Abstract
Twelve Kunming mice were randomly divided into two groups (n = 6), and administered with distilled water containing 0 mg/L and 160 mg/L HgCl2 respectively, with an experimental period of 3 days. Our results showed that mercury exposure significantly reduced weight gain in mice (P < 0.01). Through pathological observation of cecum tissues, significant pathological changes were observed in cecum tissues of mice exposed to mercury. Furthermore, mercury exposure not only significantly increased malondialdehyde (MDA) content in mice (P < 0.01) but also significantly decreased superoxide dismutase (SOD) activity (P < 0.01) and glutathione peroxidase (GSH) level in mice (P < 0.01). Furthermore, high-throughput sequencing analysis showed that at the genus level some microbial populations including Clostridiales, Lactobacillus, Treponema, Oscillospira, and Desulfovibrio were significantly increased whereas some microbial populations including S24-7, Acinetobacter, and Staphylococcus were significantly decreased. Moreover, correlation analysis indicated that microorganisms were not correlated with biomarkers of oxidative stress. In summary, mercury exposure reduced the growth performance of mice, resulting in gut microbiota alterations, and led to oxidative stress by increasing the concentration of malondialdehyde (MDA) and decreasing the concentration of superoxide dismutase (SOD) and glutathione peroxidase (GSH).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mercury has been considered by World Health Organization (WHO) to be one of the most toxic heavy metals to humans, livestock, and poultry, and one of the global environmental hazards. Mercury, as the main pollutant of the environment, accumulates in human and animal bodies in the form of food chain, causing harm to human health. In the 1960s, residents of Minamata, Japan, developed severe mental illness from eating seafood contaminated with methylmercury [1]. Mercury pollution events have also occurred in other countries, such as Iraq, Brazil, Indonesia, and the USA. In recent years, with the rapid development in industry and agriculture, China’s mercury consumption has been increased significantly [2]. In addition, combustion is the most important source of mercury in the global atmosphere, with about 75% of mercury emissions coming from burning fossil fuels, especially in China, where a large amount of mercury may pose a threat to the global environment [2]. In Fig. 1, we made the map of global mercury emissions by country and sector based on UNEP Global Mercury assessment data, which was retrieved on December 20, 2019, via the link https://public.tableau.com/shared/5S8FCT7QX?:display_count=no&:showVizHome=no.
It has been confirmed that mercury exposure is associated with multiple organ injury [3, 4]. The gut is the organ that communicates with the outside world and is the first to suffer when mercury enters the body through the mouth. Oxidative stress is also thought to be an important marker of intestinal injury [5]. For example, Bollengier et al. have pointed out that oxidative stress significantly reduces the height, surface area, and volume of jejunum villi in young hens, thus affecting intestinal function [6]. Therefore, when mercury destroys the antioxidant defense ability and produces too many free radicals, it leads to the changes in oxidative stress and oxidase activity in the body, and also causes gastrointestinal diseases in animals. The gut is the main organ for the digestion, absorption, and metabolism of nutrients in the human body. It is also the place where most microorganisms gather in the body [7]. When the intestinal environment changes, the intestinal microbial community also changes, and some scholars have found that lead, phosphorus, copper, Fe, and so on can cause changes in intestinal microorganisms [8,9,10,11]. In recent years, there have been many researches on oxidative stress caused by heavy metals or gut microbiota. However, there is still a lack of articles to analyze the correlation between oxidative stress and gut microbiota.
Thus, we analyzed the effects of administering oral mercury for 3 days on oxidative stress and gut microbiota in mice. Firstly, we observed the intestinal morphology of mice. Secondly, we observed the changes of gut microbiota through 16S rRNA Miseq sequencing. Finally, we measured the expression of some biomarkers of oxidative stress and analyzed the correlation between biomarkers of oxidative stress and gut microbiota. Our result provided an important basis for the future study of the relationship between oxidative injury to the intestine and gut microbiota under mercury exposure.
