Abstract
Nano-titanium dioxide is a kind of widely used nanomaterial that exhibits various adverse outcomes. However, the role of oxidative stress in this regard remains controversial. This study aimed to evaluate whether oxidative stress is one of the toxicity mechanisms induced by nano-titanium dioxide in rats and mice model. In this meta-analysis, 64 relevant publications were included through detailed database search. The pooled results showed that nano-titanium dioxide exposure could promote the expression of oxidants, such as malonaldehyde (MDA), 8-hydroxy-2-deoxyguanosine (8-OHdG), superoxide anion (O2−), and hydrogen peroxide (H2O2). Meanwhile, the levels of antioxidant-related enzymes and molecules, such as superoxide dismutase (SOD), glutathione (GSH), glutathione peroxidase (GPx), and catalase (CAT), were reduced. Subgroup analysis revealed that different intervention routes, exposure periods, exposure dosages, and sample sources could affect the oxidative stress when exposed to nano-titanium dioxide. It was worth noting that the levels of MDA, 8-OHdG, and GSH significantly increased (P < 0.05) when the particle size of nano-titanium dioxide was < 10 nm, whereas H2O2, SOD, and GPx showed the highest effect at 10–40 nm. This study indicated that nano-titanium dioxide could cause oxidative damage by affecting the levels of enzymes and molecules involved in oxidative stress in rats and mice. And these results could provide a reference for studies of the toxicity mechanism induced by nano-titanium dioxide in the future.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
With the development of nanotechnology, nanomaterials have been widely used in various fields [1]. Nano-titanium dioxide was widely used in industrial products, medicines, food, and cosmetics [2, 3], due to its small particle size, large surface area per unit mass, and unique optical and electronic properties [4, 5]. Accordingly, nano-titanium dioxide could enter the environment and contact with people through the respiratory tract, digestive tract, and even injection [6,7,8].
Traditionally, titanium dioxide fine particles were considered to possess poor solubility and low toxicity particles [9]. However, Pelclova et al. observed nucleic acid and protein oxidation markers increased significantly in exhaled breath condensates of workers exposed to nano-titanium dioxide [10]; another prospective cohort study also suggested that inflammatory markers of workers changed [11]. These researches showed that nano-titanium dioxide could cause damage to human health in occupational environment. Moreover, a large number of animal studies and cell experiments have proven that the exposure of nano-titanium dioxide at different intervention routes, periods, dosages, sample sources, and particle sizes could cause different degrees of damage to organs in rats and mice, such as the liver, kidney, heart, lung, spleen, testis, and ovary [12,13,14]. The International Agency for Research on Cancer (IARC) classified the nano-titanium dioxide as possibly carcinogenic to humans (Category 2B) [15]. Therefore, the toxicological mechanism of nano-titanium dioxide should be investigated.
Several studies suggested that high level of inflammatory factors and apoptosis-related genes played an important role in the toxicity of nano-titanium dioxide [16]. Other studies confirmed that oxidative stress plays a major role in this respect [17]. In addition, certain experiments have shown that oxidative stress promoted the expression of inflammatory and apoptotic proteins, thus immensely increased the toxic effects of nano-titanium dioxide [18, 19]. The role of oxidative stress in the toxicity of nano-titanium dioxide should be determined.
There have been a large number of articles exploring the relationship between the toxic effects of nano-titanium dioxide and oxidative stress. Previous studies showed that after exposure to nano-titanium dioxide, the oxidative-antioxidant system experienced an imbalance; the levels of superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx) were decreased, and the level of malonaldehyde (MDA) was increased in a dose-dependent manner in the liver and brain of rats and mice [20, 21]. However, various studies which used different intervention routes, exposure periods, exposure dosages, sample sources, and particle sizes of nano-titanium dioxide obtained different conclusion, even some studies reached the opposite. Geyu et al. observed that the levels of SOD and GPx in the liver of rats increased after exposure to nano-titanium dioxide [22].
The absence of relevant evidence-based evidence is due to the lack of systematic analysis of the relationship between the toxic effects of nano-titanium dioxide and oxidative stress. Thus, systematic evaluation of these studies about oxidative damage caused by nano-titanium dioxide is necessary. This study conducted a meta-analysis of experimental studies on oxidative damage induced by nano-titanium dioxide to describe the role of oxidative stress in the damage induced by nano-titanium dioxide.
Materials and Methods
Search Strategy
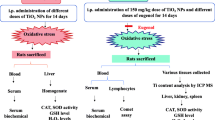
A comprehensive literature about toxicity of nano-titanium dioxide has been performed between January 1, 1980, and November 3, 2018. The studies were searched on Pubmed, Web of science, Embase, CNKI, VIP, WanFang, SinoMed databases, restricting to English and Chinese, with keywords included (nano-titanium dioxide OR nano-TiO2) AND (rats OR mice) AND (oxygenation OR oxidant). The full texts were reviewed and articles that met the research requirements were included in this meta-analysis.
Eligibility Criteria
Eligibility criteria for the meta-analysis were as follows: (1) randomized controlled adult rats or mice experiments and studies published in English and Chinese; (2) no restriction on strain and gender; (3) the experimental group was considered to be exposed to nano-titanium dioxide, the control group was the blank control group, and both of them used the oxidative damage index as the outcome; (4) the highest dosage and the longest experimental period were selected for the analysis from a range of nano-titanium dioxide dosages and periods. These oxidative damage indexes included lipid peroxidation (LPO), MDA, reactive oxygen species (ROS), 8-hydroxy-2-deoxyguanosine (8-OHdG), superoxide anion (O2−), hydrogen peroxide (H2O2), SOD, glutathione (GSH), GPx, and CAT. When response was not received from the authors, the numerical values were measured from the graphs by a digital ruler.
