Abstract
Mannheimia haemolytica is the main bacterial pathogen isolated in bovine respiratory disease (BRD), a common disease affecting calves before weaning. Previous research has shown that experimental infection with bovine herpesvirus 1, a respiratory virus, decreases plasma zinc (Zn) levels. However, changes in plasma Zn concentrations in calves experimentally infected with M. haemolytica have not been studied thus far. The objective of this study was to evaluate the effect of experimental infection with M. haemolytica on plasma Zn concentration in calves. Total leukocyte count and bovine respiratory disease (BRD) clinical score were also evaluated. We conducted a 6-day trial in 14 male Holstein calves randomly assigned to one of two groups, experimental (EG, n = 8) and control (CG, n = 6). Animals in EG were intrabronchially inoculated with M. haemolytica (6.5 × 106 CFU/mL) on day 0 of the trial. Plasma Zn levels were affected by time, treatment, and time by treatment interaction, being lower in EG compared with CG on days 1, 2, and 3. Differences in total leukocyte count were significant on day 1, observing a tendency on day 3. BRD clinical score differed between groups, being higher in EG throughout the trial. We conclude that experimental M. haemolytica infection reduced plasma Zn concentration in clinically ill calves, suggesting that the clinical condition of animals (healthy/ill) should be considered to better interpret plasma Zn values.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Zinc (Zn) is an essential trace element for normal growth and development of living organisms. It plays a key structural and regulatory role in mammal cell activity [1] and contributes to the normal function of the immune system. Innate and acquired immunity are affected by Zn deficiency [2], which is responsible for reduced phagocytic and lytic activity of polymorphonuclear cells and monocytes, reduced natural killer cell activity, T lymphocyte alterations, thymic atrophy, and lymphopenia [3, 4].
Supplementation with Zn improves the immune response in human beings and bovines [5, 6]. However, Zn overdosing may be harmful. In human beings, high doses of oral Zn (100 mg/day) have been associated with impaired immune function and alterations similar to those caused by Zn deficiency [7, 8]. On the other hand, addition of 75 mg Zn/kg dry matter to a diet covering Zn requirements elicited lower weight gain and feed efficiency in lactating feedlot heifers [9]. Considering the delicate relationship between the essential nature of Zn and its adverse effects, adequate levels of this micronutrient are needed for optimal health. Accordingly, Zn supplementation should be carefully used and limited to individuals with manifest Zn deficiency [10].
Before deciding supplementation, Zn status should be assessed. Plasma Zn concentration is the most widely used indicators of Zn status [11]. However, it is not considered reliable because it may vary due to different factors, without accurately reflecting the current Zn status. In rodents, plasma Zn concentration decreases during acute phase response, inflammatory processes, trauma, infection, and stress [12,13,14]. In bovines under stress and/or disease conditions, changes are scarcely known.
Bovine respiratory disease (BRD) is the most common disease affecting calves before weaning [15] and the major cause of disease and death in feedlot cattle [16]. The disease is the result of a complex interaction among stress, viruses, and bacteria [17]. Among the latter, M. haemolytica is the main bacterial pathogen isolated in BRD feedlot cattle [18]. Previous research performed in calves has shown that experimental infection with bovine herpesvirus 1 (BoHV-1) decreases plasma Zn levels, while bovine syncytial virus infection increases them [19,20,21]. To date, changes in plasma Zn concentrations in calves experimentally infected with M. haemolytica have not been studied.
Based on the hypothesis that M. haemolytica infection reduces plasma Zn concentration, thereby affecting its use as a measure of Zn status, the aim of this study was to assess the effect of experimental M. haemolytica infection on plasma Zn concentration in calves.
Materials and Methods
All the experimental procedures performed in the current trial were approved by the Committee for the Care and Use of Laboratory Animals (CICUAL, for its Spanish acronym), School of Veterinary Sciences, La Plata National University, Argentina, under Protocol N° 55-2-18P.
