Abstract
Metal-oxide nanoparticles (NPs), as a new emerging technological compound, promise a wide range of usage areas and consequently have the potential to cause environmental toxicology. In the present work, aluminum (Al2O3), copper (CuO), and titanium (TiO2) nanoparticles (NPs) were administered via oral gavage to mature female rats (Rattus norvegicus var. albinos) for 14 days with a dose series of 0 (control), 0.5, 5, and 50 (mg/kg b.w./day). Enzyme activities of the antioxidant system such as catalase (CAT), superoxide dismutase (SOD), glutathione peroxidase (GPx), glutathione S-transferase (GST), and glutathione reductase (GR) in the liver were measured. Transmission electron microscope (TEM) images of the liver were taken to demonstrate NP accumulation and distribution in liver tissue. Data showed that all NPs caused some significant (P > 0.05) alterations in the activities of antioxidant enzymes. CAT activity increased after CuO and TiO2 administrations, while SOD activity decreased after Al2O3 administration. The activities of enzymes associated with glutathione (GR, GPx, GST) metabolisms were also significantly altered by NPs. GPx activity increased in rats received Al2O3, CuO NPs, while GR activity increased only by Al2O3. However, there were increases (TiO2) and decreases (CuO) in GST activity in the liver of rats. TEM images of the liver demonstrated that all NPs accumulated in the liver, even at the lowest dose. This study indicated that the antioxidant enzymes in the liver of rats were affected by all NPs, suggesting the antioxidant system of rats suffered after NP administration.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Recently, there is a major concern among environmentalists who are working on the fate of metal-oxide nanoparticles (NP), as they have a wide range of usage areas (Jeng and Swanson 2006; Janrao et al. 2014). Metal-oxide NPs have a size range of 1–100 nm and contain heavy metal ions and other organic and inorganic groups which make them unique in their functional groups, electronic properties, shape, surface structure and aggregation behavior. One of the most important characteristics of NPs is high surface to volume ratio, which are valuable for industrial usage. NPs are used in medicine, textile and electronic industry, filters, toothpaste, suntan cream, toys, moisturizer, packing products, white goods, and food industry and new areas for their usage are being emerged day by day (Handy and Benjamin 2007; AshaRani et al. 2009; Schrand et al. 2010). There are controversial data on their toxic effects resulting from NP studies from different laboratories, most being due to differences in the characteristics of different NPs. Administration routes (inhalation, subcutaneous, injection etc.) of NPs are also known to have significant effects on their toxicities in mammals (Shrivastava et al. 2014; Lei et al. 2015; Hu et al. 2015). Nevertheless, studies have shown that there is general agreement on the tissue accumulation of NPs after exposure to them, suggesting they can pass through the membranes of cells and interact with vital molecules (Jeng and Swanson 2006; Schrand et al. 2010). Most important factors in NP toxicity have been reported to be the administration route, dose, metal type, and size. Smaller sizes of the same NP have been shown to be more toxic than bigger sizes (Wang et al. 2013; Elle et al. 2013).
Oxidants are produced by the metabolisms of aerobic organisms and also additional oxidant inputs come from man-made products. In normal situation, there is a balance between oxidants and antioxidants in the normal metabolism. However, this balance may change if oxidant levels increased more than the capacity of the antioxidant defense system, causing oxidative stress due to changes in the delicate balance of oxidative/antioxidative elements. This generally happens when organisms receive man-made oxidants such as toxic metals and pesticides (Winston 1991; Martinez-Alvarez et al. 2005). There are several antioxidant elements to fight with the oxidants including enzymatic and non-enzymatic elements. For example, enzymatic ones include catalases (CAT; eliminates hydrogen peroxide to water), superoxide dismutase (SOD; converts superoxide anion radical into hydrogen peroxide), glutathione-S-transferase (GST; catalyzes glutathione and xenobiotic conjugation), and glutathione peroxidase (GPx; detoxifies hydrogen and organic peroxides) (Hidalgo et al. 2002; Sanchez et al. 2005). The present study aimed to investigate only the enzymatic ones in antioxidant defense system in rat’s metabolism.