The required instructions for the qualified literature comprised the following: (1) biological model: animal species and genders; (2) study design: intervention route, exposure period, exposure dosage, sample source, and particle size of nano-titanium dioxide (less than or equal to 100 nm); (3) main results (oxidative damage indexes): LPO, MDA, ROS, 8-OHdG, O2−, H2O2, SOD, GSH, GPx, and CAT. A total of 64 published papers were included in this study.
Exclusion Criteria
Exclusion criteria were as follows: (1) duplicate publications, (2) title and abstract that were irrelevant to nano-titanium dioxide, (3) usage of non-adult animals (less than 2 months or 8 weeks in rats and 6 weeks for mice), (4) data unrelated to the oxidative damage indexes, (5) lack of appropriate controls, (6) conference or reviews.
Data Extraction
The data were extracted independently from each article by two members of the research. The following information were extracted from the complete manuscript of each qualified study: publication characteristics (title of the study, first author, publication date, and journal/magazine), data on the experimental and control groups (n, mean ± SD), subject characteristics (species, gender), intervention route, exposure period, exposure dosage, sample source, and particle size of nano-titanium dioxide. The discrepancies in the information were decided the results by Prof. LJM, when two reviewers held different opinions.
Data Analysis
The mean values for each outcome indicator differed between the experimental and control groups. Significant heterogeneity was detected (P < 0.05, I2 > 75%). Therefore, a random effects model was applied for the meta-analysis. Subgroup analysis was performed to determine the source of heterogeneity. Continuous variables were estimated as standardized mean differences (SMD) with 95% confidence intervals (95% CI) between the nano-titanium dioxide treated and control animals. All reported P values were two-sided and a significance level of 0.05 was used. Subgroup analyses were performed based on the intervention route (respiratory tract, gavage, and injection), exposure period (≤ 7, ≤ 30, and > 30 days), exposure dosage (≤ 10, ≤ 100, > 100 mg), sample source (serum, liver, kidney), and nano-titanium dioxide particle size (< 10, 10–40, > 40 nm), to determine the factors associated with the differences among study results in the outcome indicators. For additional insight, meta-regression was used to analyze the sources of heterogeneity. Publication bias was explored using funnel plots. Sensitivity analysis was performed with one study removed at a time to assess whether the results were remarkably affected by a single study. All analyses were implemented in Review Manager Version 5.2 (The Nordic Cochrane Centre, The Cochrane Collaboration, 2012) and Stata 12.0 (StataCorp., College Station, Texas, TX, USA, 2011).
Result
Study Characteristics
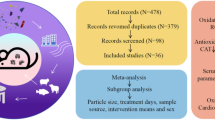
Using our search strategy, 2518 references were initially included (Fig. 1), and screened 64 studies (Table 1) were evaluated based on the exclusion criteria for the final meta-analysis. All the subjects were restricted to rats and mice, and the type and extent of oxidative damage caused by nano-titanium dioxide to animal models were investigated (Fig. 2).
Effect of nano-titanium dioxide on LPO level. Forest plot showed the effect of nano-titanium dioxide treatment on LPO in the treated and control groups. Nano-titanium dioxide treatment could not change the value of LPO (95% CI, − 12.56–11.55, Z = 0.080, P = 0.94). SMD, standardized mean difference; IV, independent variable; 95% CI, 95% confidence interval; SD, standard deviation
Effect of Nano-titanium Dioxide on Oxidants and Antioxidants
In this meta-analysis, 64 papers were identified to measure the changes in 10 indicators and calculate the oxidation and antioxidant levels in rats and mice after exposure to nano-titanium dioxide. Summary analysis showed the levels of oxidants, such as MDA, 8-OHdG, O2−, and H2O2, which were more highly expressed in the experimental group (nano-titanium dioxide treatment) than the control (Table 2). The results were described in detail in Figs. 3, 4, 5, 6, and 7. In the six evaluations of oxides, the SMD values were showed to be greater than 1, and analysis of MDA, 8-OHdG, O2−, and H2O2 was associated with significant heterogeneity (P < 0.01). For the antioxidant levels, pooled analysis of the indicators showed that SOD, GSH, GPx, and CAT levels were lower in the experimental group (nano-titanium dioxide treatment) than the control (Table 2). The results were described in detail in Figs. 8, 9, 10, and 11. In the four assessments of antioxidants, the SMD values were showed to be less than − 2, and analysis of each molecule was associated with significant heterogeneity (P < 0.01).