Animals
Male Holstein calves (n = 14; 3.5 ± 0.2 months old; 67.7 ± 7.7 kg body weight) from a dairy farm (Manantiales, Buenos Aires Province, Argentina; 35° 44′ 31.5"S 58° 06' 11.7"W) were used. The animals were housed and kept in individual tie-stalls in the Large Animals Teaching Hospital facilities of the National University of La Plata School of Veterinary Sciences (34° 91 20´´S, 57° 93′ 75′´ W). Calves were fed with alfalfa hay ad libitum and a specially formulated commercial pellet (1.8 kg/day/animal) according to the age and weight of the animals. The pellet provided 15,000 IU vitamin A, 5000 IU vitamin D, 50 mg vitamin E, 90 mg iron, 12.5 mg copper, 60 mg manganese, 90 mg Zn, 0.3 mg selenium, 1.0 mg iodine, and 0.5 mg cobalt per kilogram. At the beginning of the trial, calves were homogeneous with respect to body weight, age, and feed intake. They had good health, and clinical, hematological, and plasma Zn parameters are within the normal range for age and species. After an adaptation period of 12 days to both facilities and food intake (Table 1), they were included in the study.
Experimental Design
Calves were randomly assigned to one of two groups, experimental (EG, n = 8) and control (CG, n = 6). Animals in EG received an inoculum containing a strain of M. haemolytica during the log phase of growth, provided by the Laboratory of Bacteriological Diagnosis of the School of Veterinary Sciences. Inoculum concentration was adjusted to the 0.5 McFarland standard scale (final viable count of 6.5 × 106 colony forming units (CFU) per milliliter (mL) of bacterial inoculum) [18]. Intrabronchial inoculation of both left and right lung was performed at 8:00 a.m. with either 8 ml bacterial inoculum plus 8 ml sterile physiological solution (EG) or physiological solution only (16 mL; CG). The time of inoculation was considered as time 0 of the trial.
Blood Sample Collection
Fasting blood samples were obtained daily by jugular venipuncture at 7:00 a.m. from day 0 (before the experimental infection) to 5 (end of the trial). For plasma Zn assessment, blood was collected in 10-ml plastic tubes previously washed with nonionic detergent and nitric acid and treated with sodium heparin as anticoagulant. For total leukocyte count, plastic EDTA-containing tubes were used. Samples were kept at 4 °C until processing.
Sample Processing
For plasma Zn assessment, samples were centrifuged within 60 min after blood extraction at 3000 rpm for 5 min. The plasma thus obtained was deproteinized with 10% (w/v) trichloroacetic acid. The Zn concentration in the supernatant was determined by flame atomic absorption spectrometry using an AAnalyst 200 spectrometer (PerkinElmer, AAS, International Equipment Trading Ltd., Mundelein, USA). Internal (0, 50, 100, and 200 μg/dL Zn) and quality control standards were used [22].
Total leukocyte count was carried out within 6 h after manual extraction using a Neubauer counting chamber. Briefly, 20 μl of blood was mixed together with 380 μl Türk’s solution (final dilution, 1:20) so that erythrocytes were lysed and the leukocyte nucleus was stained. The total number of cells in four squares at the corners of the counting chamber was determined under the low power objective of the microscope (10 ×). The total number of cells per microliter of sample was calculated using the following formula: ((number of white cell count × 20 × 10)/4), where 20 is the dilution factor, 10 is the chamber depth, and 4 is the number of square counts [23].
Clinical Assessment
The clinical assessment of calves was performed daily following the criteria of the Wisconsin clinical scoring system for the diagnosis of BRD in dairy cows (www.vetmed.wisc.edu/dms/fapm/fapmtools/8calf/calf_ health_scoring_chart.pdf) [24]. The system assesses the following five clinical signs: rectal temperature, spontaneous or induced coughing, nasal discharge, ocular discharge, and ear and head position. These signs are assigned values on a 0–3 scale, which are used to determine a total BRD score. Calves in the current trial were categorized as clinically ill if their total score was ≥ 5.
Humane endpoint criteria were applied to calves severely depressed, unresponsive to stimuli, and unable to stand without assistance. The animals were humanely euthanized following the CICUAL protocol.