Various fields of industry such as the chemical industry, medical, electronics, military, biomedicine, cosmetics, and food sectors use widely nanoparticles such as Al2O3, CuO, and TiO2 (Klaine et al. 2008; Janrao et al. 2014). Like other xenobiotic, NPs are also released to the environment and taken up by mammals via various routes and possibly cause adverse effects in their metabolisms. In this study, it is aimed to investigate the effects of Al2O3, CuO, and TiO2 NPs on the antioxidant enzyme activities in the liver in female Wistar rats.
Materials and methods
Experimental protocols and enzyme analysis
Experimental animals (female Wistar Albino rats, Rattus norvegicus var. albinos) were purchased from DETAUM of Cukurova University (Turkey). The animals were kept in a room with 12 h light and 12 h dark photoperiod regime at 22 ± 1.5 °C (moisture of 48 and 56%). The weight of the rats ranged between 190 and 220 g.
Female rats were allocated to ten cages and the weight of each group of animals did not differ significantly (P > 0.05). A total of 60 female rats was used in the experiments as each experimental group consisted of 6 rats, including a control group (6 rats). All NPs were sonicated and mixed vigorously with a sonicator (Bandelin HD2200, Germany) for 20 min on the ice and well-mixed suspensions were immediately applied to the related assay to minimize agglomeration. All NPs were given to the rats with 200-μl water via oral gavage. Three sub-lethal NP dose groups (0.5, 5, 50 mg/kg b.w./day) and a control group (received only 200-μl water) were used in the experiments. All rats were fed with standard rat feed. Rats were killed with high doses of anesthesia (ketasol 10%, Harson Lab. India) after 14 days and dissected carefully using sterile equipment. Liver tissues were put in disposable tubes and stored at − 80 °C until the analysis.
Homogenization of the liver was done in homogenization buffer (1:10, w/v) containing 100 mM potassium buffer (pH 7.4), 100 mM KCl, and 1 mM EDTA for 90 s and then homogenates were centrifuged at 10,000g (Hettich Universal 30 RF) for 30 min (+ 4 °C) to obtain supernatants. All analyses were carried out in the supernatants of liver tissues of female rats. CAT activity was measured using the method of Lartillot et al. (1988). The activity was expressed as μmol H2O2/mg prot./min. The method of Livingstone et al. (1992) was used to measure the GPx activity and enzyme activity was expressed as μmol/mg prot./min. GR activity was also measured using the method of Carlberg and Mannervik (1975). The activity was expressed as μmol/mg prot./min. The method of McCord and Fridovich (1969) was used to measure SOD activity and the activity was expressed as unit/mg prot. GST activity was measured using the method of Habig et al. (1974). The activity was expressed as μmol/mg prot./min. Total protein concentrations in the liver were determined by the method of Lowry et al. (1951), using bovine serum albumin as a standard. More details about the methods were given in our previous paper (Atli et al. 2016).
Characterization of nanoparticles
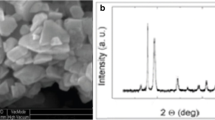
Metal-oxide nanoparticles (Al2O3, CuO and TiO2) were purchased from Nanografi (Turkey) or Sigma-Aldrich (Germany). Characteristics of NPs were as follows: Al2O3 (~ 40 nm, > 99% purity, > 30 m2/g surface area, 2.70 g/cm3 density), CuO (~ 40 nm, > 99% purity, > 20 m2/g surface area, 6.50 g/cm3 density), and TiO2 (~ 21 nm, > 99% purity, > 30 m2/g surface area, 4.26 g/cm3 density) (Canli et al. 2017). TEM images of the nanoparticles and liver tissue were obtained using a Jeol JEM-1010 TEM (80 kW) connected to a GATAN 782 ES500W Erlangshen camera. TEM images of Al2O3, CuO, and TiO2 NPs in stock solutions were presented in Fig. 1. X-ray diffraction (XRD) analysis of NPs were obtained using a Rigaku RadB SmartLab diffractometer system (CuKα1, λ = 1.5405 Å, 30 kV, 15 mA, 2θ = 10–90°, scanning rate 2°/min). XRD data showed that gamma Al2O3 NP had polycrystal structure and cubic phase, CuO NP had polycrystal structure, and monoclinic phase and TiO2 NP had polycrystal structure and tetragonal phase. Energy-dispersive X-ray (EDX) analysis was done using a field-emission scanning electron microscope (Zeiss/Supra 55 VP). Data showed that percentage atomic ratios of aluminum, copper, and titanium in Al2O3, CuO, and TiO2 nanoparticle powders were 38.26, 48.74, and 33.83, respectively. Data also showed that percentage weight ratios of aluminum, copper, and titanium in Al2O3, CuO, and TiO2 nanoparticle powders were 51.10, 79.06, and 60.49, respectively. Remaining percentages contained only oxygen atoms.