Effect of nano-titanium dioxide on MDA level. Forest plot showed the effect of nano-titanium dioxide treatment on MDA in the treated and control groups. Nano-titanium dioxide treatment could promote the level of MDA (95% CI, 4.53–6.52, Z = 10.86, P < 0.00001). SMD, standardized mean difference; IV, independent variable; 95% CI, 95% confidence interval; SD, standard deviation
Effect of nano-titanium dioxide on ROS level. Forest plot showed the effect of nano-titanium dioxide treatment on ROS in the treated and control groups. Nano-titanium dioxide treatment could not change the level of ROS (95% CI, − 0.33–3.55, Z = 1.62, P = 0.10). SMD, standardized mean difference; IV, independent variable; 95% CI, 95% confidence interval; SD, standard deviation
Effect of nano-titanium dioxide on 8-OHdG level. Forest plot showed the effect of nano-titanium dioxide treatment on 8-OHdG in the treated and control groups. Nano-titanium dioxide treatment could promote the level of 8-OHdG (95% CI, 1.74–5.93, Z = 3.58, P = 0.0003). SMD, standardized mean difference; IV, independent variable; 95% CI, 95% confidence interval; SD, standard deviation
Effect of nano-titanium dioxide on O2− level. Forest plot showed the effect of nano-titanium dioxide treatment on O2− in the treated and control groups. Nano-titanium dioxide treatment could promote the level of O2− (95% CI, 9.19–15.37, Z = 7.79, P < 0.00001). SMD, standardized mean difference; IV, independent variable; 95% CI, 95% confidence interval; SD, standard deviation
Effect of nano-titanium dioxide on H2O2 level. Forest plot showed the effect of nano-titanium dioxide treatment on H2O2 in the treated and control groups. Nano-titanium dioxide treatment could promote the level of H2O2 (95% CI, 4.09–10.57, Z = 4.43, P < 0.00001). SMD, standardized mean difference; IV, independent variable; 95% CI, 95% confidence interval; SD, standard deviation
Effect of nano-titanium dioxide on SOD level. Forest plot showed the effect of nano-titanium dioxide treatment on SOD in the treated and control groups. Nano-titanium dioxide treatment could reduce the level of SOD (95% CI, − 4.05 to − 2.56, Z = 8.71, P < 0.00001). SMD, standardized mean difference; IV, independent variable; 95% CI, 95% confidence interval; SD, standard deviation
Effect of nano-titanium dioxide on GSH level. Forest plot showed the effect of nano-titanium dioxide treatment on GSH in the treated and control groups. Nano-titanium dioxide treatment could reduce the level of GSH (95% CI, − 5.20 to − 2.05, Z = 4.52, P < 0.00001). SMD, standardized mean difference; IV, independent variable; 95% CI, 95% confidence interval; SD, standard deviation
Effect of nano-titanium dioxide on GPx level. Forest plot showed the effect of nano-titanium dioxide treatment on GPx in the treated and control groups. Nano-titanium dioxide treatment could reduce the level of GPx (95% CI, − 4.27 to − 2.28, Z = 6.46, P < 0.00001). SMD, standardized mean difference; IV, independent variable; 95% CI, 95% confidence interval; SD, standard deviation
Effect of nano-titanium dioxide on CAT level. Forest plot showed the effect of nano-titanium dioxide treatment on CAT in the treated and control groups. Nano-titanium dioxide treatment could reduce the level of CAT (95% CI, − 4.48 to − 1.58, Z = 4.09, P < 0.0001). SMD, standardized mean difference; IV, independent variable; 95% CI, 95% confidence interval; SD, standard deviation
Subgroup Analysis
The subgroup analysis explored the source of heterogeneity by intervention route (respiratory tract, gavage, and injection), period of nano-titanium dioxide treatment (≤ 7, ≤ 30, > 30 days), nano-titanium dioxide dosage (≤ 10, ≤ 100, > 100 mg), sample source (serum, liver, kidney), and particle size of nano-titanium dioxide (< 10, 10–40, > 40 nm).
The SMD values of 8-OHdG, O2−, H2O2, and GPx indicated that gavage tract administration was higher than injection (P < 0.05, see Fig. 12b, c, d, g), whereas those of GSH and CAT through injection and respiratory tract administration showed the highest level, respectively (P < 0.05, see Fig. 12f, h).
Subgroup analysis determined the effect of the nano-titanium dioxide intervention route on oxidative damage. SMD, standardized mean difference. Nano-titanium dioxide could promote the expression of 8-OHdG, O2−, H2O2, and GPx through gavage tract (P < 0.05, b, c, d, g). The SMD of GSH and CAT administered through injection and the respiratory tract showed the highest level, respectively (P < 0.05, f, h). No statistical difference was detected for other indicators. Data were expressed as SMD and 95% CI
The SMD values of MDA, 8-OHdG, and H2O2 indicated that it yielded higher levels at an exposure period > 30 days than ≤ 30 or ≤ 7 days (P < 0.05, see Fig. 13a, b, d); the SMD value of O2− at an exposure period ≤ 7 days showed the lowest level (P < 0.05, see Fig. 13c), and the SMD values of SOD and GPx at an exposure period ≤ 7 days showed the highest level (P < 0.05, see Fig. 13e, g).
Subgroup analysis determined the effect of the nano-titanium dioxide period on oxidative damage. SMD, standardized mean difference. The promotive effect of MDA, 8-OHdG, and H2O2 in exposure period > 30 days is higher than ≤ 30 days (P < 0.05, a, b, d); the O2− was showed lowest level which exposure period ≤ 7 days (P < 0.05, c), and the SOD and GPx were showed highest level which exposure period ≤ 7 days (P < 0.05, e, g). No statistical difference was detected for the other indicators. Data were expressed as SMD and 95% CI
The absolute SMD values of O2−, H2O2 at exposure dosage > 100 mg showed the highest level (P < 0.05, see Fig. 14c, d).
Subgroup analysis determined the effect of the nano-titanium dioxide dosage on oxidative damage. SMD, standardized mean difference. The absolute SMD of O2−, H2O2 were showed highest level in exposure dosage > 100 mg (P < 0.05, c, d). No statistical difference was detected for the other indicators. Data were expressed as SMD and 95% CI
The absolute SMD values of MDA, 8-OHdG, SOD, and CAT indicated that tissue samples were higher than serum samples (P < 0.05, see Fig. 15a, b, e, h). The SMD values of O2− and H2O2 indicated that their levels were higher in kidney samples than liver samples (P < 0.05, see Fig. 15c, d), and the GSH showed the opposite trend (P < 0.05, see Fig. 15f).