Euthanasia and Assessment of Pulmonary Lesions
The euthanasia procedure was performed after complete sedation and deep anesthesia with 10% xylazine-5% ketamine (1 and 4 mL, respectively). Then, the animals were sacrificed using 20 mL concentrated procaine (Eutanasico®, Equi Systems SRL, Buenos Aires, Argentina). Once death was confirmed by absence of corneal reflex by touching the surface of the eyeball and heart beats on auscultation, complete necropsy was carried out. Lungs were removed and percentage lung consolidation was assessed by direct palpation. Final pulmonary lesion was determined with the formula (0.053 × cranial segment of left cranial lobe %) + (0.049 × caudal segment of left cranial lobe %) + (0.319 × left caudal lobe %) + (0.043 × accessory lobe %) + (0.352 × right caudal lobe %) + (0.061 × right middle lobe %) + (0.060 × caudal segment of right cranial lobe %) + (0.063 × cranial segment of right cranial lobe %) [25].
Bacteriological Cultures
Routine bacteriological studies of samples from the injured lung segments of the sacrificed animals were performed at the Laboratory of Bacteriological Diagnosis, School of Veterinary Sciences. Briefly, samples were suspended in 0.7 mL of brain-heart infusion broth. A 100 μL aliquot of the suspension was incubated aerobically at 37 °C for 48 h on tryptic soy agar plates containing 5% defibrinated sheep blood at 37 °C. After culture, colonies were selected for further Gram-stained smears. The isolates were subjected to routine biochemical tests, i.e., nitrate reduction, catalase, oxidase, urease, and growth on MacConkey agar.
Statistical Analysis
Plasma Zn variations, total leukocyte count, and BRD score were evaluated using an adjusted linear mixed model with repeated measures over time, implemented in SAS PROCMIXED (9.4). Treatment (infected), time (day), and their interaction were the fixed variables, and animal was the random variable. The SLICE option of SAS was used to detect means when differences were significant. Significance was set at p < 0.05 for the main effects, p < 0.1 for their interaction, and p < 0.1 and < 0.15 for tendency, respectively. Plasma Zn levels, total leukocyte count, and BRD clinical score for different levels of the variable time (day) are presented as least square means ± the standard error of the means (LSM ± SEM).
Results
Time, treatment, and time*treatment interaction affected plasma Zn levels (p < 0.001, = 0.04, and < 0.001, respectively), which were lower in EG compared with CG on days 1 (46 vs 108 μg/dL, p < 0.01), 2 (71 vs 109 μg/dL, p = 0.03), and 3 (69 vs 107 μg/dL, p = 0.03), respectively (Fig. 1).
Plasma zinc concentration, total leukocyte count, and BRD clinical score in calves experimentally infected with Mannheimia haemolytica. Day 0: Values recorded before the experimental infection. CG, control group (n = 6; solid line). EG, experimental group (n = 8; dotted line) infected with M. haemolytica (6.5 × 106 CFU/mL). Three calves from EG were humanely euthanized (one on day 1 of the trial and two on day 2). * p < 0.05; ** p < 0.01
Time (day) elicited a significant effect on total leukocyte count (p < 0.001), whereas inoculation with M. haemolytica tended to decrease it (p = 0.07). Time and treatment showed a significant interaction (p < 0.001). Differences in total leukocyte count were significant on day 1 (EG, 16.7 × 103 μL; CG, 9.8 × 103 μL; p < 0.001), while a tendency was observed on day 3 (EG, 11.5 × 103 μL; CG, 7.8 × 103 μL; p = 0.06) (Fig. 1).
Time, treatment, and time*treatment interaction affected BRD clinical score (p < 0.001), which was higher in EG throughout the trial (Fig. 1).
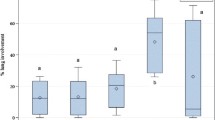
Three EG calves with severe depression, unresponsiveness to stimuli, and which did not eat or drink were humanely euthanized. The macroscopic anatomic pathology diagnosis was acute fibrinous bronchopneumonia, without lesions in other organs and systems. At necropsy of the calf sacrificed on day 1 (7:30 p.m.), pulmonary consolidation (35.46%) and plasma Zn concentration of 14 μg/dL were observed. The two calves sacrificed on day 2 of the trial (5:00 and 7:00 p.m.) had 40.5 and 42.61% pulmonary consolidation and 31 and 38 μg/dL plasma Zn level, respectively (Fig. 2).
