17–22. https://doi.org/10.1016/j.jembe.2010.06.005
Lee IS, Ohs CL, Broach JS, DiMaggio MA, Watson CA (2018) Determining live prey preferences of larval ornamental marine fish utilizing fluorescent microspheres. Aquaculture 490:125–135. https://doi.org/10.1016/j.aquaculture.2018.01.035
Leising AW, Pierson JJ, Halsband-Lenk C, Horner R, Postel J (2005) Copepod grazing during spring blooms: Does Calanus pacificus avoid harmful diatoms? Prog Oceanogr 67:384–405. https://doi.org/10.1016/j.pocean.2005.09.008
Li J, Wang Y, Liang Y, Huang J, Liu Y, Lu J (2019) Production of aldehydes in diatoms and dinoflagellates and the detrimental effect on copepod grazers. Int J Agric Biol 22:763–768. https://doi.org/10.17957/IJAB/15.1127
Liao IC, Su HM, Chang EY (2001) Techniques in finfish larviculture in Taiwan. Aquaculture 200:1–31. https://doi.org/10.1016/S0044-8486(01)00692-5
Liu GX, Xu DH (2010) Feeding, egg production and laboratory culture of Schmackeria poplesia Shen (Copepoda: Calanoida). Aquac Res 41:1817-1826. https://doi.org/10.1111/j.1365-2109.2010.02566.x
Low JS, Chew LL, Ng CC, Goh HC, Lehette P, Chong VC (2018) Heat shock response and metabolic stress in the tropical estuarine copepod Pseudodiaptomus annandalei converge at its upper thermal optimum. J Therm Biol 74:14–22. https://doi.org/10.1016/j.jtherbio.2018.02.012
Lundholm N, Krock B, John U, Skov J, Cheng J, Pančić M, Wohlrab S, Rigby K, Nielsen TG, Selander E (2018) Induction of domoic acid production in diatoms—Types of grazers and diatoms are important. Harmful Algae 79:64–73. https://doi.org/10.1016/j.hal.2018.06.005
Martin-Jézéquel V, Hildebrand M, Brzezinski MA (2000) Silicon metabolism in diatoms: implications for growth. J Phycol 36:821–840. https://doi.org/10.1046/j.1529-8817.2000.00019.x
Mendes C, Chambel J, Lopes J, Calado R, Maranhão P (2016) Effect of different diets on growth of the ciliate protozoan Euplotes sp. Front Mar Sci. Conference. https://doi.org/10.3389/conf.FMARS.2016.04.00032
Michels J, Gorb SN (2015a) Mandibular gnathobases of marine planktonic copepods–feeding tools with complex micro-and nanoscale composite architectures. Beilstein J Nanotechnol 6:674–685. https://doi.org/10.3762/bjnano.6.68
Michels J, Gorb SN (2015b) Mandibular gnathobases of marine planktonic copepods—structural and mechanical challenges for diatom frustules. In: Hamm C (eds) Evolution of lightweight structures. Springer, Dordrecht, pp 59–73. https://doi.org/10.1007/978-94-017-9398-8_4
Miller C, Nelson D, Weiss C, Soeldner A (1990) Morphogenesis of opal teeth in calanoid copepods. Mar Biol 106:91–101. https://doi.org/10.1007/BF02114678
Mitchell JG, Seuront L, Doubell MJ, Losic D, Voelcker NH, Seymour J, Lal R (2013) The role of diatom nanostructures in biasing diffusion to improve uptake in a patchy nutrient environment. PLoS One 8:e59548. https://doi.org/10.1371/journal.pone.0059548
Nogueira N, Sumares B, Andrade CAP, Afonso A (2018) The effects of temperature and photoperiod on egg hatching success, egg production and population growth of the calanoid copepod, Acartia grani (Calanoida: Acartiidae). Aquac Res 49:93–103. https://doi.org/10.1111/are.13437
Nurhidayati T, Purnobasuki H, Muslihatin W, Ferdianto A, Purwani KI (2023) The influences of N and Si on growth and lipid content of microalgae Skeletonema costatum. AIP Conference Proceedings 2554:090005. https://doi.org/10.1063/5.0106112
Ohs CL, Chang KL, Grabe SW, DiMaggio MA, Stenn E (2010) Evaluation of dietary microalgae for culture of the calanoid copepod Pseudodiaptomus pelagicus. Aquaculture 307:225–232. https://doi.org/10.1016/j.aquaculture.2010.07.016
Oliveira MD, Monteiro MPC, Robbs PG, Leite SGF (1999) Growth and chemical composition of Spirulina maxima and Spirulina platensis biomass at different temperatures. Aquac Int 7:261–275. https://doi.org/10.1023/A:1009233230706
Pai SC, Riley JP (1994) Determination of nitrate in the presence of nitrite in natural waters by flow injection analysis with a non-quantitative on-line cadmium redactor. Int J Environ Anal Chem 57:263–277. https://doi.org/10.1080/03067319408027460
