Abstract
This work aimed to evaluate the salivary concentration of chemical elements in patients undergoing orthodontic treatment with fixed orthodontic appliances and removable aligners. Twelve Angle Class I and II orthodontic patients undergoing treatment with conventional fixed appliances and 15 patients treated with removable aligners provided unstimulated whole saliva samples before treatment (pre) and after 3 months of treatment (post). The concentration and secretion rate of chemical elements in saliva were determined by total reflection X-ray fluorescence. Differences from pre to post and between groups were determined with the paired T test or Wilcoxon test, and two-way ANOVA, considering P < 0.05. The concentrations of S, Cl, and K decreased, while Zn increased significantly (P < 0.05) between pre and post treatment with the fixed appliance treatment. The salivary secretion rate of S was decreased from pre to post in the fixed appliance group. No differences in the concentration and secretion rate of chemical elements were detected from pre to post in the Invisalign group. Fe secretion rate presented an interaction between time and treatment, with lower secretion at post (P = 0.02) in the Invisalign group. Increased Br secretion rate and decreased Rb, Fe, P, and K in Invisalign patients suggested a better salivary electrolyte profile regarding periodontal bone remodeling. No significant alterations in ions associated with metal corrosion and inflammatory reactions were detected in orthodontic patients under dental plaque control.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The use of orthodontic fixed appliances can alter the composition of saliva trace elements and minerals when the metal alloys are exposed to the oral environment, temperature, eating habits, salivation, and mechanical agents [1, 2]. Metal corrosion may lead to the release of several metals and chemical elements into saliva [3,4,5,6]. Increased levels of titanium (Ti) have been detected in saliva samples after 6 months of orthodontic fixed appliances [7]. Dental plaque and dentifrices can also induce corrosion of orthodontic metallic appliances and the release of iron (Fe), chrome (Cr), nickel (Ni), and aluminum (Al) [8, 9]. A clinical study demonstrated that metallic ions released from metallic orthodontic appliances could be incorporated into oral mucosa and cause genotoxicity in epithelial cells [10]. The release of metal elements from metallic fixed appliances or altered electrolyte content in saliva may be associated with oxidative stress and predisposition to dental surface decalcification [11]. The use of orthodontic appliances is also associated with an increased predisposition to periodontal inflammation due to deficient plaque control and the dynamic of orthodontic forces [12]. In this sense, the concentration of chemical elements associated with bone turnovers, such as calcium (Ca), zinc (Zn), selenium (Se), manganese (Mn), and phosphorus (P) [13,14,15], may be modified during orthodontic treatment.
The use of removable aligners may be advantageous regarding salivary homeostasis, since as they can be easily cleaned, patients present better oral hygiene. In addition, removable aligners do not contain metallic components [16]. The Invisalign system (Align Technology, San José, CA, USA) aims to move the teeth through a series of removable aligners that can be removed to eat, drink, and brush teeth [16]. Lower levels of periodontal inflammation are observed since the aligners do not present any obstruction to oral hygiene, such as brackets, wires, elastic, or bands [17, 18]. Lower levels of inflammatory reaction may also be associated with the accuracy of orthodontic movements, avoiding excessive stress on periodontal tissues [19, 20]. In this way, it is hypothesized that treatment with removable aligners causes less disturbance in salivary ion content associated with bone metabolism, inflammatory defenses, and metallic corrosion. A study comparing fixed appliances and Invisalign aligners demonstrated increased disturbances in potassium (K), chlorine (Cl), Ca, and P electrolytes after 3 and 9 months only in fixed appliances [11]. The results suggested that fixed appliances induced more disturbances in the chemical composition of saliva than removable aligners.
The present study aimed to evaluate the concentration of chemical elements present in metallic ions, essential trace elements, and minerals in the saliva of patients undergoing conventional orthodontic treatment with metallic fixed appliances and removable aligners before and after 3 months of orthodontic treatment. The study hypothesis was that removable aligners induce fewer alterations in salivary electrolyte content.