Materials and Methods
Animal Feeding and Sample Collection
Twelve 8-week-old female mice were obtained from the Experimental Animal Center of Nanchang University. Each mouse weighed about 25 g. After a week’s adaptation period, the mice were randomly divided into two groups: control group and Hg group. The mice were placed in a constant environment (temperature 25 °C, humidity 42 °C, light/dark cycle 12 h and 12 h, with plenty of water and food). All mice were reared according to the feeding standard of mice in the laboratory of School of Animal Science and Technology, Jiangxi Agricultural University. The mice in the control group were fed with 5 ml of pure water every day, and the mice in the Hg group were fed with 5 ml of water containing HgCl2 (160 mg/L) every day, and the experimental period was 3 days. The selection of HgCl2 dose is based on the reasonable dose of acute toxicity test in mice (1/20 ~ 1/5 of the median lethal dose of LD50) [4].
The mice fasted food and water within 12 h before sampling. In addition, 1 ml of blood was collected from each mouse’s eyeball. After the blood was placed for half an hour, the blood was centrifuged at 3500 r/min for 10 min at 4 °C [12]. The supernatant was collected, and the purpose of separating serum was achieved. The contents of the cecum were collected, and then, the cecal tissue was cut into 1cm3 cubes and fixed with slice fixation solution. All experimental protocols were approved by the Committee for the Care and Use of Experimental Animals, Jiangxi Agricultural University, Jiangxi, China (no. JXAULL-20200098).
Histopathological Examination
Cecal tissues were rinsed with saline, and then fixed in 4% paraformaldehyde. The fixed cecal tissue was rinsed, dehydrated, made transparent, and then waxed. The paraformaldehyde-fixed samples were embedded in paraffin and stained with hematoxylin and eosin (H&E). The crypt depth and villus height were measured by image processing and analysis system. Goblet cells were identified and enumerated by light microscope for every 100 villous epithelial cells in the same field [13].
Transmission Electron Microscopy
The cecal tissues were rapidly cut into pieces smaller than 1 mm3, and immersed in 2.5% glutaraldehyde solution at 4 °C. These fragments were immersed in a special electron microscope fixative. The fixed specimens were sent to Wuhan Seville Biotechnology Co., Ltd. for transmission electron microscope preparation, projection microscope observation, and image acquisition.
Measurement of Biomarkers of Oxidative Stress
The blood samples place at 37 °C for 2 h and then centrifuge the samples at 3500 r at 4 °C for 15 min to the separation of serum and stored at − 20 °C before analysis. Then, according to the instructions of the manufacturer of Nanjing Jincheng Company, the separated serum was used to determine SOD activity, MDA, and GSH concentration.
16S rRNA Miseq Sequencing and Bioinformatic Analysis
Microbial genomic DNA was extracted from cecal contents for the PCR amplification. After quantification of DNA concentration using Nanodrop, each sample was diluted to the concentration of 1 × 109 mol/μL in the Tris-EDTA buffer and pooled together. Then, according to the manufacturer’s instructions, we used the Illumina MiSeq sequencing system to sequence 20 μL of the pooled mixture. Quantification of the PCR products was performed on the microplate reader (BioTek, FLX800 FTX-800) real-time PCR instrument. The V3-V4 region of 16S rRNA gene of gut microbiota was sequenced using Illumina MiSeq 2 × 250 bp, the high-throughput platform. Raw pyrosequencing reads obtained from the sequencer were denoised using the Titanium PyroNoise software. We adopted widely used methods as described in previous studies to analyze the resulting pyrosequencing reads.
Statistical Analysis
The SPSS 20.0 software (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. All results are expressed as mean ± SE. Analysis of variance and t test were used to compare between groups. P < 0.05 is “significant;” P < 0.01 is “extremely significant” [14]. Pearson was measured by R language, and the relationship between intestinal flora and biomarkers of oxidative stress was analyzed.
Results
Effects of Mercury on Cecal Histopathological Changes
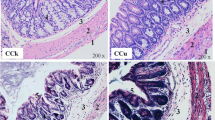
The weights of mice were presented in the format of mean ± SD. Compared with CK group (41.6623 ± 5.32666 g), the Hg group (34.6169 ± 4.50942 g) were significantly decreased (P < 0.01). As shown in Fig. 2a, compared with CK group, there was obvious hyperemia on the surface of the cecum of mice in Hg group. As shown in Fig. 2 a and c, in the cecal tissue of the Hg group, there were observed histopathological lesions consisting of shortening and atrophy of colorectal gland, increased thickness of inner muscularis and outer muscularis, widened submucosa, mild to moderate necrosis of enterocytes, and a decrease in the number of goblet cells.