Statistical analysis
A statistical package program (SPSS 15, Chicago, IL, USA) was used for data analysis. All data were checked for their homogeneities to be able to evaluate their distributions. Data with normal or close to normal distribution were analyzed directly with one-way ANOVA test or a non-parametric test (Kruskal-Wallis test) was applied if they did not have a normal distribution. Data were presented as mean and standard error of mean and presented as figures indicating their statistical results (P < 0.05) on the figures.
Results
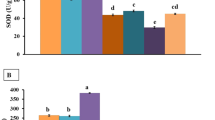
There was no considerable rat mortality (3 dead out of 60 rats, 5%). Rats also did not show any apparent health problem following the oral administrations of NPs for 14 days. Data were presented as mean of six measurements and associated standard errors for the antioxidant enzymes in the liver (Figs. 2 and 3). Statistically significant (P < 0.05) data were marked as asterisks on the figures. Data showed that all NPs caused significant (P > 0.05) alterations in antioxidant enzyme activities. CAT activity (control value 310 ± 17 U/mg protein) increased significantly after Cu-NP and Ti-NP administrations (Fig. 2a), while SOD activity (control value 223 ± 16 U/mg protein) decreased significantly after Al-NP administration (Fig. 2b). The activities of enzymes associated with glutathione metabolism (GR, GPx, GST) were also significantly altered by NPs, most alterations being increasing trend. GPx activity (control value 1.25 ± 0.04 U/mg protein) increased significantly after Al-NP and Cu-NP administrations (Fig. 3a). GST activity (control value 0.62 ± 0.03 U/mg protein) also increased significantly by Cu-NP, while its activity decreased by Ti-NP (Fig. 3b). GR activity (control value 0.08 ± 0.007 U/mg protein) was increased significantly by Al-NP, but not by Cu-NP and Ti-NP (Fig. 3c).
Effects of NPs a the activity of antioxidant enzymes associated with glutathione in the liver of rats received NPs orally for 14 days. GPx (a), GST (b), and GR (c). Data are expressed as mean (n = 6) ± standard errors. *Indicate significant (P < 0.05) differences resulted from the Duncan tests between control and individual group
TEM images of liver tissues were presented in Fig. 4 for rats received the lowest NP dose (0.5 mg/kg/day). TEM images of control (Fig. 4a) rats demonstrate that there was no NP presence in the liver, though the images (Fig. 4b–d) from rats received NPs clearly show the accumulation of all NPs in the liver.
Discussion
As NPs are able to pass from biological membranes and penetrate inside the cytoplasm and nucleus, they have the potential to cause the oxidative stress in animals (Jeng and Swanson 2006; Shrivastava et al. 2014). Thus, NP toxicity has been being studied extensively in different groups of animals in the toxicology laboratories in the world. The present data also demonstrated the accumulation of Al2O3, CuO, and TiO2, supporting the previous statements. Our previous papers dealing with accumulation of these NPs also support the present data; there were considerable NP accumulation in fish tissues (Canli et al. 2018).