Material and Methods
Experimental Design
This is a prospective cohort study in patients undergoing orthodontic treatment with removable aligners and conventional fixed appliances. Patients were selected from two orthodontic clinics, the Integrale (Londrina, PR, Brazil) and Rotta Institute (Presidente Prudente, SP, Brazil), from August to December 2018. The patients provided unstimulated whole saliva samples before and after 3 months of the beginning of orthodontic treatment. The participants gave written informed consent before data collection and all study procedures were approved by the Research Ethics Committee of the State University of Londrina, protocol no. 2.682.872.
The number of subjects per study group was determined based on changes in the saliva Ni concentration of patients undergoing orthodontic treatment with conventional fixed appliances before (1.1 ± 0.7 μg/l) and after 3 months of treatment (3.1 ± 1.3 μg/l) using graphite furnace atomic absorption spectrophotometry [4]. Ni is a metallic component of fixed appliances that is most susceptible to corrosion in the oral environment and is associated with hypersensitivity [21]. A minimum of five patients per group was required to achieve a statistical power of 80% and a lower alpha error of 5%. Considering possible dropouts, 15 patients per group were included in the sample.
Subjects
Thirty patients aged 16 to 35 years, paired for the use of fixed orthodontic appliances and removable aligners, were included in the study. Inclusion criteria were Angle Class I malocclusion with the indication for orthodontic treatment and no history of previous orthodontic treatment. Subjects requiring dental extraction, presenting clinical and radiographic signs of periodontal disease, external or internal dental resorption, a history of dental trauma, previous endodontic or orthodontic treatment, metallic restorations, and requiring the use of mini-implants were excluded from the selection. Smokers, diabetic patients, or those on continuous medication for inflammatory diseases and bone diseases were also excluded.
Before initiation and during orthodontic treatment, patients underwent dental prophylaxis, dental plaque control, and oral hygiene instruction. During orthodontic treatment, patients were under the supervision of two periodontists, to properly control dental plaque accumulation and avoid the development of gingival inflammation due to poor oral hygiene. During treatment, patients maintained healthy clinical aspects of gingival tissues, without signs of gingivitis and gingival recession, and no visible dental plaque accumulation.
Patients were classified into two study groups:
-
Conventional orthodontic treatment: patients treated with fixed appliances with metal brackets (Morelli Roth Max, Sorocaba, São Paulo, Brazil) and metallic orthodontic wires (Orthometric, Marília, São Paulo, Brazil). Metal brackets were composed of NiCr alloys and orthodontic wires by NiTi alloys.
-
Removable aligner treatment: use of removable aligners (Invisalign, Align Technology, San Jose, California, USA). The aligners were composed of a polyurethane polymer [22].
Saliva Sampling
Unstimulated whole saliva samples were collected at rest immediately before orthodontic appliance installation and after 3 months of treatment. Patients were instructed not to eat, drink, or brush their teeth 1 h before saliva collection. They rinsed their mouth for 1 min with distilled water and then spontaneously salivated into sterile graduated collection tubes for 2 min. Saliva samples were centrifuged at 4000g for 4 min and supernatants were immediately frozen at − 20 °C. Saliva samples were collected between 10:00 and 16:00, at the same hour, in pre and post moments.
Total Reflection X-ray Fluorescence
Saliva samples were thawed at 25 °C. Then, 10 μl of the sample and 10 μl of the ultrapure gallium (Ga) standard solution (cat. 170319, Merck Millipore, Darmstadt, Germany) were pipetted onto an acrylic disc and oven dried at 50 °C for 30 min. The background value of each disc was read before placing the sample and standard. After drying, each sample was read in triplicate, discounting background values. For each sample, the test-retest reliability was performed three times and was higher than 10% for each chemical element.
Saliva spectra were obtained by the total reflection X-ray fluorescence system—TXRF S2 Picofox (Bruker, Berlin, Germany)—located at the Applied Nuclear Physics Laboratory of the State University of Londrina (UEL) and evaluated by SPECTRA software (Bruker, Berlin, Germany). The saliva samples and standard were irradiated with a Mo target X-ray tube at 50 kV and 602 μA for a 200-s exposure time (Bruker, Berlin, Germany). The method consists of the detection and quantification (in the ppb range) of the characteristic radiation emitted by the chemical elements present in the sample after these are excited by the incident X-rays. The range elemental detection limits vary from aluminum (Z = 13) to yttrium (Z = 39). Detection accuracy varies from aluminum (Z = 13) to yttrium (Z = 39) for K-series radiation. The accuracy of the system was checked before each experiment with certified standard solutions recommended by the manufacturer.