Pančić M, Torres RR, Almeda R, Kiørboe T (2019) Silicified cell walls as a defensive trait in diatoms. Proc Royal Soc B 286:20190184. https://doi.org/10.1098/rspb.2019.0184
Pandey BD, Yeragi S (2004) Preliminary and mass culture experiments on a heterotrichous ciliate, Fabrea salina. Aquaculture 232:241–254. https://doi.org/10.1016/S0044-8486(03)00459-9
Parsons TR, Maita Y, Lalli CM (1984a) Determination of chlorophylls and total carotenoids: spectrophotometric method. A manual of chemical and biological methods for seawater analysis. Pergamon Press, Oxford, pp 101–104. https://doi.org/10.1016/B978-0-08-030287-4.50032-3
Parsons TR, Maita Y, Lalli CM (1984b) Determination of chlorophylls and total carotenoids: spectrophotometric method. A manual of chemical & biological methods for seawater analysis, pp 101–104
Pinto CS, LlP S-S, Santos PJP (2001) Development and population dynamics of Tisbe biminiensis (Copepoda: Harpacticoida) reared on different diets. Aquaculture 198:253–267. https://doi.org/10.1016/S0044-8486(00)00582-2
Pohnert G (2005) Diatom/copepod interactions in plankton: the indirect chemical defense of unicellular algae. ChemBioChem 6:946–959. https://doi.org/10.1002/cbic.200400348
Pondaven P, Gallinari M, Chollet S, Bucciarelli E, Sarthou G, Schultes S, Jean F (2007) Grazing-induced changes in cell wall silicification in a marine diatom. Protist 158:21–28. https://doi.org/10.1016/j.protis.2006.09.002
Rahman NA, Teh JC, Katayama T, Wahid ME, Nagao N, Yamada Y, Takahashi K (2022) Effect of newly isolated high antioxidant diatom on the reproduction and stress tolerance of the marine copepod, Acartia erythraea under crowding stress. Aquac Res 53:5365–5374. https://doi.org/10.1111/are.16019
Rasdi NW, Qin JG (2018) Impact of food type on growth, survival and reproduction of the cyclopoid copepod Cyclopina kasignete as a potential live food in aquaculture. Aquac Int 26:1281–1295. https://doi.org/10.1007/s10499-018-0283-x
Rayner TA, Jørgensen NO, Blanda E, Wu CH, Huang CC, Mortensen J, Hwang JS, Hansen BW (2015) Biochemical composition of the promising live feed tropical calanoid copepod Pseudodiaptomus annandalei (Sewell 1919) cultured in Taiwanese outdoor aquaculture ponds. Aquaculture 441:25–34. https://doi.org/10.1016/j.aquaculture.2015.01.034
Romann J, Valmalette JC, Chauton MS, Tranell G, Einarsrud MA, Vadstein O (2015) Wavelength and orientation dependent capture of light by diatom frustule nanostructures. Sci Rep 5:1–6. https://doi.org/10.1038/srep17403
Ryderheim F, Grønning J, Kiørboe T (2022) Thicker shells reduce copepod grazing on diatoms. Limnol Oceanogr Letters 7:435–442. https://doi.org/10.1002/lol2.10243
Shikata T, Nukata A, Yoshikawa S, Matsubara T, Yamasaki Y, Shimasaki Y, Oshima Y, Honjo T (2009) Effects of light quality on initiation and development of meroplanktonic diatom blooms in a eutrophic shallow sea. Mar Biol 156:875–889. https://doi.org/10.1007/s00227-009-1131-3
Sommer U, Stibor H, Katechakis A, Sommer F, Hansen T, 2002. Pelagic food web configurations at different levels of nutrient richness and their implications for the ratio fish production: primary production. In: Vadstein O, Olsen Y (eds) Sustainable increase of marine harvesting: Fundamental mechanisms and new concepts. Springer, Dordrecht, pp 11–20. https://doi.org/10.1007/978-94-017-3190-4_2
Tammilehto A, Nielsen TG, Krock B, Møller EF, Lundholm N (2015) Induction of domoic acid production in the toxic diatom Pseudo-nitzschia seriata by calanoid copepods. Aquat Toxicol 159:52–61. https://doi.org/10.1016/j.aquatox.2014.11.026
Tew KS, Meng PJ, Lin HS, Chen JH, Leu MY (2013) Experimental evaluation of inorganic fertilization in larval giant grouper (Epinephelus lanceolatus Bloch) production. Aquac Res 44:439–450. https://doi.org/10.1111/j.1365-2109.2011.03051.x