Pulmonary consolidation of three calves experimentally infected with Mannheimia haemolytica. Pulmonary consolidation (35.46%) was observed in the calf sacrificed on day 1 of the trial (ID 15; 7:30 p.m.). Calves identified as 11 and 12 and sacrificed on day 2 of the trial (5:00 and 7:00 p.m.) presented 40.5 and 42.61% pulmonary consolidation, respectively
M. haemolytica was isolated in all bacteriological cultures of samples collected at necropsy of the three animals.
Discussion
According to our hypothesis, experimental infection with M. haemolytica decreases plasma Zn concentration in calves. During an infection, the release of interleukin1β (IL-1β) and 6 (IL-6) could be involved in plasma Zn reduction [13, 26]. Both cytokines increase Zn transporter ZIP14 gene transcription, mainly in the liver [14]. The localization of ZIP14 at the plasma membrane of hepatocytes facilitates Zn uptake from blood [26]. Additionally, an increased gene transcription of membrane transporter ZIP8 has been described in blood monocytes in response to infection, thereby decreasing plasma Zn content [27]. Such reduction could be due to a redistribution of Zn in the organism as part of nutritional immunity, where pathogenic microorganisms are deprived of essential nutrients needed for survival [28, 29]. Moreover, Zn redistribution increases hepatic Zn levels; the micronutrient participates in liver antioxidant defense system and would reduce oxidative stress damage during infection [30]. The additional provision of Zn to the liver during infection increases liver Zn bioavailability at a time of high Zn requirement due to the increased synthesis of acute phase proteins and cytokines [31].
Studies performed in bovines evaluating other response parameters to bacterial or viral infection have also reported decreased plasma Zn levels. On the one hand, decreased plasma Zn levels 24 h after Staphylococcus aureus intramammary infection and increased somatic cell elimination in milk [32]. On the other, plasma Zn concentrations with concurrent morbidity peak (evidenced by increased rectal temperature, nasal and ocular discharge, anorexy, and depression) were found to be decreased 4 days after intranasal herpes virus 1 inoculation of calves [20].
In our study, after the experimental challenge with M. haemolytica, plasma Zn levels in EG calves were below 80 μg/dL, which is the lower limit of the marginal range described in the literature [33]. The lowest Zn levels were recorded in euthanized animals, suggesting their association with the most severe lesions caused by M. haemolytica. This finding was reinforced by the fact that the lowest Zn values were recorded on the day with the highest total leukocyte count and BRD clinical score. Mannheimia haemolytica produces a localized lesion in the lungs; thus, the decrease in plasma Zn could be attributed to a systemic response to infection, which would involve proteins of the immune response. Interestingly, interleukins such as IL-6 have the capacity of diminishing plasma Zn, also causing leukocytosis characterized by rapid neutrophilia [34]. Although we did not examine plasma IL-6 concentrations in this study, values could be remarkably increased at the time when the animals presented the lowest plasma Zn values and the highest leukocyte count.
Despite plasma Zn concentration is altered during infectious processes, it remains the most widely used indicator to evaluate Zn status in bovines [35]. However, the clinical condition of animals (healthy/ill) should also be considered to avoid diagnostic errors, considering that both Zn deficiency and excess are harmful for animal health.
