Abstract
The aim of this study was to investigate the long-term effects of low-dose lead exposure on bone microstructure in mice. Ten SPF 12-week-old male C57BL/6J mice were randomly divided into two groups: control (deionized water) and lead exposure (150 ppm of lead acetate in drinking water). After 24 weeks treatment, mice were weighed and the left femurs were collected and stored at − 80 °C. The right femurs of the mice were scanned by Micro-CT for three-dimensional reconstruction, and bone mineral density, bone volume fraction, trabeculae thickness, trabeculae number, and trabeculae separation were measured. The right tibia was collected to investigate histopathological changes in H&E-stained sections. The gene expression of osteoprotegerin (OPG), RANKL, and runt-related transcription factor 2 (Runx2) was determined using real-time PCR. The bone density of femoral cancellous bone and the number of cancellous bone trabeculae in the lead exposure group were both significantly decreased compared with the control group. Bone marrow stromal cell numbers were decreased following lead administration, and lipid droplet vacuoles were observed in the lead group. Levels of OPG were significantly decreased in the lead group, and lead also inhibited the expression of Runx2 compared with the control group. Long-term exposure to low doses of lead can cause bone damage without inducing other obvious symptoms through decreasing bone density and the number of cancellous bone trabeculae, further suppressing bone formation. It suggests that lead may exacerbate bone loss and osteoporosis, especially in the elderly.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lead (Pb) has been recognized as a toxic metal that is found naturally in soil for many years. Its extensive use and presence in the environment is a recognized public health problem worldwide [1]. Despite the many policies and procedures made to decrease the amount of lead in the environment, it remains a pervasive toxicant affecting numerous biological systems [2,3,4]. A study by the World Health Organization (WHO) found that lead poisoning was linked to 853,000 deaths worldwide in 2013 [5].
Bone is the hard component of the endoskeleton of vertebrates, with complex internal and external structures [6]. Environmental lead enters the body primarily through the respiratory and digestive tracts. Initially, lead is distributed in the body in the form of lead salts bound to plasma proteins. After a few weeks, more than 94% of lead is deposited in bone as insoluble lead phosphate [7, 8]. Bone lead is a biomarker of past exposure with a half-life of approximately 20 years, which increases with age [9, 10]. Bone formation and growth is controlled by a series of complex feedback processes that depend on biological and environmental factors. Large amounts of lead accumulating in bone can affect bone mineral deposition, development and maturation. Studies have shown that occupational lead exposure at high levels can lead to decreased bone density, osteoporosis and osteoarthritis, even deformity of bone development, fractures, affecting the quality of life [11, 12]. In addition, recent clinical and basic science research has suggested that even low levels of lead exposure may adversely affect both the developing and adult skeleton. In a study of rats exposed to low levels of lead in their drinking water over a lifetime, Beier et al. found adverse effects on bone. Lead exposure caused a reduction in BMD sufficient to reduce biomechanical strength and increase fracture risk [13]. They also reported that Pb targeted progenitor MSCs and altered their ability to differentiate into appropriate cell lineages. Pb also increased the in vitro formation of adipogenic cells and decreased the formation of osteoblasts [14]. However, a cohort study found that adolescent children with high blood lead levels (BLL) (23.6 μg/dl) had higher BMD measurements of the vertebras and femoral heads compared with those with low blood lead cohort (6.5 μg/dl BLL) [15]. Likewise, an animal experiment found elevated lifetime lead exposure produces an increase in bone mass in adolescent mice [16]. The exact effects of lead exposure on bone depend on dosing, duration of exposure, and animal species. The topic remains ill-defined at present. Most people have a detectable amount of Pb in their skeleton in industrial world currently [17]. It’s essential to understand the impact of Pb on bone structure.
The purpose of this study was to investigate the effects of long-term low-dose Pb exposure on bone of adult male mice and explore the underlying mechanism. Bone microstructure was detected by microcomputed tomography (Micro-CT). Biomarkers for bone formation and resorption were measured by quantitative real-time (qRT)-PCR in bone tissue. This study may provide more clues about the effects of long-term low-dose Pb exposure on bone metabolism and suggest bone health should be monitored in populations chronically exposed to Pb.