Studies carried out so far have demonstrated that metal-oxide NPs have lower toxic effects compared to the similar levels of dissolved metals (Ema et al. 2016). Despite this, it seems that NPs are not innocent compounds and show serious toxic effects when taken in substantial amounts (Bahadar et al. 2016). In our previous studies, we demonstrated that orally administered Al2O3, CuO, and TiO2 NPs altered significantly the levels of serum biomarkers and erythrocyte enzyme activities in rats (Canli and Canli 2017; Canli et al. 2017). The effect order of NPs was TiO2 > CuO > Al2O3 for the antioxidant enzymes, though it changed in the serum biomarkers as TiO2 showed the lowest effects. There was an outstanding increase in the level of serum bilirubin, suggesting liver damage. Similarly, orally administered NPs (TiO2, ZnO, and Al2O3) altered the activities of antioxidant enzymes and caused elevated production of ROS in male mice (Shrivastava et al. 2014). The production of ROS has been shown in the literature, suggesting the failure of antioxidant system following different NP exposures of rats (Yu et al. 2014; Hu et al. 2015; Lei et al. 2015). Studies carried out with NPs showed that the size of NPs is a very important factor to take into account (Vinardell et al. 2015). Likewise, administration routes of NPs in mammals such as oral, inhalation subcutaneous, or vein injection also affect the effects caused by them (Wang et al. 2013; Elle et al. 2013; Jeng and Swanson 2006; Ema et al. 2016). So, it seems that toxic effects caused by NPs are likely to vary with the type, size, administration route, and biology of test animals. Interestingly, metals (e.g., Mg) with very low toxic effects might also cause serious toxicity in the antioxidant status of rats when they administered in the nanoparticle form (MgO) (Kiranmai and Reddy 2013).
The present study demonstrated that Al2O3, CuO, and TiO2 NPs accumulated in the liver of rats, suggesting they passed the intestinal wall, entered the blood stream, and distributed to the liver. Nonetheless, none of NPs caused considerable mortality within 14 days, even at higher concentrations. Like the present study, data regarding the antioxidant system mostly come from the liver, because the liver is known to be a detoxification organ of the body. All aerobic organisms face oxidative stress after the antioxidant defense system cannot cope with the extra oxidant production in the metabolisms (Winston 1991). When oxidative/antioxidative balance alters in anyways, oxidative stress would be unavoidable, causing serious harms for the vital molecules such as proteins, enzymes, or the genetic materials. Antioxidant enzymes, in this respect, play significant roles in the elimination of reactive oxygen species (ROS) produced by organisms itself or due to xenobiotic taken into the metabolisms. Several studies suggested the negative relationship between ROS increase and antioxidant system success after NP administrations in mammals (Sha et al. 2011; Yu et al. 2014; Hu et al. 2015; Lei et al. 2015).