Statistical Analysis
The Shapiro-Wilk test was used to evaluate data distribution. Data with normal distribution are described as mean and standard deviation, and data without normal distribution are described as medians and 25 to 75% quartiles. For comparison between pre and post moments, the Student’s t test (parametric) and Wilcoxon test (non-parametric) were performed. The effect of time and treatment was identified with the two-way ANOVA test. If the assumption of sphericity of data was violated by the Mauchly’s test, the Greenhouse-Geisser correction was applied. Differences were considered significant if P < 0.05.
Results
Three (10%) patients in the fixed appliance group did not attend the data collection after 3 months and were excluded from the study. Twenty-seven patients, 12 with conventional fixed braces (21.8 ± 7.8 years, 6 men) and 15 with removable aligners—Invisalign® (25.3 ± 12.1 years, 9 men)—were included in the study, with no significant between-group differences in age (P < 0.05, Student T test) and sex (P < 0.05, Fisher’s exact test).
All saliva samples contained P, sulfur (S), Cl, K, Ca, Zn, bromine (Br), and rubidium (Rb). Al, P, Ti, Cr, Mn, Fe, Ni, copper (Cu), strontium (Sr), and cobalt (Co) were not found in all saliva samples (Table 1) at pre and post moments, in both groups.
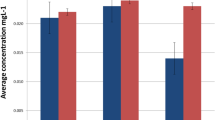
The concentrations of S, Cl, and K decreased, while Zn increased significantly (P < 0.05) between pre and post treatment with the fixed appliance treatment (Table 2). No differences in chemical element concentrations were detected in the removable aligner group from pre to post (Table 2). The chemical elements (Sr, Ti, Ni, Co) that were detected in less than four samples in pre or post moments in both study groups are not included in the tables and statistical analysis. The two-way ANOVA test detected effects of time with decreasing concentrations of P (P = 0.04), S (P = 0.04), K (P = 0.02), and Fe (P = 0.04) in both groups. A treatment effect was observed in reduced concentrations of Br (P = 0.03) in the removable aligners group compared with the fixed appliance group.
The secretion rate of chemical elements was determined through multiplying ion concentration by saliva flow rate. The S secretion rate significantly decreased in the fixed appliance group (Table 3). A time effect was observed through decreasing P (P = 0.03), K (P = 0.009), and Fe (P = 0.04), and increasing Rb (P = 0.009) and Br (P = 0.02) saliva secretion rate. Fe secretion rate presented an interaction between time and treatment, with lower secretion at post (P = 0.02) in the removable aligner group.
Discussion
This is the first study to identify the salivary electrolyte profile associated with periodontal status and metal corrosion in patients treated with removable aligners compared with metallic fixed appliances. The main findings of the study demonstrated that significant changes in ion concentration and secretion rates occur during orthodontic treatment, mainly in fixed appliance patients. Only non-significant changes occurred in the removable appliance treatment group from pre to post analysis. The use of the TXRF technique allowed the analysis of several elements of clinical importance in a single analysis. The chemical elements detected may be altered due to the corrosion of orthodontic alloys, alteration of oral homeostasis, local inflammation, and accumulation of microbial biofilm [11, 23,24,25]. In the present study, caution was taken to maintain satisfactory oral hygiene to avoid the development of gingivitis and altered salivary composition due to the excessive release of inflammatory products or dental plaque compounds. Oral hygiene control might reduce the influence of dental plaque corrosion on metallic alloys.
The presence of altered ionic profiles in saliva samples of orthodontic patients using fixed appliances presented contradictory results in the literature. Some studies evaluated metallic ion release in saliva from orthodontic patients during the initial stages of treatment and did not find significant differences [5, 26, 27]. On the other hand, increased levels of Fe, Ni, and Cr were detected a few hours after insertion of metallic fixed appliances or after long periods of treatment [28,29,30]. Different methods of analysis, sample collection, time, and type of treatment might account for the contradictory findings [3,4,5, 30]. An in vitro study demonstrated that metallic ions, including Co, Ni, Ti, Cr, and Fe, were released from metallic bands, wires, and brackets, over 2 to 3 months of immersion in artificial saliva [25, 31]. However, in the present study, no longer-term effects of chemical elements associated with metal corrosion were detected in the fixed appliance group. This result suggests that metal corrosion was no longer responsible for the ion imbalance in saliva.