The histological analysis of rectal and cecal tissues (HE. 50 ×). a Apparent observation of cecum in mice. b and c The histological analysis of cecal tissues (HE. 100 ×). The two groups are CK group (b) and the Hg group (c). The numbers in the figures indicated (1) colorectal gland, (2) inner muscularis, (3) submucosa, and (4) outer muscularis
Analysis of Intestinal Morphology
As shown in Fig. 3a, the length of the colonic glands in the Hg group was significantly lower than that in the CK group (P < 0.01). As shown in Fig. 3b, the crypt depth in the Hg group was also significantly lower than that in the CK group (P < 0.01). As shown in Fig. 3c, the number of goblet cells in the Hg group was also significantly lower than that in the CK group (P < 0.01).
Effects of Mercury Exposure on Cecal Ultrastructure
As shown in Fig. 4, the CK group showed normal mitochondria morphology. On the contrary, mercury exposure caused significant mitochondrial damage in cecum, including rupture of internal and external mold and fracture of internal crest.
Effects of Mercury Exposure on Oxidative Stress
As shown in Fig. 5, mercury exposure not only could dramatically increase MDA (Fig. 5a) formation but also obviously lessened SOD (Fig. 5b) and GSH (Fig. 5c) in mice.
Analysis of Intestinal Flora Differences
In order to study the differences between gut microbiology composition in different groups, we detected the difference of relative abundance of microbiota in mice. As shown in Fig. 6, at the phylum level, compared with the CK group, Firmicutes, Actinobacteria, and Spirochaetes were significantly increased in the Hg group. At the same time, Bacteroidetes and Proteobacteria were decreased in the Hg group. At the genus level, Clostridiales, Lactobacillus, Treponema, Oscillospira, and Desulfovibrio were increased in the Hg group. S24-7, Acinetobacter, and Staphylococcus were decreased in the Hg group.
Correlation Analysis
Figure 7 shows the relationships between the relative abundance of composition of intestinal microbiota and biomarkers of oxidative stress. We found there was no significant correlation between biomarkers of oxidative stress including MDA, SOD, and GSH and gut microorganisms (P > 0.05). However, Actinobacteria is significantly negatively correlated to Bacteroidetes. There is strong positive correlation between Spirochaetes and Proteobacteria. Acidobacteria is significantly negatively correlated to Verrucomicrobia.
Discussion
To the best of our knowledge, there are some studies showing that mercury is related with and may cause kidney, nerve, reproductive system, and immune system injury, but there have been few studies on the effects of mercury exposure on intestinal injury [3, 4, 15, 16]. In this study, we found that mercury affected the body weight, induced pathological changes in intestinal tissues and gut microbiota, and caused oxidative stress in mice. These results provide some basis for the study of the relationship between gut microbiota and oxidative stress under mercury exposure.
Some studies have shown that mercury can cause toxic effects through oxidative stress, thereby changing cell functions and eventually leading to pathological or physiological damage of various cells, and even cell death. What is more, mercury’s toxicity is considered to be related with strengthening of oxidative stress by either ROS overproduction or reducing the antioxidant defense system [4]. On the one hand, mercury poisoning can increase the production of free radicals, which lead to the increase of membrane lipid peroxidation by attacking the cell membrane and eventually produce MDA. Mercury, on the other hand, binds to mercaptan proteins, leading to the consumption of the powerful cellular antioxidant GSH [17, 18]. At the same time, SOD is an antioxidant enzyme in all mammalian cells, which plays a protective role in cell damage [19]. Our results showed that mercury significantly increased the content of MDA in vivo and decreased the activity of SOD and GSH. This also demonstrated that exposure to mercury induced oxidative damage in mice. In addition, some scholars found that oxidative stress caused intestinal mucosal injury, and significantly reduced activity of intestinal mucosal marker enzymes such as sodium-potassium ATPase and sucrose, which also resulted in significantly reduced glucose transport capacity of mesenteric membrane [20]. Moreover, oxidative damage also resulted in reduced expression of proteins associated with the tight junctions between intestinal epithelial cells [21]. This also demonstrated that oxidative damage caused by exposure to mercury damaged the integrity of intestinal cells in mice.