Materials and Methods
Animals
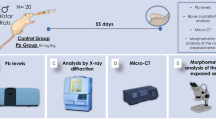
Twelve-week-old male C57BL/6J mice were purchased from the SLAC Laboratory for this experiment (SLAC, Shanghai, China). Animal care followed the Guide for the Care and Use of Laboratory Animals, and the study protocols were approved by the Soochow University Institutional Animal Care and Use Committee (SCKK2017-0006). Animals were housed five per cage in polycarbonate cages with corncob bedding under standard conditions (23 ± 2 °C, 12-h light-dark cycle, and 50% ± 10% humidity) and provided water and food ad libitum. After acclimatization to laboratory conditions for 1 week, the mice were randomly divided into two groups (5 animals/group): control group (Control) and low-dose lead group (Pb). Mice in the lead group were administered 150 ppm lead acetate dissolved in the drinking water.
Sample Collection
At week 24, the mice were sacrificed. Blood samples were collected for hematological analysis. Femurs were harvested and isolated from any soft tissues. Left femurs were cleaned in saline solution and stored at − 80 °C for quantitative real-time PCR, and right femurs were fixed in 10% formalin for 3 days for Micro-CT analysis.
Hematological Analysis
Hematological parameters, such as red blood cell number (RBC), hemoglobin concentration (HGB), hematocrit value (HCT), mean corpuscular hemoglobin concentration (MCHC), total white blood cell number (WBC), and platelet count (PLT) were determined using an automatic five-classification blood cell analyzer (CELL-DYN RUBY, Abbott, America).
Micro-CT Analysis
Right femurs were scanned and analyzed for cortical and trabecular bone by Micro-CT (SkyScan 1174, Bruker, Kontich, Belgium). The acquisition settings were X-ray voltage = 50 kV, X-ray current = 800 μA, filter = 0.5 mm aluminum, rotation step = 0.7°, and image pixel size = 10.3 μm. After scanning, images were reconstructed using NRecon software (Bruker, Kontich, Belgium). Parameters for cortical and trabecular bone were performed using CTAn software (Bruker, Kontich, Belgium), and 3D image reconstruction was performed using CTvox software (Bruker, Kontich, Belgium). For trabecular and cortical bone parameters, the volume of interest (VOI = 100 slices) was selected with reference to the distal growth plate. The trabecular and cortical bone regions started ~ 0.7 mm and ~ 5 mm, respectively, from the growth plate and extended towards the proximal end of the femur. The cortical bone parameters included cortical BMD and cortical thickness (Ct.Th). The cancellous bone parameters of the femoral metaphysis included trabecular BMD, bone volume to total tissue volume ratio (BV/TV), trabecular number (Tb.N), trabecular thickness (Tb.Th), and trabecular spacing (Tb.Sp).
Bone Histology
Right tibias of mice were fixed in 10% formalin for 3 days and decalcified for 4 weeks in 10% EDTA. Specimens were embedded in paraffin and cut in 5-μm sections through the longitudinal axis. Sections were stained with hematoxylin and eosin (H&E), mounted onto glass slides, and examined under a light microscope (DM2000, Leica; Germany). Microphotographs (× 40) were taken using a camera attachment.