Tew KS, Chang YC, Meng PJ, Leu MY, Glover DC (2016) Towards sustainable exhibits–application of an inorganic fertilization method in coral reef fish larviculture in an aquarium. Aquac Res 47:2748–2756. https://doi.org/10.1111/are.12725
Tulsankar SS, Cole AJ, Gagnon MM, Fotedar R (2021) Temporal variations and pond age effect on plankton communities in semi-intensive freshwater marron (Cherax cainii, Austin and Ryan, 2002) earthen aquaculture ponds in Western Australia. Saudi J Biol Sci 28:1392–1400. https://doi.org/10.1016/j.sjbs.2020.11.075
Vargas CA, Escribano R, Poulet S (2006) Phytoplankton food quality determines time windows for successful zooplankton reproductive pulses. Ecology 87:2992–2999. https://doi.org/10.1890/0012-9658(2006)87[2992:PFQDTW]2.0.CO;2
Vaulot D, Olson R, Merkel S, Chisholm S (1987) Cell-cycle response to nutrient starvation in two phytoplankton species, Thalassiosira weissflogii and Hymenomonas carterae. Mar Biol 95:625–630. https://doi.org/10.1007/BF00393106
Walter TC, Ohtsuka S, Castillo LV (2006) A new species of Pseudodiaptomus (Crustacea: Copepoda: Calanoida) from the Philippines, with a key to pseudodiaptomids from the Philippines and comments on the status of the genus Schmackeria. Proc Biol Soc Wash 119:202–221. https://doi.org/10.2988/0006-324X(2006)119[202:ANSOPC]2.0.CO;2
Wang B, Xin M, Wei Q, Xie L (2018) A historical overview of coastal eutrophication in the China Seas. Mar Pollut Bull 136:394–400. https://doi.org/10.1016/j.marpolbul.2018.09.044
Wu F, Dai M, Huang H, Qi Z (2018) Plankton ciliate community responses to different aquatic environments in Nan’ao Island, a representative mariculture base in the South China Sea. Mar Freshw Res 70:426–436. https://doi.org/10.1071/MF18153
Xu H, Shi Z, Zhang X, Pang M, Pan K, Liu H (2021) Diatom frustules with different silica contents affect copepod grazing due to differences in the nanoscale mechanical properties. Limnol Oceanogr 66:3408–3420. https://doi.org/10.1002/lno.11887
Yeh HD, Questel JM, Maas KR, Bucklin A (2020) Metabarcoding analysis of regional variation in gut contents of the copepod Calanus finmarchicus in the North Atlantic Ocean. Deep Sea Res Part II Top Stud Oceanogr 180:104738. https://doi.org/10.1016/j.dsr2.2020.104738
Yin K, Qian PY, Chen JC, Hsieh DP, Harrison PJ (2000) Dynamics of nutrients and phytoplankton biomass in the Pearl River estuary and adjacent waters of Hong Kong during summer: preliminary evidence for phosphorus and silicon limitation. Mar Ecol Prog Ser 194:295–305. https://doi.org/10.3354/meps194295
Zhang A, Zhang J, Liu S, Xuan J, Zhu Z (2020) Evaluation of silicic acid sources for spring diatom blooms on the continental shelf: Insights from stable silicon isotopes in the East China Sea. J Geophys Res Oceans 125:e2019JC015478. https://doi.org/10.1029/2019JC015478
Acknowledgements
This work was supported by grants from Taiwan Ministry of Science and Technology (MOST) with grant number MOST 110-2611-M-291-001, and Taiwan National Science and Technology Council (NSTC) with grant number NSTC 111-2611-M-110-028 to KST.
Funding
The research project was partially sponsored by Taiwan’s Ministry of Science and Technology (MOST) with grant number MOST 110–2611-M-291–001, and Taiwan’s National Science and Technology Council (NSTC) with grant number NSTC 111–2611-M-110–028 to KST.
Author information
Authors and Affiliations
Contributions
Guo-Kai Hong: Data curation, Formal analysis, Writing- Original draft preparation, Visualization, Investigation. Kwee Siong Tew: Conceptualization, Methodology, Supervision, Validation, Writing- Reviewing and Editing, Funding acquisition.
Corresponding author
Ethics declarations
Ethical approval
NA
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Handling editor: Brian Austin
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hong, GK., Tew, K.S. Assessing the effects of silicate addition on phytoplankton composition and copepod production in an inorganic fertilization system. Aquacult Int 32, 1119–1134 (2024). https://doi.org/10.1007/s10499-023-01208-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-023-01208-2