Although S is released by salivary glands, increased levels of salivary S may be produced by dental plaque sulfur compound-producing bacteria and are associated with halitosis [32]. Clinical studies reported increased levels of volatile sulfur-containing compounds and malodor in orthodontic patients treated with fixed appliances [33, 34]. Moreover, an in vitro study demonstrated that corrosion of fixed appliances in artificial saliva released S ions [3]. Therefore, it was expected that a small increase in salivary S might occur in fixed appliance patients. Contrary to our hypothesis, a significant drop in S concentration was observed in fixed appliance patients. In Invisalign patients, no significant changes in S concentration were detected. This effect was expected since a previous study found no significant change in volatile sulfur compounds during Invisalign orthodontic treatment and observed a good level of oral hygiene in patients during treatment [16]. In the present study, patients were supervised by two periodontists in order to prevent dental plaque accumulation and gingivitis. This may have accounted for decreasing levels of sulfur-containing compounds in saliva. Moreover, the presence of sulfur-reducing bacteria accelerates metal corrosion [24], which could increase the release of metallic ions. However, reduced formation of oral biofilm may have reduced both products with sulfur content and sulfur-reducing bacteria, as well as metallic corrosion.
A previous study revealed that fixed appliances increased salivary concentrations of Ni and Cr [4, 6]. In the present study, the brackets selected, according to the manufacturer, are produced in a single body by the MIM (metal injection molding) system, in stainless steel (CrNi) with treated bases, and have low nickel content and stainless steel resistance. The results suggest that no substantial corrosion of the alloy occurred, so low levels of these elements were observed. As expected, no significant changes in other metallic ions were observed in Invisalign-treated patients, since the removable plates are made of urethane plastic materials [22].
Salivary electrolyte concentration may be altered in the first month after placement of fixed appliances, returning to normal levels after 3 months [35]. The authors reported that saliva flow rate and Cl concentration significantly increased, whereas P, Ca, and K concentrations decreased after placement of fixed appliances [35]. On the other hand, in another study, no differences in K concentration were found after 1 month of treatment [27]. A study demonstrated that some alterations in saliva ion content may occur after more than 3 months [11]. The authors reported decreased levels of Cl only after 9 months of treatment, but no effects on K after 3 and 9 months of fixed appliance orthodontic treatment [11]. In the present study, Cl and K concentrations significantly decreased in fixed appliance patients, suggesting conventional treatment could induce imbalance in salivary buffer capacity. However, this imbalance may not be attributed to modulation of Cl and K secretion by salivary glands, since salivary secretion rate was not significantly altered from pre to post. During production of hypertonic saliva in the acini of salivary glands, the sympathetic nervous system activates the transport of Cl− by chlorine channels and induces the subsequent passive transport of Na+ and K+ [36]. Thus, decreased levels of K were expected since Cl levels were reduced. The mechanism of altered Cl and K salivary levels in fixed appliance patients is not known, but may be related to increased stress caused by pain during treatment or altered saliva flow secretion [27, 35]. No significant differences in Cl and K were observed in patients treated with removable appliances, suggesting the treatment may have had a less negative impact on saliva secretion and saliva buffering.
A previous study reported increased levels of Ca and P after 3 and 9 months in fixed appliance and removable aligner-treated patients [11]. No significant differences were detected in either group in the present study. However, caution was taken to avoid dental plaque accumulation, which may account for dental decalcification, and increasing levels of Ca and phosphate.
Other factors associated with orthodontic treatment could contribute to changes in the concentration of chemical elements in saliva. Even controlling for dental plaque accumulation and preventing gingivitis, soft tissue inflammation caused by mechanical injury, bone remodeling of periodontal tissue, and inflammation caused by orthodontic movement could change salivary content [15, 17, 18, 37]. Ca, Zn, P, and Mg play an important role in bone metabolism, and differentiation and activation of osteoblasts and osteoclasts [13,14,15]. Other salivary ions such as Mn, Fe, Zn, Cu, and Se have been associated with antioxidant defenses and were altered during inflammatory processes [13, 38]. Results of the present study suggest that neither ions associated with bone turnover nor those associated with oxidative stress and inflammatory reactions were significantly affected by orthodontic treatments with fixed appliances or removable aligners.