Quantitative Real-Time-PCR
This approach was taken to further investigate the possible mechanisms by which Pb exposure affects bone formation and resorption to better understand disorders such as osteoporosis. The left femur was ground to powder in liquid nitrogen using a mortar and pestle. Total RNA was extracted from the powder using Trizol according to the manufacturer’s instructions. cDNA was synthesized from 500 ng total RNA using a reverse transcription kit (Hifair® II 1st Strand cDNA Synthesis SuperMix, YEASEN, Shanghai, China) according to the manufacturer’s protocol. PCRs were performed using the Applied Biosystems 7500 Real-time PCR Systems and Hieff™ qPCR SYBR® GREEN Master Mix kit (YEASEN, Shanghai, China). The PCR conditions were 5 min at 95 °C followed by 40 cycles of two-step PCR denaturation at 95 °C for 10 s, and annealing at 60 °C for 30 s. The melting curve phase followed the default settings of the instrument. The GAPDH gene was used to normalize the expression of target genes in the two groups. The primer sets for real-time quantitative PCR analysis are listed as follows:
GAPDH: forward (TCCTTCCCGCTGACCAAA); reverse
(TGTCCGTTGTCTTTCCTGTCAA)
OPG: forward (TCCTTCCCGCTGACCAAA); reverse
(TGTCCGTTGTCTTTCCTGTCAA)
RANKL: forward (TCCTTCCCGCTGACCAAA); reverse
(TGTCCGTTGTCTTTCCTGTCAA)
Runx2: forward (GACTGTGGTTACCGTCATGGC);reverse
(ACTTGGTTTTTCATAACAGCGGA)
Statistical Analysis
Statistical analysis was conducted using SPSS 21.0 software. Measurement data are presented as mean and standard deviation (SD). The statistical differences between two groups were evaluated by unpaired Student’s t test, and P < 0.05 was considered statistically significant.
Results
Effect of Pb on Body Weight
After 24 weeks of treatment, the bodyweights of mice in the control group increased slightly, while that in the lead group did not change significantly. There was no statistical difference between the two groups at the end of the experiment (Fig. 1).
Effect of Pb on Hematologic System
Lead exposure did not affect red blood cell count or hemoglobin concentration in mice in the lead group. There was no significant difference in white blood cell count or platelet concentration between the two groups (Fig. 2).
Effect of Pb on BMD and Morphology of Trabecular Bone
Radiographs of Pb-exposed animals showed a decrease in femoral trabecular bone as visualized by increased radiolucency in these regions. Micro-CT analysis demonstrated that, compared with the control animals, Pb exposure significantly decreased BMD at the distal femur (Fig. 3).
Micro-CT Analysis of the Distal Femur
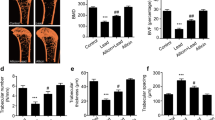
Quantitative measures using Micro-CT analysis of trabecular bone at the distal femur showed a significant decrease in Tb.N in the mice exposed to lead. Similarly, BV/TV and Tb.Th in the lead-treated group decreased compared with the control group. There was no difference in Tb.Sp between the two groups (Fig. 4b).
Effects of Pb on trabecular bone in femur. a Images are of distal femur and are representative of transverse sections from control and Pb-exposed mice. b Quantitative analysis of trabecular bone by micro-CT. BV/TV: bone volume to total tissue volume ratio; Tb.N: trabecular number; Tb.Th: trabecular thickness; Tb.Sp: trabecular spacing. The data are shown as mean ± SD, *P < 0.05
Micro-CT analysis of the femoral midshaft showed that Pb had no effect on cortical bone, neither in cortical BMD nor in Ct.Th (Fig. 5b).
Histological Analysis of Proximal Tibia
Bone marrow stromal cells were decreased following chronic administration of Pb intervention. Also, lipid droplet vacuoles were observed in the Pb group but not in the controls (Fig. 6).
Quantitative Real-Time-PCR Analysis of Bone Formation and Bone Resorption
The gene expressions of OPG/RANKL and Runx2 were quantitated. Levels of OPG were significantly decreased in mice in the Pb-treated group, and there was no effect of treatment in the expression level of RANKL between the two groups (Fig. 7a,b). In addition, Pb inhibited the expression of Runx2 compared with the control group (Fig. 7c).
Discussion
Toxicity due to lead exposure is associated with many health problems not only in the occupational staff but also in the general public [18,19,20]. Many studies have indicated that lifetime low-level lead exposures have a negative impact on human health [21, 22], even at blood lead levels < 10 μg/dL, which is the current adopted threshold of concern as defined by the US Centers for Disease Control and Prevention. In our study, lead exposure did not cause anemia during 24 weeks, which indicate that even low-level lead exposure in adult mice can lead to skeletal changes. The results are consistent with the results of epidemiological studies [23], proving adverse effect of lead on bone microstructure by in vivo experiments.