The intestinal mucosa of animals is the main site of nutrient digestion and absorption. The changes in villi structure or intestinal gland structure will directly affect nutrient absorption. Mercury is a potent poison that can cause gastrointestinal disorders, esophagitis, and blood in the stool [22]. What is more, Zhang et al. proposed that HgCl2 and MeHg cause mild inflammation of the ileus villi in mice [23]. Through the pathological observation of the intestinal tract, we found that mercury exposure can cause intestinal gland atrophy, moderate necrosis of intestinal epithelial cells, and decreased goblet cell number in the cecum. These pathological changes also confirmed the destruction of the integrity of the intestinal epithelial cells. We found significant weight loss in the Hg group. These results showed that mercury exposure caused intestinal injury in mice and affected their growth performance. In addition, some studies have shown that intestinal oxidative damage also causes intestinal villi to shorten, recess depth increased, directly affecting intestinal digestion and absorption [6]. In our results, we found that the cecal tissue showed a shortening in the height of colonic gland, a deepening of the depth of crypt, and a decrease in the number of goblet cells, so our study shows that not only mercury is damaging the integrity of the cecum but also oxidative stress is indirectly damaging the cecum.
Mitochondria play a vital role in determining the cell destiny [4]. Oxidative stress can not only lead to intestinal damage but also leads to metabolic disorders and impaired mitochondrial function [5]. When the body’s oxidative capacity and antioxidant defense capacity are reduced, ROS production increases, oxygen and phosphorus decrease, and ATP production decreases, which leads to the impairment of mitochondrial function [24]. Our results showed that the rupture of internal and external mold and fracture of internal crest occurred in the mitochondria after exposure to mercury. Therefore, when mercury exposure causes oxidative damage to the intestinal tract, it also damages the structure of the intestinal mitochondria, leading to mitochondrial dysfunction.
In addition, the results of our current study demonstrated that mercury exposure not only causes oxidative stress, it also causes perturbed cecal microbiota in mice. To elucidate the underlying mechanisms of mercury injury to intestinal tract, we detected different populations in the gut microbiota. At the phylum level, mercury increased the abundance of Firmicutes, Actinobacteria, and Spirochaetes. It is considered that most of the bacteria in these three phyla are anaerobes. Some scholars proposed that mercury poisoning would increase the formation of free radicals, inhibit the activity of GSH-Px, and thus inhibit the formation of oxygen [25]. Furthermore, mercury reduces the production of oxygen, reduces the content of oxygen in the intestinal tract, and increases the production of anaerobic bacteria. Therefore, mercury poisoning can increase the growth of anaerobic bacteria such as Clostridiales, Lactobacillus, Treponema, Oscillospira, and Desulfovibrio, and decrease the abundance of aerobic bacteria such as S24-7, Acinetobacter, and Staphylococcus. In summary, we have shown that mercury exposure induced changes in gut microbial composition in mice. This finding offers a novel pathway in studying the mechanisms involved in the development of mercury poisoning.
The correlation analysis can further reveal that the upregulation or downregulation of expression in biomarkers of oxidative stress is associated with some specific bacteria [26]. However, we found that MDA, SOD, and GSH of oxidative stress were not strongly correlated with intestinal microorganisms. We only found correlations between certain bacteria and other bacteria. Therefore, the relationship between biomarkers of oxidative stress and intestinal microorganisms needs more experimental research.
Strong Points of the Study
Our manuscript mainly contains the experimental results, which show oxidative stress and intestinal microbial changes caused by mercury in mice, such as pathological observation, detection of biomarkers of oxidative stress, and gut microbial analysis. The innovation lies in the association between the expression of biomarkers of oxidative stress and gut microorganisms, and the results and evidences motivate future study on the pathogenesis of intestinal injury caused by heavy metals.
Limitations of the Study
One significant limitation of our study is that we found that MDA, SOD, and GSH of oxidative stress were not strongly correlated with intestinal microorganisms. We only found correlations between certain bacteria and other bacteria. Therefore, the relationship between biomarkers of oxidative stress and intestinal microorganisms needs more experimental research.
Conclusion
In summary, the results of our study showed that mercury exposure in mice affected the body weight, caused cecal histopathological lesions, and altered cecal ultrastructure of mitochondria. Meanwhile, mercury exposure led to oxidative stress by increasing the concentration of malondialdehyde (MDA) and decreasing the concentration of superoxide dismutase (SOD) and glutathione peroxidase (GSH). Furthermore, exposure to mercury caused changes in microbial composition, structure, and diversity of microbial flora in mice.