Subgroup analysis determined the effect of the nano-titanium dioxide sample sources on oxidative damage. SMD, standardized mean difference. The absolute SMD values of MDA, 8-OHdG, SOD, and CAT indicated that tissue samples were higher than serum samples (P < 0.05, a, b, e, h); the SMD values of O2−, H2O2 indicated that kidney samples were higher than liver samples (P < 0.05, c, d); the GSH showed the opposite trend (P < 0.05, f). No statistical difference was detected for the other indicators. Data were expressed as SMD and 95% CI
The absolute SMD values of MDA, 8-OHdG, and GSH indicated that particle size < 10 nm were higher than < 40 or 10–40 nm (P < 0.05, see Fig. 16a, b, e), whereas those of H2O2, SOD, and GPx showed the highest effect at 10–40 nm (P < 0.05, see Fig. 16c, d, f).
Subgroup analysis determined the effect of the nano-titanium dioxide particle size on oxidative damage. SMD, standardized mean difference. The absolute SMD values of MDA, 8-OHdG, and GSH particle size < 10 nm were higher than < 40 nm or 10–40 nm (P < 0.05, a, b, e), and the absolute SMD value of H2O2, SOD, and GPx showed the highest effect at 10–40 nm (P < 0.05, c, d, f). No statistical difference was detected for the other indicators. Data were expressed as SMD and 95% CI
Meta-regression
The meta-regression analysis showed that intervention route was significantly associated with the differences in H2O2, GSH, and GPx. The experiment period was significantly associated with the differences in O2−, H2O2, and GPx. The dosage was significantly associated with the differences in GPx and CAT. Meanwhile, MDA, O2−, H2O2, GSH, and GPx significantly varied in different sample sources. Nano-titanium dioxide particle size was significantly associated with the differences in MDA, H2O2, and CAT (Table 3).
Sensitivity Analysis
Sensitivity analysis was implemented to evaluate the robustness of our results. All results were located on the two sides of the midline with no notable deviation (Fig. 17). These results indicate that no individual study influenced the combined results.
Publication Bias
The funnel plot for the studies revealed that all indicators except GSH exhibited publication bias (Egger’s test, P < 0.05). Thus, a trim-and-fill method was used to identify and correct the asymmetry of funnel plot caused by publication bias (Fig. 18).
Discussion
Animal experiments in rats and mice showed that nano-titanium dioxide particles could produce ROS accumulated in organs, cause imbalance of the oxidation-antioxidant system, and lead to oxidative damage in animal tissues [6, 38]. The results indicated that nano-titanium dioxide significantly increased the levels of oxidants, such as MDA, O2−, and H2O2, and reduced the levels of anti-oxidative enzymes, such as SOD, GSH, GPx, and CAT. Meanwhile, nano-titanium dioxide induced breakage of DNA strands resulting in production of 8-OHdG. In addition, the results indicated that oxidative damage caused by nano-titanium dioxide was related to intervention route, period of nano-titanium dioxide treatment, nano-titanium dioxide dosage, sample source, and particle size of nano-titanium dioxide.
The oxidation process of nano-titanium dioxide is complex [58]. Nano-titanium dioxide penetrated the cell membrane and produced ROS [77, 79]. These ROS (O2− and H2O2) could bind to LPO to destroy the cell membrane permeability. The reaction of ROS and unsaturated fatty acids in the membrane could enhance LPO and decompose numerous free radicals [80]. Meanwhile, MDA as an intermediate causes the free radical chain reaction by catalyzing O2 to form O2−, and produces a number of ROS [27]. In addition, ROS could cause DNA oxidative damage and produce a large amount of 8-OHdG by acting on mitochondria [58]. The antioxidant system is activated when the level of oxides is overexpressed. First, SOD catalyzes O2− to form H2O2. Then, GSH, GPx, and CAT work collectively to generate H2O from H2O2 in order to remove excess free radicals and peroxides [41, 53, 69]. The oxidative mechanism of nano-titanium dioxide is shown in Fig. 19. Numerous factors affect the role of enzymes and related molecules involved in oxidative stress caused by nano-titanium dioxide, and these might explain the heterogeneity among studies.
The subgroup analysis of this study indicated that the levels of 8-OHdG, O2−, H2O2, and GPx significantly increased through gavage administration rather than injection. This condition might be attributed to the longer period exposure in gavage; the continuous activation of oxidation system leads to serious oxidative damage to the body, so that the recovery might be slower [20]. Compared with serum, nano-titanium dioxide could significantly alter the levels of oxidative- and antioxidant-related molecules in the liver and kidney. The reason might be owed to nano-titanium dioxide which mainly acts on the liver and kidney [81, 82].
In this meta-analysis, long exposure period and high dosage could increase the levels of MDA, 8-OHdG, O2−, and H2O2. Some studies support the time-dependent and dose-dependent effects of toxicity induced by nano-titanium dioxide [27, 58]. With the decreased of nano-titanium dioxide particle size, the surface activity increased and clearance capacity of organisms gradually decreased [83, 84]. When the particle size of nano-titanium dioxide was small, high levels of MDA, 8-OHdG were observed; GSH activity of antioxidant enzymes was inhibited. Besides, the levels of H2O2, SOD, and GPx were higher at a particle size of 10–40 nm, suggesting the possible non-linear relationship between the toxicity effect of nano-titanium dioxide and particle size. The meta-analysis of Chang et al. showed that the proportion of positive studies on nano-titanium dioxide–induced cytotoxicity at a particle size of 10–40 nm for 24 h was higher than that with a particle size greater than other groups [85].