BMD is an indicator of bone health, and decreased BMD is an important biomarker for fracture risk. In the present study, lead significantly decreased BMD after 24 weeks of treatment, producing an osteoporosis phenotype in this group. Several studies have shown the potential impact of lead exposure on the incidence of osteoporosis and the likely mechanisms involved of its toxic effects [24, 25]. In recent years, Micro-CT has been widely used to observe the internal structure of bones and is recognized as the gold standard for assessing bone micro-architecture [26]. In this study, right femurs were scanned by Micro-CT. Bones are composed of numerous trabeculae, which are irregularly arranged within the bones. From a mechanical point of view, the strength of bone depends not only on the amount of internal material content but also on the arrangement of the trabeculae between each other, namely the internal structure of bone. Once trabecular bone decreases, becomes thin and spare, bone will become brittle [27, 28]. The accumulation of lead in bone over time has a negative effect on bone formation that results in the decrease in the number of trabeculae [29], consistent with the results of the present study. Data from the present study also demonstrated a decrease in trabecular thickness, which may be associated with lead toxic effects on bone mineralization.
The RANKL released by osteoblasts binds to the RANK on the surface of osteoclasts and then promotes the differentiation and activation of osteoclasts through the NF-κB, JNK, protein kinase B, and other pathways [30,31,32]. OPG can competitively inhibit the binding between RANK and RANKL and suppress the function of osteoclasts [33]. In our study, mice in the Pb-treated group showed a significant decrease in mRNA levels of OPG in bone tissue. Monir AU reported the increase in the biomarkers for bone resorption in serum of mice treated with Pb [34]. Both of the results indicated increased activation of osteoclasts. After the bone development is complete in the young adult, the bone still maintains the cycle of bone formation and resorption throughout adult life [35]. Our results showed the decrease in the biomarkers for bone formation (Runx2), provides an explanation for the decrease in BMD and trabecular number. Once bone metabolic balance is perturbed, superabundant osteoclasts and reduced bone formation will cause osteolysis. Furthermore, it is known that Pb exposure facilitates adipose cell formation [36]. After lead exposure, bone mesenchymal stem cells differentiated to adipocyte increase and fewer osteoblasts are generated [14]. In present study, lipid droplet vacuoles were observed in the tibias of the Pb-treated group and gene expression associated with bone formation was downregulated. These data support an association between Pb-depressed bone formation and increased adipogenesis.
In conclusion, our study demonstrated that in the absence of classic diseases caused by the lead, low-level lead exposure decreased bone formation and promoted bone resorption, which resulted in deterioration in bone microstructure. The study supports the association between the increased blood lead levels and an increased risk of fractures in older women and provides a mechanism to explain the associations [23]. Bone is the major reservoir of body lead and bone lead value increases with age. Elevated bone lead value has been found in population who have either been occupationally or environmentally exposed. Our study suggests that bone health should be monitored in Pb-exposed population, especially in the elderly.