Abbreviations
- MDA:
-
malondialdehyde
- GSH:
-
glutathione peroxidase
- SOD:
-
superoxide dismutase
- WHO:
-
World Health Organization
References
Liu J, Xu X, Yu S, Cheng H, Hong Y, Feng X (2014) Mercury pollution in fish from South China Sea: levels, species-specific accumulation, and possible sources. Environ Res 131:160–164. https://doi.org/10.1016/j.envres.2014.03.004
Jiang GB, Shi JB, Feng XB (2006) Mercury pollution in China. An overview of the past and current sources of the toxic metal. Environ Sci Technol 40(12):3673–3678. https://doi.org/10.1021/es062707c
de Burbure C, Buchet JP, Leroyer A, Nisse C, Haguenoer JM, Mutti A, Smerhovsky Z, Cikrt M, Trzcinka-Ochocka M, Razniewska G, Jakubowski M, Bernard A (2006) Renal and neurologic effects of cadmium, lead, mercury, and arsenic in children: evidence of early effects and multiple interactions at environmental exposure levels. Environ Health Perspect 114(4):584–590. https://doi.org/10.1289/ehp.8202
Li S, Baiyun R, Lv Z, Li J, Han D, Zhao W, Yu L, Deng N, Liu Z, Zhang Z (2019) Exploring the kidney hazard of exposure to mercuric chloride in mice:Disorder of mitochondrial dynamics induces oxidative stress and results in apoptosis. Chemosphere 234:822–829. https://doi.org/10.1016/j.chemosphere.2019.06.096
Liu B, Yu H, Baiyun R, Lu J, Li S, Bing Q, Zhang X, Zhang Z (2018) Protective effects of dietary luteolin against mercuric chloride-induced lung injury in mice: involvement of AKT/Nrf2 and NF-kappaB pathways. Food Chem Toxicol 113:296–302. https://doi.org/10.1016/j.fct.2018.02.003
Bollengier-Lee S, Mitchell MA, Utomo DB, Williams PE, Whitehead CC (1998) Influence of high dietary vitamin E supplementation on egg production and plasma characteristics in hens subjected to heat stress. Br Poult Sci 39(1):106–112. https://doi.org/10.1080/00071669889466
Yuan M, Li D, Zhang Z, Sun H, An M, Wang G (2018) Endometriosis induces gut microbiota alterations in mice. Hum Reprod 33(4):607–616. https://doi.org/10.1093/humrep/dex372
Rueda-Ruzafa L, Cruz F, Roman P, Cardona D (2019) Gut microbiota and neurological effects of glyphosate. Neurotoxicology 75:1–8. https://doi.org/10.1016/j.neuro.2019.08.006
Kou H, Fu Y, He Y, Jiang J, Gao X, Zhao H (2019) Chronic lead exposure induces histopathological damage, microbiota dysbiosis and immune disorder in the cecum of female Japanese quails (Coturnix japonica). Ecotoxicol Environ Saf 183:109588. https://doi.org/10.1016/j.ecoenv.2019.109588
Ruan Y, Wu C, Guo X, Xu Z, Xing C, Cao H, Zhang C, Hu G, Liu P (2019) High doses of copper and mercury changed cecal microbiota in female mice. Biol Trace Elem Res 189(1):134–144. https://doi.org/10.1007/s12011-018-1456-1
Skrypnik K, Suliburska J (2018) Association between the gut microbiota and mineral metabolism. J Sci Food Agric 98(7):2449–2460. https://doi.org/10.1002/jsfa.8724
Skrypnik K, Bogdanski P, Sobieska M, Suliburska J (2020) Hepcidin and erythroferrone correlate with hepatic Iron transporters in rats supplemented with multispecies probiotics. Molecules 25(7):1674. https://doi.org/10.3390/molecules25071674
Li S, Jiang X, Luo Y, Zhou B, Shi M, Liu F, Sha A (2019) Sodium/calcium overload and Sirt1/Nrf2/OH-1 pathway are critical events in mercuric chloride-induced nephrotoxicity. Chemosphere 234:579–588. https://doi.org/10.1016/j.chemosphere.2019.06.095
Skrypnik K, Bogdański P, Schmidt M, Suliburska J (2019) The effect of multispecies probiotic supplementation on iron status in rats. Biol Trace Elem Res 192(2):234–243. https://doi.org/10.1007/s12011-019-1658-1