References
Rosa DE, Fazzio LE, Picco SJ, Furnus CC, Mattioli GA (2008) Metabolismo y deficiencia de zinc en bovinos. Analecta Vet 30(2):34–44
Bonaventura P, Benedetti G, Albarède F, Miossec P (2015) Zinc and its role in immunity and inflammation. Autoimmun Rev 14(4):277–285
Hujanen ES, Seppä ST, Virtanen K (1995) Polymorphonuclear leukocyte chemotaxis induced by zinc. Copper and nickel in vitro. Biochim Biophys Acta 1245(2):145–152
King LE, Frentzel JW, Mann JJ, Fraker PJ (2005) Chronic zinc deficiency in mice disrupted T cell lymphopoiesis and erythropoiesis while B cell lymphopoiesis and myelopoiesis were maintained. J Am Coll Nutr 24(6):494–502
Prasad AS (2009) Zinc: role in immunity. Oxidative stress and chronic inflammation. Curr Opin Clin Nutr Metab Care 12(6):646–652
Wang RL, Liang JG, Lu L, Zhang LY, Li SF, Luo XG (2013) Effect of zinc source on performance, zinc status, immune response, and rumen fermentation of lactating cows. Biol Trace Elem Res 152(1):16–24
Ibs KH, Rink L (2003) Zinc-altered immune function. J Nutr 133(5):1452–1456
Bogden JD, Oleske JM, Lavenhar MA, Munves EM, Kemp FW, Bruening KS, Holding KJ, Denny TN, Guarino MA, Holland BK (1990) Effects of one year of supplementation with zinc and other micronutrients on cellular immunity in the elderly. J Am Coll Nutr 9(3):214–225
Nunnery GA, Vasconcelos JT, Parsons CH, Salyer GB, Defoor PJ, Valdez FR, Galyean ML (2007) Effects of source of supplemental zinc on performance and humoral immunity in beef heifers. J Anim Sci 85(9):2304–2313
Overbeck S, Rink L, Haase H (2008) Modulating the immune response by oral zinc supplementation: a single approach for multiple diseases. Arch Immunol Ther Exp 56(1):15–30
Kincaid RL (1999) Assessment of trace mineral status of ruminants: a review. Proc Am Soc Anim Sci 77(1):1–10
Lichten LA, Cousins RJ (2009) Mammalian zinc transporters: nutritional and physiologic regulation. Annu Rev Nutr 29:153–176
Aydemir TB, Chang SM, Guthrie GJ, Maki AB, Ryu MS, Karabiyik A et al (2012) Zinc transporter ZIP14 functions in hepatic zinc, Iron and glucose homeostasis during the innate immune response (endotoxemia). PLoS One 7(10):e48679
Aburto-Luna V, Treviño S, Santos-López G, Moroni-González D, Calva-Cruz O, Aguilar-Alonso P, León-Chávez BA, Brambila E (2017) Hepatic mobilization of zinc after an experimental surgery and its relationship with inflammatory cytokines release and expression of metallothionein and Zip14 transporter. Inflamm Res 66(2):167–175
Murray CF, Fick LJ, Pajor EA, Barkema HW, Jelinski MD, Windeyer MC (2016) Calf management practices and associations with herd-level morbidity and mortality on beef cow-calf operations. Animal 10(3):468–477
Duff GC, Galyean ML (2007) Board-invited review: recent advances in management of highly stressed. Newly received feedlot cattle. J Anim Sci 85(3):823–840
Fazzio LE, Landoni MF (2010) Comparación de la eficacia de oxitetraciclina y tilmicosina en el tratamiento metafiláctico de la enfermedad respiratoria bovina en animales de feedlot. Analecta Vet 30(2):35–40
Rice JA, Carrasco-Medina L, Hodgins DC, Shewen PE (2007) Mannheimia haemolytica and bovine respiratory disease. Anim Health Res Rev 8(2):117–128
Verhoeff J, Van der Ban M, van Nieuwstadt AP (1984) Bovine respiratory syncytial virus infections in young dairy cattle: clinical and haematological findings. Vet Rec 114(1):9–12
Orr CL, Hutcheson DP, Grainger RB, Cummins JM, Mock RE (1990) Serum copper, zinc, calcium and phosphorus concentrations of calves stressed by bovine respiratory disease and infectious Bovine Rhinotracheitis. J Anim Sci 68:2893–2900
Soltesova H, Nagyova V, Tothova C, Nagy O (2015) Haematological and blood biochemical alterations associated with respiratory disease in calves. Acta Vet Brno 84:249–256