Conclusion
The results of this meta-analysis indicated that nano-titanium dioxide could cause oxidative damage in organs of rats and mice. Nano-titanium dioxide elevated the levels of oxidative enzymes and decreased the levels of antioxidants. Further studies showed that higher dosage, longer period, and smaller particle size of nano-titanium dioxide promote the formation of oxides when exposed to the liver and kidney, resulting in the imbalance of oxidation-antioxidant system and body damage. This paper might supplement the regulatory toxicity mechanism of nano-titanium dioxide, and future research whether nano-titanium dioxide could result oxidative damage to human body should be confirmed.
Limitation
The limitation of this study is the significant heterogeneity of combined effects. Crystal and surface conditions should be the focus of future research due to their significant influence on nano-titanium dioxide–induced toxicity. However, these factors were excluded in this study, as they were rarely mentioned in the articles. Instead, this study included animal manuscripts, and cell publications were disregarded.
References
Wang Z, Zhang T, Huang F, Wang Z (2018) The reproductive and developmental toxicity of nanoparticles: a bibliometric analysis. Toxicol Ind Health 34(3):169–177. https://doi.org/10.1177/0748233717744430
Weir A, Westerhoff P, Fabricius L, Hristovski K, Goetz N (2012) Titanium dioxide nanoparticles in food and personal care products. Environ Sci Technol 46(4):2242–2250. https://doi.org/10.1021/es204168d
Pugazhendhi A, Tnji E, Karuppusamy I, Kathirvel B (2018) Inorganic nanoparticles: a potential cancer therapy for human welfare. Int J Pharm 539(1-2):1–29. https://doi.org/10.1016/j.ijpharm.2018.01.034
Chen X, Mao SS (2007) Titanium dioxide nanomaterials: synthesis, properties, modifications, and applications. Chem Rev 107(7):2891–2959. https://doi.org/10.1021/cr0500535
Kumar PSM, Ponnusamy VK, Deepthi KR (2018) Controlled synthesis of Pt nanoparticle supported TiO2 nanorods as efficient and stable electrocatalysts for the oxygen reduction reaction. J Mater Chem A 6(46):23435–23444
Shakeel M, Jabeen F, Qureshi NA, Fakhr EAM (2016) Toxic effects of titanium dioxide nanoparticles and titanium dioxide bulk salt in the liver and blood of male Sprague-Dawley rats assessed by different assays. Biol Trace Elem Res 173(2):405–426. https://doi.org/10.1007/s12011-016-0677-4
Shrivastava R, Raza S, Yadav A, Kushwaha P, Flora SJ (2014) Effects of sub-acute exposure to TiO2, ZnO and Al2O3 nanoparticles on oxidative stress and histological changes in mouse liver and brain. Drug Chem Toxicol 37(3):336–347. https://doi.org/10.3109/01480545.2013.866134
Li YS, Ootsuyama Y, Kawasaki Y, Morimoto Y, Higashi T, Kawai K (2018) Oxidative DNA damage in the rat lung induced by intratracheal instillation and inhalation of nanoparticles. J Clin Biochem Nutr 62(3):238–241. https://doi.org/10.3164/jcbn.17-70
Valberg PA, Bruch J, Mccunney RJ (2009) Are rat results from intratracheal instillation of 19 granular dusts a reliable basis for predicting cancer risk? Regul Toxicol Pharm 54(1):72–83. https://doi.org/10.1016/j.yrtph.2009.02.008
Glass DC, Mazhar M, Xiang S, Dean P, Simpson P, Priestly B, Plebanski M, Abramson M, Sim MR, Dennekamp M (2017) Immunological effects among workers who handle engineered nanoparticles. Occup Environ Med 74(12):868–876. https://doi.org/10.1136/oemed-2016-104111
Rollerova E, Tulinska J, Liskova A, Kuricova M, Kovriznych J, Mlynarcikova A, Kiss A, Scsukova S (2015) Titanium dioxide nanoparticles: some aspects of toxicity/focus on the development. Endocr Regul 49(2):97–112. https://doi.org/10.4149/endo_2015_02_97
Hongbo S, Magaye R, Castranova V, Jinshun Z (2013) Titanium dioxide nanoparticles: a review of current toxicological data. Part Fibre Toxicol 10(1):15. https://doi.org/10.1186/1743-8977-10-15
Zhang X, Li W, Yang Z (2015) Toxicology of nanosized titanium dioxide: an update. Arch Toxicol 89(12):2207–2217. https://doi.org/10.1007/s00204-015-1594-6
Chang X, Zhang Y, Tang M, Bei W (2013) Health effects of exposure to nano-TiO2: a meta-analysis of experimental studies. Nanoscale Research Letters 8(1):51–51. https://doi.org/10.1186/1556-276X-8-51
IARC (2006) Cobalt in hard metals and cobalt sulfate, gallium arsenide, indium phosphide and vanadium pentoxide. IARC Monogr Eval Carcinog Risks Hum 86:1–294
Shahin NN, Mohamed MM (2017) Nano-sized titanium dioxide toxicity in rat prostate and testis: possible ameliorative effect of morin. Toxicol Appl Pharmacol 334:129–141. https://doi.org/10.1016/j.taap.2017.08.014
Cui YL (2012) Molecular mechanisms of liver injury of mice caused by exposure to titanium dioxide nanoparticles. Dissertation, University of Suzhou. 37-38.