Sutton DJ, Tchounwou PB (2007) Mercury induces the externalization of phosphatidyl-serine in human renal proximal tubule (HK-2) cells. Int J Environ Res Public Health 4(2):138–144. https://doi.org/10.3390/ijerph2007040008
Pal PB, Pal S, Das J, Sil PC (2012) Modulation of mercury-induced mitochondria-dependent apoptosis by glycine in hepatocytes. Amino Acids 42(5):1669–1683. https://doi.org/10.1007/s00726-011-0869-3
Ansaldo M, Najle R, Luquet CM (2005) Oxidative stress generated by diesel seawater contamination in the digestive gland of the Antarctic limpet Nacella concinna. Mar Environ Res 59(4):381–390. https://doi.org/10.1016/j.marenvres.2004.06.003
Troost FJ, Saris WH, Haenen GR, Bast A, Brummer RJ (2003) New method to study oxidative damage and antioxidants in the human small bowel: effects of iron application. Am J Physiol Gastrointest Liver Physiol 285(2):G354–G359. https://doi.org/10.1152/ajpgi.00422.2002
McCord JM, Fridovich I (1968) The reduction of cytochrome c by milk xanthine oxidase. J Biol Chem 243(21):5753–5760
Deng Q, Xu J, Yu B, He J, Zhang K, Ding X, Chen D (2010) Effect of dietary tea polyphenols on growth performance and cell-mediated immune response of post-weaning piglets under oxidative stress. Arch Anim Nutr 64(1):12–21. https://doi.org/10.1080/17450390903169138
Kawauchiya T, Takumi R, Kudo Y, Takamori A, Sasagawa T, Takahashi K, Kikuchi H (2011) Correlation between the destruction of tight junction by patulin treatment and increase of phosphorylation of ZO-1 in Caco-2 human colon cancer cells. Toxicol Lett 205(2):196–202. https://doi.org/10.1016/j.toxlet.2011.06.006
Rice KM, Walker EM Jr, Wu M, Gillette C, Blough ER (2014) Environmental mercury and its toxic effects. J Prev Med Public Health 47(2):74–83. https://doi.org/10.3961/jpmph.2014.47.2.74
Zhang BB, Liu YM, Hu AL, Xu SF, Fan LD, Cheng ML, Li C, Wei LX, Liu J (2019) HgS and Zuotai differ from HgCl2 and methyl mercury in intestinal Hg absorption, transporter expression and gut microbiome in mice. Toxicol Appl Pharmacol 379:114615. https://doi.org/10.1016/j.taap.2019.114615
Ye F, Li X, Li L, Lyu L, Yuan J, Chen J (2015) The role of Nrf2 in protection against Pb-induced oxidative stress and apoptosis in SH-SY5Y cells. Food Chem Toxicol 86:191–201. https://doi.org/10.1016/j.fct.2015.10.009
El-Saeed GSM, Abdel Maksoud SA, Bassyouni HT, Raafat J, Agybi MH, Wahby AA, Aly HM (2016) Mercury toxicity and DNA damage in patients with Down syndrome. Med Res J 15(1):22–26. https://doi.org/10.1097/01.MJX.0000483973.37399.e7
Yang J, Zhang X, Xie Y, Song C, Sun J, Zhang Y, Giesy JP, Yu H (2017) Ecogenomics of zooplankton community reveals ecological threshold of ammonia nitrogen. Environ Sci Technol 51(5):3057–3064. https://doi.org/10.1021/acs.est.6b05606
Funding
This project was supported by the National Natural Science Foundation of China grant (no. 31960723; 31460679 Beijing, P. R. China) awarded to PL and the Natural Science Foundation of Jiangxi Province grant (no. 20171ACB21026; 2017ACB20012) awarded to PL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yulan Zhao and Changming Zhou are equal first author.
Rights and permissions
About this article
Cite this article
Zhao, Y., Zhou, C., Guo, X. et al. Exposed to Mercury-Induced Oxidative Stress, Changes of Intestinal Microflora, and Association between them in Mice. Biol Trace Elem Res 199, 1900–1907 (2021). https://doi.org/10.1007/s12011-020-02300-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-020-02300-x