A time effect was detected for decreased secretion rates of P, K, Fe, and Rb in patients treated with removable aligners and fixed appliance patients. Increased salivary K, Fe, and Rb concentrations were observed in patients with periodontal disease [39]. The presence of increased Fe concentration in saliva may be associated with hemoglobin release due to periodontal bleeding [39]. Since our patients were under periodontal surveillance, decreased levels of Fe released by gingival inflammation were expected. However, oral hygiene may be more easily performed by patients under treatment with removable aligners and may have reversed any subclinical sign of periodontal inflammation more quickly. Rb is a trace element present in bone and is associated with osteogenesis [40, 41]. The decreased secretion of Rb and P may be associated with different effects of fixed appliances and removable aligners in bone tissue remodeling. Increased salivary concentrations of Rb and K were also associated with periodontal disease, where increased inflammatory bone destruction is present [39].
A treatment effect favoring Br secretion rate was observed in Invisalign patients. Although the biological role of Br in live organisms is not completely understood, it is an essential element for remodeling of the basement membrane in the tissue remodeling process [42]. In this way, modulation of Br levels could improve tissue repair during orthodontic treatment, suggesting that orthodontic treatment with removable aligners may promote a better environment for periodontal repair.
Conclusions
We concluded that the electrolytic imbalances in S, Cl, and K observed in fixed appliance patients suggest the treatment influenced salivary buffer capacity. An increased Br secretion rate and decreased Rb, Fe, P, and K in the removable aligner group compared with changes in the fixed appliance group suggest a better salivary electrolyte profile regarding periodontal bone remodeling. No significant alterations in ions associated with metal corrosion and inflammatory reactions were detected in orthodontic patients under orthodontic treatment with concurrent dental plaque control, treated with removable aligners or metallic fixed appliances.
Data Availability
The authors will provide spreadsheets and research data if required by the editorial board at any moment, or to other researchers after publication of the text.
References
Martin-Camean A, Jos A, Puerto M, Calleja A, Iglesias-Linares A, Solano E, Camean AM (2015) In vivo determination of aluminum, cobalt, chromium, copper, nickel, titanium and vanadium in oral mucosa cells from orthodontic patients with mini-implants by inductively coupled plasma-mass spectrometry (ICP-MS). J Trace Elem Med Biol 32:13–20. https://doi.org/10.1016/j.jtemb.2015.05.001
Natarajan M, Padmanabhan S, Chitharanjan A, Narasimhan M (2011) Evaluation of the genotoxic effects of fixed appliances on oral mucosal cells and the relationship to nickel and chromium concentrations: an in-vivo study. Am J Orthod Dentofac Orthop 140(3):383–388. https://doi.org/10.1016/j.ajodo.2010.07.027
Mikulewicz M, Chojnacka K, Wozniak B, Downarowicz P (2012) Release of metal ions from orthodontic appliances: an in vitro study. Biol Trace Elem Res 146(2):272–280. https://doi.org/10.1007/s12011-011-9233-
Dwivedi A, Tikku T, Khanna R, Maurya RP, Verma G, Murthy RC (2015) Release of nickel and chromium ions in the saliva of patients with fixed orthodontic appliance: an in-vivo study. Natl J Maxillofac Surg 6(1):62–66. https://doi.org/10.4103/0975-5950.168224
Amini F, Rahimi H, Morad G, Mollaei M (2013) The effect of stress on salivary metal ion content in orthodontic patients. Biol Trace Elem Res 155(3):339–343. https://doi.org/10.1007/s12011-013-9812-7