Sang XZ (2014) Molecular mechanisms of immune function impaired of mouse spleen following exposure to titanium dioxide nanoparticles. Dissertation, University of Suzhou.66-69.
Liu HL (2011) The biotoxicity of three kinds of typical nanomaterials. Dissertation, Chinese academy of military medical sciences.50-61.
Jia X, Wang S, Zhou L, Sun L (2017) The potential liver, brain, and embryo toxicity of titanium dioxide nanoparticles on mice. Nanoscale Res Lett 12(1):478. https://doi.org/10.1186/s11671-017-2242-2
Meena R, Kumar S, Paulraj R (2015) Titanium oxide (TiO2) nanoparticles in induction of apoptosis and inflammatory response in brain. J Nanopart Res 17(491). doi: https://doi.org/10.1007/s11051-015-2868-x
Liang G, Pu Y, Yin L, Liu R, Ye B, Su Y, Li Y (2009) Influence of different sizes of titanium dioxide nanoparticles on hepatic and renal functions in rats with correlation to oxidative stress. J Toxicol Env Heal A 72(12):740–745. https://doi.org/10.1080/15287390902841516
Abdou KH, Moselhy WA, Mohamed HM, El-Nahass ES, Khalifa AG (2019) Moringa oleifera leaves extract protects titanium dioxide nanoparticles-induced nephrotoxicity via Nrf2/HO-1 signaling and amelioration of oxidative stress. Biol Trace Elem Res 187(1):181–191. https://doi.org/10.1007/s12011-018-1366-2
Alidadi H, Khorsandi L, Shirani M (2018) Effects of quercetin on tubular cell apoptosis and kidney damage in rats induced by titanium dioxide nanoparticles. Malaysian Journal of Medical Sciences 25(2):2–81. https://doi.org/10.21315/mjms2018.25.2.8
Al-Rasheed NM, Faddah LM, Mohamed AM, Abdel BN, Al-Rasheed NM, Mohammad RA (2013) Potential impact of quercetin and idebenone against immuno-inflammatory and oxidative renal damage induced in rats by titanium dioxide nanoparticles toxicity. J Oleo Sci 62(11):961–971
Elnagar A, Ibrahim A, Soliman AM (2018) Histopathological effects of titanium dioxide nanoparticles and the possible protective role of N-acetylcysteine on the testes of male albino rats. Int J Fertil Steril 12(3):249–256. https://doi.org/10.22074/ijfs.2018.5389
Attia HF, Soliman MM, Abdel-Rahman GH, Nassan MA, Ismail SA, Farouk M, Solcan C. (2013). Hepatoprotective effect of N-Acetylcystiene on the toxic hazards of titanium dioxide nanoparticles. Am J Pharmacol Toxicol 10(6). https://doi.org/10.3844/ajptsp.2013.141.147
Canli EG, Atli G, Canli M (2017) Response of the antioxidant enzymes of the erythrocyte and alterations in the serum biomarkers in rats following oral administration of nanoparticles. Environ Toxicol Pharmacol 50:145–150. https://doi.org/10.1016/j.etap.2017.02.007
Chen AJ (2016) Central neurotoxicity through gustatory nerve pathway by instillation of ZnO and TiO2 nanoparticles. Dissertation, University of Southern Medical. 26-28.
Chen Q, Zou H, Xing M (2012) Effects of different sized titanium dioxide nanoparticles on oxidative stress in rats. Zhejiang J Pre Med 24(11):1–3
Duan YM (2011) The molecular and immune mechanisms of the mouse liver and spleen damaged by exposure to nanoparticulate TiO2. Dissertation, University of Suzhou.36-41.
El-Shenawy NS, Al-Harbi MS, Al HF (2016) Hormonal and organ-specific dysfunction induced by the interaction between titanium dioxide nanoparticles and salicylic acid in male mice. J Basic Clin Physiol Pharmacol 27(4):425–435. https://doi.org/10.1515/jbcpp-2015-0124
Zhiyi F, Yihe J, Bing L, Yinhua Z, Yixin M (2007) Study on the effect of nanosized and microsized titanium dioxide for spermatogenesis function of male rats. Pre Med Forum (02):137–140
Fadda LM, Mohamed AM, Ali HM, Hagar H, Aldossari M (2018) Prophylactic administration of carnosine and melatonin abates the incidence of renal toxicity induced by an over dose of titanium dioxide nanoparticles. J Biochem Mol Toxicol 32(3):e22040. https://doi.org/10.1002/jbt.22040
Grissa I, Ezzi L, Chakroun S, Mabrouk A, Saleh AB, Braham H, Haouas Z, Cheikh HB (2017) Rosmarinus officinalis L. ameliorates titanium dioxide nanoparticles and induced some toxic effects in rats’ blood. Environ Sci Pollut Res Int 24(13):12474–12483. https://doi.org/10.1007/s11356-017-8848-1
Gui SX (2014) Molecular mechanisms of kidney injury following exposure to titanium dioxide nanoparticles in mice. Dissertation, University of Suzhou.45-47.