In the present study, all NPs caused alterations in the activities of the antioxidant enzymes. There were increases in CAT activity after CuO and TiO2 administration, while SOD activity decreased after Al2O3 administration. The activities of GR, GPx, and GST, which are the enzymes of glutathione metabolisms, were also altered by NP administrations. The activities of these enzymes mostly increased except a decrease in GST activity of rats received CuO NP. Canli and Canli (2017) suggested that rats might have oxidative stress following the oral administration of Al2O3, CuO, and TiO2 NPs, as the total oxidant levels in the serum increased considerably, while the total antioxidant levels did not behave in the same manner. Syama et al. (2013) demonstrated that there was an increase in DNA adduct, a fall in cell viability and a decrease in antioxidant enzyme levels in the liver of mice after ZnO NP administration. Shrivastava et al. (2014) pointed the evidences on oxidative stress in the erythrocytes, liver, and brain of male mice following 21 days of oral administration of TiO2, ZnO, and Al2O3 NPs. Their findings support the present data as they showed the alterations in antioxidant enzyme activities related to significant production of ROS after NP administration. Song et al. (2012) studied the oxidative DNA damage and micronuclei induction in mice injected with several metal-oxide nanoparticles (AgO, CuO, Fe2O3, Fe3O4, TiO2). They found that all nanoparticle-treated groups showed the evidences of oxidative DNA damage and micronuclei formation and they concluded that the metal nanoparticles caused genotoxicity and oxidative stress. Abdelhalim and Jarrar (2012) demonstrated the histological effects of gold nanoparticles in the liver of rats, suggesting induced histological changes might be the reason for the injured hepatocytes due to gold NPs. They also indicated that histological alterations were dependent on the size of gold NPs, the smaller ones being more toxic. Histological toxicity of nanoparticles (chromium) was also demonstrated in the brain and kidney of Wistar rats by Fatima et al. (2017). The toxic effects of NPs might be due to substantial accumulation of NPs in the organ of animals (Park et al. 2015). The authors showed that aluminum NPs administered orally for 13 weeks accumulated in the liver and kidney of mice and caused pathological lesion in the liver and kidneys and toxic effects in some blood parameters. Similarly, Sarhan and Hussein (2014) indicated that injection of silver NPs to albino rats could have severe cytotoxic effects on the structure and function of these organs and caused a significant increase in serum creatinine, urea, and aspartate and alanine aminotransferases levels. Multiway toxic effects of NPs (titanium) were demonstrated by Rizk et al. (2017), as they found the oxidative stress, biochemical disturbances, and genotoxicity in mice after chronic treatment titanium NPs.
Conclusion
The present data showed that aluminum, copper, and titanium NPs are able to passed from intestinal gut wall and entered into the blood stream and accumulated in the liver of female rats following oral administrations. Following NP accumulation, several adverse effects of NPs on the activities of antioxidant enzymes in the liver were detected. Nevertheless, the present data also indicate that there are needs to carry out further research on NP toxicity in mammals to enlighten better the reasons for induced toxicity. Additionally, further studies on the effects of NPs should also be done for the other vertebrates with different ecological needs.
References
Abdelhalim MA, Jarrar BM (2012) Histological alterations in the liver of rats induced by different gold nanoparticle sizes, doses and exposure duration. J Nanobiotechnology 10:1–10. https://doi.org/10.1186/1477-3155-10-5
AshaRani PV, Mun GLK, Hande MP, Valiyaveettil S (2009) Cytotoxicity and genotoxicity of silver nanoparticles in human cells. ACS Nano 3:279–290. https://doi.org/10.1021/nn800596w