Lages RB, Bridi EC, Perez CA, Basting RT (2017) Salivary levels of nickel, chromium, iron, and copper in patients treated with metal or esthetic fixed orthodontic appliances: a retrospective cohort study. J Trace Elemen Med Biol 40:67–71. https://doi.org/10.1016/j.jtemb.2016.12.011
Jurela A, Verzak Z, Brailo V, Skrinjar I, Sudarevic K, Jankovic B (2018) Salivary electrolytes in patients with metallic and ceramic orthodontic brackets. Acta Stomatol Croat 52(1):32–36. https://doi.org/10.15644/asc52/1/5
Kameda T, Oda H, Ohkuma K, Sano N, Batbayar N, Terashima Y, Sato S, Terada K (2014) Microbiologically influenced corrosion of orthodontic metallic appliances. Dent Mater J 33(2):187–195. https://doi.org/10.4012/dmj.2013-297
Brandao GA, Simas RM, de Almeida LM, da Silva JM, Meneghim Mde C, Pereira AC, de Almeida HA, Brandao AM (2013) Evaluation of ionic degradation and slot corrosion of metallic brackets by the action of different dentifrices. Dental Press J Orthod 18(1):86–93. https://doi.org/10.1590/s2176-94512013000100019
Fernandez-Minano E, Ortiz C, Vicente A, Calvo Guirado JL, Ortiz AJ (2011) Metallic ion content and damage to the DNA in oral mucosa cells of children with fixed orthodontic appliances. Biometals 24(5):935. https://doi.org/10.1007/s10534-011-9448-z
Dallel I, Ben Salem I, Merghni A, Bellalah W, Neffati F, Tobji S, Ben Amor A, Mastouri M (2020) Influence of orthodontic appliance type on salivary parameters during treatment. Angle Orthod 90:532–538. https://doi.org/10.2319/082919-562.1
Verrusio C, Iorio-Siciliano V, Blasi A, Leuci S, Adamo D, Nicolo M (2018) The effect of orthodontic treatment on periodontal tissue inflammation: a systematic review. Quintessence Int 49(1):69–77. https://doi.org/10.3290/j.qi.a39225
Dermience M, Lognay G, Mathieu F, Goyens P (2015) Effects of thirty elements on bone metabolism. J Trace Elemen Med Biol 32:86–106. https://doi.org/10.1016/j.jtemb.2015.06.005
Mahdavi-Roshan M, Ebrahimi M, Ebrahimi A (2015) Copper, magnesium, zinc and calcium status in osteopenic and osteoporotic post-menopausal women. Clin Cases Miner Bone Metab 12(1):18–21. https://doi.org/10.11138/ccmbm/2015.12.1.018
Saghiri MA, Orangi J, Asatourian A, Sorenson CM, Sheibani N (2016) Functional role of inorganic trace elements in angiogenesis part III: (Ti, Li, Ce, As, Hg, Va, Nb and Pb). Crit Rev Oncol Hematol 98:290–301. https://doi.org/10.1016/j.critrevonc.2015.10.004
Schaefer I, Braumann B (2010) Halitosis, oral health and quality of life during treatment with Invisalign((R)) and the effect of a low-dose chlorhexidine solution. J Orofac Orthop 71(6):430–441. https://doi.org/10.1007/s00056-010-1040-6
Miethke RR, Brauner K (2007) A Comparison of the periodontal health of patients during treatment with the Invisalign system and with fixed lingual appliances. J Orofac Orthop 63(3):223–231. https://doi.org/10.1007/s00056-007-0655-8
Miethke RR, Vogt S (2005) A comparison of the periodontal health of patients during treatment with the Invisalign system and with fixed orthodontic appliances. J Orofac Orthop 63(3):219–229. https://doi.org/10.1007/s00056-005-0436-1
Kravitz ND, Kusnoto B, BeGole E, Obrez A, Agran B (2009) How well does Invisalign work? A prospective clinical study evaluating the efficacy of tooth movement with Invisalign. Am J Orthod Dentofac Orthop 135(1):27–35. https://doi.org/10.1016/j.ajodo.2007.05.018
Ke Y, Zhu Y, Zhu M (2019) A comparison of treatment effectiveness between clear aligner and fixed appliance therapies. BMC Oral Health 19(1):24. https://doi.org/10.1186/s12903-018-0695-z
Saito M, Arakaki R, Yamada A, Tsunematsu T, Kudo Y, Ishimaru N (2016) Molecular mechanisms of nickel allergy. Int J Mol Sci 17(2). https://doi.org/10.3390/ijms17020202