Gao GD (2013) Nano-titanium dioxide exposure induced reproductive toxicity and alterations of gene expression in mice. Dissertation, University of Suzhou. 89
Hassanein KM, El-Amir YO (2017) Protective effects of thymoquinone and avenanthramides on titanium dioxide nanoparticles induced toxicity in Sprague-Dawley rats. Pathol Res Pract 213(1):13–22. https://doi.org/10.1016/j.prp.2016.08.002
Hassanein K, El-Amir YO (2018) Ameliorative effects of thymoquinone on titanium dioxide nanoparticles induced acute toxicity in rats. Int J Vet Sci Med 6(1):16–21. https://doi.org/10.1016/j.ijvsm.2018.02.002
Hu RP (2013) Molecular mechanisms of impairment of spatial recognition memory in mice following exposure to titanium dioxide nanoparticles. Dissertation, University of Suzhou.55
Jafari A, Rasmi Y, Hajaghazadeh M, Karimipour M (2018) Hepatoprotective effect of thymol against subchronic toxicity of titanium dioxide nanoparticles: biochemical and histological evidences. Environ Toxicol Pharmacol 58:29–36. https://doi.org/10.1016/j.etap.2017.12.010
Jin YL, Yang M, Su SB, Chen JY, Liu M (2014) Protective effect of propolis on redox damage induced by nano-TiO2 in rats. Chin Occup Med 41(02):147–150
Khorsandi L, Orazizadeh M, Mansori E, Fakhredini F (2015) Glycyrrhizic acid attenuated lipid peroxidation induced by titanium dioxide nanoparticles in rat liver. Bratisl Lek Listy 116(6):383–388
Khorsandi L, Orazizadeh M, Moradi-Gharibvand N, Hemadi M, Mansouri E (2017) Beneficial effects of quercetin on titanium dioxide nanoparticles induced spermatogenesis defects in mice. Environ Sci Pollut Res Int 24(6):5595–5606. https://doi.org/10.1007/s11356-016-8325-2
Li YW (2009) Studies on the acute hazards of different sized nano-titanium dioxide particles to mice. Dissertation, University of Hebei Medical. 27
Caixia Yu (2018) Effects and mechanisms of lung injury induced by nitrogen-droped nano-titanium dioxide. Dissertation, University of Guizhou Medical. 28–29
Lin L, Song GL, Zeng HM, Hongmei Z, Zhengzheng W, Longlong L, Lu S, Zhangrui X, Wei L (2017) Effect of nano titanium dioxide on the kidney female mouse. J Shihezi hihezi hihezi hihezi hihezi hihezi Univniv 35(03):344–348
Liu HT (2014) Liver injury in mice caused by nano titanium dioxide and its molecular mechanism. Dissertation, University of Suzhou. 22-24.
Liu LL, Song GL, Niu Q, Ruling M, Yusong D, Zhenzhen W, Hongmei Z, Lin L (2018) The study of the nephrotoxicity on male mice induced by Nano-TiO2. J Shihezi Univ 36(03):278–281
Liu ZM (2016) Research for the nervous system and cardiovascular system toxicity of nano-titanium dioxide. Dissertation, University of Central China normal. 22-24.
Martins AJ, Azevedo LF, de Souza RC, Carneiro M, Venancio VP, Almeida MR, Antunes LMG, Carvalho HR, Rodrigues JL, Ogunjimi AT, Adeyemi JA, Barbosa FJ (2017) Evaluation of distribution, redox parameters, and genotoxicity in Wistar rats co-exposed to silver and titanium dioxide nanoparticles. J Toxicol Environ Health A 80(19-21):1156–1165. https://doi.org/10.1080/15287394.2017.1357376
Meena R, Kajal K, P R (2015) Cytotoxic and genotoxic effects of titanium dioxide nanoparticles in testicular cells of male Wistar rat. Appl Biochem Biotechnol 175(2):825–840. https://doi.org/10.1007/s12010-014-1299-y
Morgan A, Galal MK, Ogaly HA, Ibrahim MA, Abd-Elsalam RM, Noshy P (2017) Tiron ameliorates oxidative stress and inflammation in titanium dioxide nanoparticles induced nephrotoxicity of male rats. Biomed Pharmacother 93:779–787. https://doi.org/10.1016/j.biopha.2017.07.006
Morgan A, Ibrahim MA, Galal MK, Ogaly HA, Abd-Elsalam RM (2018) Innovative perception on using tiron to modulate the hepatotoxicity induced by titanium dioxide nanoparticles in male rats. Biomed Pharmacother 103:553–561. https://doi.org/10.1016/j.biopha.2018.04.064
Morgan AM, Ibrahim MA, Noshy PA (2017) Reproductive toxicity provoked by titanium dioxide nanoparticles and the ameliorative role of tiron in adult male rats. Biochem Biophys Res Commun 486(2):595–600. https://doi.org/10.1016/j.bbrc.2017.03.098
Niu LH, Dong HT, Yang LS, Bao S, Rong Z, Yujie N (2015) Effects of different particle sizes of nano-titanium dioxide on the antioxidant capacity of mice. J Hebei Med Univ 36(02):184–187
Rizk MZ, Ali SA, Hamed MA, El-Rigal NS, Aly HF, Salah HH (2017) Toxicity of titanium dioxide nanoparticles: effect of dose and time on biochemical disturbance, oxidative stress and genotoxicity in mice. Biomed Pharmacother 90:466–472. https://doi.org/10.1016/j.biopha.2017.03.089
Sheng L, Wang X, Sang X, Ze Y, Zhao X, Liu D, Gui S, Sun Q, Cheng J, Cheng Z, Hu R, Wang L, Hong F (2013) Cardiac oxidative damage in mice following exposure to nanoparticulate titanium dioxide. J Biomed Mater Res A 101(11):3238–3246. https://doi.org/10.1002/jbm.a.34634
Shukla RK, Kumar A, Vallabani NV, Pandey AK, Dhawan A (2014) Titanium dioxide nanoparticle-induced oxidative stress triggers DNA damage and hepatic injury in mice. Nanomedicine (Lond) 9(9):1423–1434. https://doi.org/10.2217/nnm.13.100
Song G, Lin L, Liu L, Wang K, Ding Y, Niu Q, Mu L, Wang H, Shen H, Guo S (2017) Toxic effects of anatase titanium dioxide nanoparticles on spermatogenesis and testicles in male mice. Pol J Environ Stud 26(6):2739–2745. https://doi.org/10.15244/pjoes/70807
Song MF, Wang YW, Wang C, Long Z, Kailiang P, Yayin L (2015) Oxidative DNA damage in liver of mice induced by titanium dioxide nanoparticles. J Environ Occup Med 32(04):358–361
Sun QQ (2014) Long-term exposure to titanium dioxide nanoparticles induced pulmonary toxicity in mice and its molecular mechanisms. Dissertation, University of Suzhou. 28-29.