References
Tong S, Schirnding YE, Von PT (2000) Environmental lead exposure: a public health problem of global dimensions. Bull World Health Organ 78(9):1068
Kasperczyk A, Dobrakowski M, Czuba ZP, Horak S, Kasperczyk S (2015) Environmental exposure to lead induces oxidative stress and modulates the function of the antioxidant defense system and the immune system in the semen of males with normal semen profile. Toxicol Appl Pharmacol 284(3):339–344
Wildemann TM, Mirhosseini N, Siciliano SD, Weber LP (2015) Cardiovascular responses to lead are biphasic, while methylmercury, but not inorganic mercury, monotonically increases blood pressure in rats. Toxicology 328:1–11
Murata K, Araki S (2010) Autonomic nervous system dysfunction in workers exposed to lead, zinc, and copper in relation to peripheral nerve conduction: a study of R-R interval variability. Am J Ind Med 20(5):663–671
Caito S, Acba L, Mmb P, Aschner M (2017) Toxicology of lead and its damage to mammalian organs. Metal Ions in Life Sciences 17
Parfitt AM (1988) Bone histomorphometry: standardization of nomenclature, symbols and units (summary of proposed system). Bone 9(1):67–69
Polák J, O’Flaherty EJ (1995) Physiologically based models of lead exposure in children. Toxicol Lett 78(4):67
Hu H, Rabinowitz M, Smith D (1998) Bone lead as a biological marker in epidemiologic studies of chronic toxicity: conceptual paradigms. Environ Health Perspect 106(1):1–8
Skerfving S, Nilsson U (1992) Assessment of accumulated body burden of metals. Toxicology letters 64-65 spec no:17
Gamblin C, Gordon CL, Muir DCF, Chettle DR, Webber CE (1994) In vivo measurements of bone lead content in residents of Southern Ontario. Appl Radiat Isot 45(10):1035–1038
Berlin K, Gerhardsson L, Börjesson J, Lindh E, Lundström N, Schütz A, Skerfving S, Edling C (1995) Lead intoxication caused by skeletal disease. Scand J Work Environ Health 21(4):296–300
Potula V, Henderson A, Kaye W (2005) Calcitropic hormones, bone turnover, and lead exposure among female smelter workers. Arch Environ Occup Health 60(4):195–204
Beier EE, Maher JR, Sheu TJ, Cory-Slechta DA, Berger AJ, Zuscik MJ, Puzas JE (2012) Heavy metal lead exposure, osteoporotic-like phenotype in an animal model, and depression of Wnt signaling. Environ Health Perspect 121(1):97–104
Beier EE, Inzana JA, Sheu TJ, Shu L, Puzas JE, Mooney RA (2015) Effects of combined exposure to lead and high-fat diet on bone quality in juvenile male mice. Environ Health Perspect 123(10):935–943. https://doi.org/10.1289/ehp.1408581
Campbell JR, Rosier RN, Novotny L, Puzas JE (2004) The association between environmental lead exposure and bone density in children. Environ Health Perspect 112(11):1200–1203. https://doi.org/10.1289/ehp.6555
Beier EE, Holz JD, Sheu TJ, Puzas JE (2016) Elevated lifetime lead exposure impedes osteoclast activity and produces an increase in bone mass in adolescent mice. Toxicol Sci : Off J Soc Toxicol 149(2):277–288. https://doi.org/10.1093/toxsci/kfv234
Bleecker ML, McNeill FE, Lindgren KN, Masten VL, Ford DP (1995) Relationship between bone lead and other indices of lead exposure in smelter workers. Toxicol Lett 77(1–3):241–248
Wedeen RP, Maesaka JK, Weiner B, Lipat GA, Lyons MM, Vitale LF, Joselow MM (1975) Occupational lead nephropathy. Am J Med 59(5):630–641
Karimooy HN, Mood MB, Hosseini M, Shadmanfar S (2010) Effects of occupational lead exposure on renal and nervous system of workers of traditional tile factories in Mashhad (northeast of Iran). Toxicol Ind Health 26(9):633–638
Evans M, Fored CM, Nise G, Bellocco R, Nyrén O, Elinder CG (2010) Occupational lead exposure and severe CKD: a population-based case-control and prospective observational cohort study in Sweden. Am J Kidney Dis 55(3):497–506
C C (1985) Preventing lead poisoning in young children--United States. MMWR Morb Mortal Wkly Rep 34 (5):66–73
Campbell JR, Auinger P (2007) The association between blood lead levels and osteoporosis among adults: results from the Third National Health and Nutrition Examination Survey (NHANES III). Environ Health Perspect 115(7):1018–1022