Fang D, Li F, Zhang Y, Bai Y, Wu BM (2020) Changes in mechanical properties, surface morphology, structure, and composition of Invisalign material in the oral environment. Am J Orthod Dentofac Orthop 157(6):745–753. https://doi.org/10.1016/j.ajodo.2019.05.023
Imani MM, Mozaffari HR, Ramezani M, Sadeghi M (2019) Effect of fixed orthodontic treatment on salivary nickel and chromium levels: a systematic review and meta-analysis of observational studies. Dent J 7 (1). https://doi.org/10.3390/dj7010021
Mystkowska J, Niemirowicz-Laskowska K, Lysik D, Tokajuk G, Dabrowski JR, Bucki R (2018) The role of oral cavity biofilm on metallic biomaterial surface destruction-corrosion and friction aspects. Int J Mol Sci 19 (3). https://doi.org/10.3390/ijms19030743
Wendl B, Wiltsche H, Lankmayr E, Winsauer H, Walter A, Muchitsch A, Jakse N, Wendl M, Wendl T (2017) Metal release profiles of orthodontic bands, brackets, and wires: an in vitro study. J Orofac Orthop 78(6):494–503. https://doi.org/10.1007/s00056-017-0107-z
Eliades T, Athanasiou AE (2002) In vivo aging of orthodontic alloys: implications for corrosion potential, nickel release, and biocompatibility. Angle Orthod 72(3):222–237. https://doi.org/10.1043/0003-3219(2002)072<0222:IVAOOA>2.0.CO;2
Silva Andrade A, Marcon Szymanski M, Hashizume LN, Santos Mundstock K, Ferraz Goularte J, Hauber Gameiro G (2018) Evaluation of stress biomarkers and electrolytes in saliva of patients undergoing fixed orthodontic treatment. Minerva Stomatol 67(4):172–178. https://doi.org/10.23736/S0026-4970.18.04025-6
Gjerdet NR, Erichsen ES, Remlo HE, Evjen G (1991) Nickel and iron in saliva of patients with fixed orthodontic appliances. Acta Odontol Scand 49(2):73–78. https://doi.org/10.3109/00016359109005889
Matos de Souza R, Macedo de Menezes L (2008) Nickel, chromium and iron levels in the saliva of patients with simulated fixed orthodontic appliances. Angle Orthod 78(2):345–350. https://doi.org/10.2319/111806-466.1
Amini F, Jafari A, Amini P, Sepasi S (2012) Metal ion release from fixed orthodontic appliances--an in vivo study. Eur J Orthod 34(1):126–130. https://doi.org/10.1093/ejo/cjq181
Karnam SK, Reddy AN, Manjith CM (2012) Comparison of metal ion release from different bracket archwire combinations: an in vitro study. J Contemp Dent Pract 13(3):376–381. https://doi.org/10.5005/jp-journals-10024-1154
Krespi YP, Shrime MG, Kacker A (2006) The relationship between oral malodor and volatile sulfur compound-producing bacteria. Otolaryngol Head Neck Surg 135(5):671–676. https://doi.org/10.1016/j.otohns.2005.09.036
Costacurta M, Petrini M, Biferi V, Arcuri C, Spoto G, Docimo R (2019) The correlation between different techniques for the evaluation of oral malodour in children with and without orthodontic treatment. Eur J Paediatr Dent 20(3):233–236. https://doi.org/10.23804/ejpd.2019.20.03.12
Sokucu O, Akpinar A, Ozdemir H, Birlik M, Calisir M (2016) The effect of fixed appliances on oral malodor from beginning of treatment till 1 year. BMC Oral Health 16:14. https://doi.org/10.1186/s12903-016-0174-3
Li Y, Hu B, Liu Y, Ding G, Zhang C, Wang S (2009) The effects of fixed orthodontic appliances on saliva flow rate and saliva electrolyte concentrations. J Oral Rehabil 36(11):781–785. https://doi.org/10.1111/j.1365-2842.2009.01993.x
Proctor GB (2016) The physiology of salivary secretion. Periodontol 70(1):11–25. https://doi.org/10.1111/prd.12116
Karkhanechi M, Chow D, Sipkin J, Sherman D, Boylan RJ, Norman RG, Craig RG, Cisneros GJ (2013) Periodontal status of adult patients treated with fixed buccal appliances and removable aligners over one year of active orthodontic therapy. Angle Orthod 83(1):146–151. https://doi.org/10.2319/031212-217.1
Chakraborty S, Tewari S, Sharma RK, Narula SC, Ghalaut PS, Ghalaut V (2014) Impact of iron deficiency anemia on chronic periodontitis and superoxide dismutase activity: a cross-sectional study. J Periodontal Implant Sci 44(2):57–64. https://doi.org/10.5051/jpis.2014.44.2.57