Atli G, Canli EG, Eroglu A, Canli M (2016) Characterization of antioxidant system parameters in four freshwater fish species. Ecotoxicol Environ Saf 126:30–37. https://doi.org/10.1016/j.ecoenv.2015.12.012
Bahadar H, Maqbool F, Niaz K, Abdollahi M (2016) Toxicity of nanoparticles and an overview of current experimental models. Iran Biomed J 20:1–11
Canli EG, Canli M (2017) Effects of aluminum, copper, and titanium nanoparticles on some blood parameters in Wistar rats. Turk J Zool 41:259–266. https://doi.org/10.3906/zoo-1512-23
Canli EG, Atli G, Canli M (2017) Response of the antioxidant enzymes of the erythrocyte and alterations in the serum biomarkers in rats following oral administration of nanoparticles. Environ Toxicol Pharmacol 50:145–150. https://doi.org/10.1016/j.etap.2017.02.007
Canli EG, Dogan A, Canli M (2018) Serum biomarker levels alter following nanoparticle (Al2O3, CuO, TiO2) exposures in freshwater fish (Oreochromis niloticus). Environ Toxicol Pharmacol 62:181–187. https://doi.org/10.1016/j.etap.2018.07.009
Carlberg I, Mannervik B (1975) Purification and characterization of the flavoenzyme glutathione reductase from rat liver. J Biol Chem 250:5475–5480
Elle RE et al (2013) Dietary exposure to silver nanoparticles in Sprague-Dawley rats: effects on oxidative stress and inflammation. Food Chem Toxicol 60:297–301. https://doi.org/10.1016/j.fct.2013.07.071
Ema M, Hougaard KS, Kishimoto A, Honda K (2016) Reproductive and developmental toxicity of carbon-based nanomaterials: a literature review. Nanotoxicology 10:391–412. https://doi.org/10.3109/17435390.2015.1073811
Fatima R, Akhtar K, Hossain MM, Ahmad R (2017) Chromium oxide nanoparticle-induced biochemical and histopathological alterations in the kidneys and brain of Wistar rats. Toxicol Ind Health 33:911–921. https://doi.org/10.1177/0748233717735266
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S-transferases the first enzymatic step in mercapturic acid formation. Biol Chem 249:7130–7139
Handy RD, Benjamin JS (2007) Toxic effects of nanoparticles and nanomaterials: implications for public health, risk assessment and the public perception of nanotechnology. Health Risk Soc 9:125–144
Hidalgo MC, Exposito A, Palma JM, de la Higuera M (2002) Oxidative stress generated by dietary Zn-deficiency: studies in rainbow trout (Oncorhynchus mykiss). Int J Biochem Cell Biol 34:183–193. https://doi.org/10.1016/S1357-2725(01)00105-4
Hu H, Guo Q, Wang C, Ma X, He H, Oh Y, Feng Y, Wu Q, Gu N (2015) Titanium dioxide nanoparticles increase plasma glucose via reactive oxygen species-induced insulin resistance in mice. J Appl Toxicol 35:1122–1132. https://doi.org/10.1002/jat.3150
Janrao KK, Gadhave MV, Banerjee SK, Gaikwad DD (2014) Nanoparticle induced nanotoxicity: an overview. Asian J Biomed Pharma Sci 4(32):1–7
Jeng HA, Swanson J (2006) Toxicity of metal oxide nanoparticles in mammalian cells. J Environ Sci Health A 41:2699–2711. https://doi.org/10.1080/10934520600966177
Kiranmai G, Reddy AR (2013) Antioxidant status in MgO nanoparticle-exposed rats. Toxicol Ind Health 29:897–903. https://doi.org/10.1177/0748233712446723
Klaine SJ, Alvarez PJ, Batley GE, Fernandes TF, Handy RD, Lyon DY, Lead JR (2008) Nanomaterials in the environment: behavior, fate, bioavailability, and effects. Environ Toxicol Chem 27:1825–1851. https://doi.org/10.1897/08-090.1
Lartillot S, Kedziora P, Athias A (1988) Purification and characterization of a new fungal catalase. Prep Biochem 18:241–246. https://doi.org/10.1080/00327488808062526
Lei R, Yang B, Wu C, Liao M, Ding R, Wang Q (2015) Mitochondrial dysfunction and oxidative damage in the liver and kidney of rats following exposure to copper nanoparticles for five consecutive days. Toxicol Res 4(2):351–364
Livingstone DR, Lips F, Martinez PG, Pipe RK (1992) Antioxidant enzymes in the digestive gland of the common mussel Mytilus edulis. Mar Biol 112:265–276. https://doi.org/10.1007/BF00702471