Piper HG, Higgins G (1967) Estimation of trace metals in biological material by atomic absorption spectrophotometry. Proc Assoc Clin Biochem 7:190–195
Ióvine E, Selva AA (1985) El laboratorio en la clínica: metodología analítica, fisiopatología e interpretación semiológica. 3ra edición. Buenos Aires. Argentina
Love W, Lehenbauer T, Van Eenennaam A, Drake C, Kass P, Farver T, Aly S (2016) Sensitivity and specificity of on-farm scoring systems and nasal culture to detect bovine respiratory disease complex in pre weaned dairy calves. J Vet Diagn Investig 28(2):119–128
Fajt VR, Apley MD, Roth JA, Frank DE, Brogden KA, Skogerboe TL, Shostrom VK, Chin YL (2003) The effects of danofloxacin and tilmicosin on neutrophil function and lung consolidation in beef heifer calves with induced Pasteurella (Mannheimia) haemolytica pneumonia. J Vet Pharmacol Ther 26(3):173–179
Liuzzi JP, Lichten LA, Rivera S, Blanchard RK, Aydemir TB, Knutson MD, Ganz T, Cousins RJ (2005) Interleukin-6 regulates the zinc transporter Zip14 in liver and contributes to the hypozincemia of the acute-phase response. Proc Natl Acad Sci U S A 102(19):6843–6848
Besecker BY, Exline MC, Hollyfield J, Phillips G, Disilvestro RA, Wewers MD, Knoell DL (2011) A comparison of zinc metabolism, inflammation, and disease severity in critically ill infected and noninfected adults early after intensive care unit admission. Am J Clin Nutr 93(6):1356–1364
Corbin BD, Seeley EH, Raab A, Feldmann J, Miller MR, Torres VJ, Anderson KL, Dattilo BM, Dunman PM, Gerads R, Caprioli RM, Nacken W, Chazin WJ, Skaar EP (2008) Metal chelation and inhibition of bacterial growth in tissue abscesses. Science 319(5865):962–965
Gammoh NZ, Rink L (2017) Zinc in infection and inflammation. Nutrients 9(6):E624
Sakaguchi S, Iizuka Y, Furusawa S, Ishikawa M, Satoh S, Takayanagi M (2002) Role of Zn2+ in oxidative stress caused by endotoxin challenge. Eur J Pharmacol 451(3):309–316
Alker W, Haase H (2018) Zinc and sepsis. Nutrients 10(8):E976
Middleton JR, Luby CD, Viera L, Tyler JW, Casteel S (2004) Short communication: influence of Staphylococcus aureus intramammary infection on serum copper, zinc and iron concentrations. J Dairy Sci 87(4):976–979
Enjalbert F, Lebreton P, Salat O (2006) Effects of copper, zinc and selenium status on performance and health in commercial dairy and beef herds: retrospective study. J Anim Physiol Anim Nutr (Berl) 90(11–12):459–466
Suwa T, Hogg JC, English D, Van Eeden SF (2000) Interleukin-6 induces demargination of intravascular neutrophils and shortens their transit in marrow. Am J Physiol Heart Circ Physiol 279(6):H2954–H2960
Underwood EJ, Suttle NF (1999) The mineral nutrition of livestock. CABI Publishing, London
Acknowledgments
Thanks are due to A. Di Maggio for manuscript correction and edition.
Funding
This work was supported by Grant 11/V257 from UNLP.
Author information
Authors and Affiliations
Contributions
EMG, JMA and LEF designed the study. RML, NS and GA assisted with data collection. GAM and EMG analyzed the data. LEF and JMA coordinated the experiments and revised the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflict of interests.
Ethical approval
All the experimental procedures performed in the current trial were approved by the Committee for the Care and Use of Laboratory Animals (CICUAL, for its Spanish acronym), School of Veterinary Sciences, La Plata National University, Argentina, under Protocol N° 55-2-18P.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Galarza, E.M., Lizarraga, R.M., Streitenberger, N. et al. Assessment of Plasma Zinc and Total Leukocyte Count in Calves Experimentally Infected with Mannheimia haemolytica. Biol Trace Elem Res 199, 120–125 (2021). https://doi.org/10.1007/s12011-020-02145-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-020-02145-4