Inonu E, Hakki SS, Kayis SA, Nielsen FH (2019) The association between some macro and trace elements in saliva and periodontal status. Biol Trace Elem Res 197:35–42. https://doi.org/10.1007/s12011-019-01977-z
Ouyang Z, Huang Q, Liu B, Wu H, Liu T, Liu Y (2019) Rubidium chloride targets Jnk/p38-mediated NF-kappaB activation to attenuate osteoclastogenesis and facilitate osteoblastogenesis. Front Pharmacol 10:584. https://doi.org/10.3389/fphar.2019.00584
Scancar J, Milacic R, Benedik M, Bukovec P (2000) Determination of trace elements and calcium in bone of the human iliac crest by atomic absorption spectrometry. Clin Chim Acta 293(1-2):187–197. https://doi.org/10.1016/s0009-8981(99)00239-9
McCall AS, Cummings CF, Bhave G, Vanacore R, Page-McCaw A, Hudson BG (2014) Bromine is an essential trace element for assembly of collagen IV scaffolds in tissue development and architecture. Cell 157(6):1380–1392. https://doi.org/10.1016/j.cell.2014.05.009
Acknowledgments
The authors thank the Integrale (Londrina, PR, Brazil) and Rotta Institute (Presidente Prudente, SP, Brazil) for authorization to recruit patients and technical support in data sampling.
Funding
This study was supported by the Annual Research Award Program, 2018, Align Technology, Inc. (São José, California – USA) in the form of purchasing consumables and equipment; and the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Brazil) for grants to ACZ (n. 1763037/2017) and BGD (n.1760771/2017).
Author information
Authors and Affiliations
Contributions
ACZ: Master student in Dentistry responsible for research project design, patient selection, radiographic analysis, hygiene and periodontal control of patients, collection of biological samples, data analysis, and scientific writing. BGD: Master student in Dentistry responsible for research project design, patient selection, hygiene and periodontal control of patients, collection of biological samples, data analysis, and scientific writing. DCMMS: Master student in Dentistry responsible for patient selection, radiographic analysis, dental plaque control, collection of biological samples, data analysis, and scientific writing. LOR: Orthodontist responsible for research project design, patient diagnostics, treatment planning and execution, data analysis, and scientific writing. EIJ: Doctor in physics responsible for TXRF analysis and data spectra analysis, statistical analysis, and scientific writing. ACA: Doctor in physics responsible for research project design, TXRF analysis, statistical analysis, and scientific writing. SPR: Research project coordinator.
Corresponding author
Ethics declarations
Conflicts of Interest
This work received financial support from Align technology Inc., manufacturer of Invisalign orthodontic appliances. This project was awarded the Annual Research Award Program, 2018, promoted by Align technology Inc. which financed the purchase of research reagents and equipment.
Ethics Approval
All study procedures were approved by the Research Ethics Committee of the State University of Londrina, protocol no. 2.682.872. The study was performed in accordance with the recommendations of the Declaration of Helsinki.
Consent to Participate
The participants gave written informed consent before data collection.
Consent for Publication
Not applicable.
Code Availability
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Campos Zeffa, A., Dias, B.G., Silva, D.C.M.S. et al. Influence of Conventional or Invisalign Orthodontic Treatment on Mineral and Trace Element Salivary Levels: Longitudinal Study with Total Reflection X-ray Fluorescence. Biol Trace Elem Res 199, 2565–2572 (2021). https://doi.org/10.1007/s12011-020-02396-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-020-02396-1