Khalil N, Cauley JA, Wilson JW, Talbott EO, Morrow L, Hochberg MC, Hillier TA, Muldoon SB, Cummings SR (2008) Relationship of blood lead levels to incident nonspine fractures and falls in older women: the study of osteoporotic fractures. J Bone Miner Res 23(9):1417–1425. https://doi.org/10.1359/jbmr.080404
Á-L P, Lee CM, Conti MI, Terrizzi AR, González-López S, Martínez MP (2017) Effects of chronic lead exposure on bone mineral properties in femurs of growing rats. Toxicology 377
Carmouche JJ, Puzas JE, Zhang X, Tiyapatanaputi P, Coryslechta DA, Gelein R, Zuscik M, Rosier RN, Boyce BF, O’Keefe RJ (2005) Lead exposure inhibits fracture healing and is associated with increased chondrogenesis, delay in cartilage mineralization, and a decrease in osteoprogenitor frequency. Environ Health Perspect 113(6):749–755
Ashton JR, West JL, Badea CT (2015) In vivo small animal micro-CT using nanoparticle contrast agents. Front Pharmacol 6:256. https://doi.org/10.3389/fphar.2015.00256
Van RB, Majumdar S, Pistoia W, Newitt DC, Kothari M, Laib A, Rüegsegger P (1998) Assessment of cancellous bone mechanical properties from micro-FE models based on micro-CT, pQCT and MR images. Technol Health Care Off J Eur Soc Eng Med 6(5–6):413–420
Res JBM (2010) Growing C57Bl/6 mice increase whole bone mechanical properties by increasing geometric and material properties. Journal of Bone & Mineral Research the Official Journal of the American Society for Bone & Mineral Research 14(12):2159–2166
Alvarez-Lloret P, Lee CM, Conti MI, Terrizzi AR, Gonzalez-Lopez S, Martinez MP (2017) Effects of chronic lead exposure on bone mineral properties in femurs of growing rats. Toxicology 377:64–72. https://doi.org/10.1016/j.tox.2016.11.017
Yuan LQ, Zhu JH, Wang HW, Liang QH, Xie H, Wu XP, Zhou H, Cui RR, Sheng ZF, Zhou HD (2011) RANKL is a downstream mediator for insulin-induced osteoblastic differentiation of vascular smooth muscle cells. Bone 47(12):S452–S452
Bezerra MC, Carvalho JF, Prokopowitsch AS, Pereira RMR (2005) RANK, RANKL and osteoprotegerin in arthritic bone loss. Braz J Med Biol Res 38(2):161–170
Udagawa N, Takahashi N, Jimi E, Matsuzaki K, Tsurukai T, Itoh K, Nakagawa N, Yasuda H, Goto M, Tsuda E (1999) Osteoblasts/stromal cells stimulate osteoclast activation through expression of osteoclast differentiation factor/RANKL but not macrophage colony-stimulating factor. Bone 25(5):517–523
Thomas GP, Baker SU, Eisman JA, Gardiner EM (2001) Changing RANKL/OPG mRNA expression in differentiating murine primary osteoblasts. J Endocrinol 170(2):451–460
Monir AU, Gundberg CM, Yagerman SE, van der Meulen MC, Budell WC, Boskey AL, Dowd TL (2010) The effect of lead on bone mineral properties from female adult C57/BL6 mice. Bone 47(5):888–894. https://doi.org/10.1016/j.bone.2010.07.013
Lei J, Liu MQ, Yap AUJ, Fu KY (2012) Condylar subchondral formation of cortical bone in adolescents and young adults. Br J Oral Maxillofac Surg 51(1):63–68
Tencerova M, Figeac F, Ditzel N, Taipaleenmaki H, Nielsen TK, Kassem M (2018) High-fat diet-induced obesity promotes expansion of bone marrow adipose tissue and impairs skeletal stem cell functions in mice. J Bone Miner Res: Off J Am Soc Bone Miner Res 33(6):1154–1165. https://doi.org/10.1002/jbmr.3408
Funding
This work was supported by National Natural Science Foundation of China (grant number 81773414) and National Natural Science Foundation of China (grant number 81673151).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Animal care followed the Guide for the Care and Use of Laboratory Animal, and the study protocols were approved by the Soochow University Institutional Animal Care and Use Committee.
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sheng, Z., Wang, S., Zhang, X. et al. Long-Term Exposure to Low-Dose Lead Induced Deterioration in Bone Microstructure of Male Mice. Biol Trace Elem Res 195, 491–498 (2020). https://doi.org/10.1007/s12011-019-01864-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-019-01864-7