Lowry OH, Rosebrough N, Farra NJ, Randall RJ (1951) Protein measurements with the folin phenol reagent. J Biol Chem 193:265–275
Martinez-Alvarez RM, Morales AE, Sanz A (2005) Antioxidant defenses in fish: biotic and abiotic factors. Rev Fish Biol Fish 15:75–88. https://doi.org/10.1007/s11160-005-7846-4
McCord JM, Fridovich I (1969) Superoxide dismutase: an enzymatic function for erythrocuprein (hemocuprein). J Biol Chem 244:6049–6055
Park EJ, Sim J, Kim Y, Han BS, Yoon C, Lee S, Cho MH, Lee BS, Kim JH (2015) A 13-week repeated-dose oral toxicity and bioaccumulation of aluminum oxide nanoparticles in mice. Arch Toxicol 89:371–379. https://doi.org/10.1007/s00204-014-1256-0
Rizk MZ, Ali SA, Hamed MA, El-Rigal NS, Aly HF, Salah HH (2017) Toxicity of titanium dioxide nanoparticles: effect of dose and time on biochemical disturbance, oxidative stress and genotoxicity in mice. Biomed Pharmacother 90:466–472. https://doi.org/10.1016/j.biopha.2017.03.089
Sanchez W, Palluel O, Meunier L, Coquery M, Porcher JM, Ait-Aissa S (2005) Copper-induced oxidative stress in three-spined stickleback: relationship with hepatic metal levels. Environ Toxicol Pharmacol 19:177–183. https://doi.org/10.1016/j.etap.2004.07.003
Sarhan OM, Hussein RM (2014) Effects of intraperitoneally injected silver nanoparticles on histological structures and blood parameters in the albino rat. Int J Nanomedicine 9:1505–1517. https://doi.org/10.2147/IJN.S56729
Schrand AM, Rahman MF, Hussain SM, Schlager JJ, Smith DA, Ali SF (2010) Metal-based nanoparticles and their toxicity assessment. WIREs Nanomed Nanobiotechnol 2:544–568. https://doi.org/10.1002/wnan.103
Sha BY, Gao W, Wang SQ, Xu F, Lu TJ (2011) Cytotoxicity of titanium dioxide nanoparticles differs in four liver cells from human and rat. Compos Part B Eng 42:2136–2144. https://doi.org/10.1016/j.compositesb.2011.05.009
Shrivastava R, Raza S, Yadav A, Kushwaha P, Flora SJS (2014) Effects of sub-acute exposure to TiO2, ZnO and Al2O3 nanoparticles on oxidative stress and histological changes in mouse liver and brain. Drug Chem Toxicol 37:336–347. https://doi.org/10.3109/01480545.2013.866134
Song MF, Li YS, Kasai H, Kawai K (2012) Metal nanoparticle-induced micronuclei and oxidative DNA damage in mice. J Clin Biochem Nutr 50:211–216. https://doi.org/10.3164/jcbn.11-70
Syama S, Sreekanth PJ, Varma HK, Mohanan PV (2013) Effect of zinc oxide nanoparticles on cellular oxidative stress and antioxidant defense mechanisms in mouse liver. Toxicol Environ Chem 95:495–503. https://doi.org/10.1080/02772248.2013.789606
Vinardell MP, Sorde A, Diaz J, Baccarin T, Mitjans M (2015) Comparative effects of macro-sized aluminum oxide and aluminum oxide nanoparticles on erythrocyte hemolysis: influence of cell source, temperature, and size. J Nanopart Res 17:80–90. https://doi.org/10.1007/s11051-015-2893-9
Wang DG, Guo DD, Bi HS, Wu QX, Tian QM, Du YX (2013) Zinc oxide nanoparticles inhibit Ca2+-ATPase expression in human lens epithelial cells under UVB irradiation. Toxicol in Vitro 27:2117–2126. https://doi.org/10.1016/j.tiv.2013.09.015
Winston GW (1991) Oxidants and antioxidants in aquatic animals. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol 100:173–176. https://doi.org/10.1016/0742-8413(91)90148-M
Yu ZX, Ze Y, Wang L, Liu D, Hong J, Li B (2014) Changes of serum parameters of TiO2 nanoparticle-induced atherosclerosis in mice. J Hazard Mater 280:364–371. https://doi.org/10.1016/j.jhazmat.2014.08.015
Acknowledgements
This study was produced from PhD Thesis of Dr. E.G. Canli, except nanoparticle characterizations and supported by the research fund (FDK-2017-8197) of Cukurova University (Turkey). We thank Dr. G. Atli for her help.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Canli, E.G., Ila, H.B. & Canli, M. Response of the antioxidant enzymes of rats following oral administration of metal-oxide nanoparticles (Al2O3, CuO, TiO2). Environ Sci Pollut Res 26, 938–945 (2019). https://doi.org/10.1007/s11356-018-3592-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-3592-8