Sun Q, Tan D, Ze Y, Sang X, Liu X, Gui S, Cheng Z, Cheng J, Hu R, Gao G, Liu G, Zhu M, Zhao X, Sheng L, Wang L, Tang M, Hong F (2012) Pulmotoxicological effects caused by long-term titanium dioxide nanoparticles exposure in mice. J Hazard Mater 235:47–53. https://doi.org/10.1016/j.jhazmat.2012.05.072
Sun Q, Tan D, Zhou Q, Liu X, Cheng Z, Liu G, Zhu M, Sang X, Gui S, Cheng J, Hu R, Tang M, Hong F (2012) Oxidative damage of lung and its protective mechanism in mice caused by long-term exposure to titanium dioxide nanoparticles. J Biomed Mater Res A 100A(10):2554–2562. https://doi.org/10.1002/jbm.a.34190
Tian JZ, Guo HC, Yue XL, Yi L, Yue Z, Weiyi W, Yaning W, Rong Z, Yujie N (2014) Protection of vitamin C on the cardiac injury induced by nano-titanium dioxide in mice. Chin J Pharmacol Toxicol 28(02):227–232
Wang S (2009) Target organs selectivity induced by intratracheally instilling titanium dioxide nanoparticles in rats. Dissertation, University of Southeast. 14-15.
Wang WB, Li CL, An LH, Zang FM, Lihua Y, Hua Y, Meng E, Huinan Z, Jie L (2013) Effects of nano-TiO2 exposure on oxidative stress and hippocampus structure in rats. J Environ Health 30(12):1054–1057
Wang WB (2014) Effect of nano-titanium dioxide on the central nervous system in rat and the mechanism. Dissertation, University of Shandong.24-33.
Wang J, Chen C, Liu Y, Jiao F, Li W, Lao F, Li Y, Li B, Ge C, Zhou G, Gao Y, Zhao Y, Chai Z (2008) Potential neurological lesion after nasal instillation of TiO2 nanoparticles in the anatase and rutile crystal phases. Toxicol Lett 183(1-3):72–80. https://doi.org/10.1016/j.toxlet.2008.10.001
Wang Y, Chen Z, Ba T, Pu J, Chen T, Song Y, Gu Y, Qian Q, Xu Y, Xiang K, Wang H, Jia G (2013) Susceptibility of young and adult rats to the oral toxicity of titanium dioxide nanoparticles. Small 9(9-10SI):1742–1752. https://doi.org/10.1002/smll.201201185
Wang Y, Chen ZJ, Ba T, Ji P, Xiaoxing C, Guang J (2014) Effects of TiO2 nanoparticles on antioxidant function and element content of liver and kidney tissues in young and adult rats. J Peking Univ 46(3):395–399. https://doi.org/10.3969/j.issn.1671-167X.2014.03.011
Chang XH, Chen LS, Yang T, Meng T, Bei W (2014) Experimental study on subacute pulmonary toxicity induced by intra-tracheal instillation of nano-TiO2 in rats. J Southeast Univ (3):616–620. https://doi.org/10.3969/j.issn.1001-0505.2014.03.029
Yu X, Zhao X, Ze Y, Wang L, Liu D, Hong J, Xu B, Lin A, Zhang C, Zhao Y, Li B, Hong F (2014) Changes of serum parameters of TiO2 nanoparticle-induced atherosclerosis in mice. J Hazard Mater 280:364–371. https://doi.org/10.1016/j.jhazmat.2014.08.015
Zhang T, Tang M, Wang S, Yang Y, Mingyu L, Bing Y (2008) Effects of lung perfusion with nano-titanium dioxide particles on the oxidative damage of rat viscera. Acta Sci Natu Univ Nankaiensis (03):24–28
Zhang WJ (2009) Study on toxicological and biological activity of nano-TiO2. Dissertation, Tianjin University of Technology. 13-14.
Zhao XY (2013) Effects of different sized nano-titanium dioxide particles on the oxidative stress and DNA damage in rats after intratracheal administration. Dissertation, University of Hebei Medical.33-36.
Zhao J, Li N, Wang S, Zhao X, Wang J, Yan J, Ruan J, Wang H, Hong F (2010) The mechanism of oxidative damage in the nephrotoxicity of mice caused by nano-anatase TiO2. J Exp Nanosci 5(5):447–462. https://doi.org/10.1080/17458081003628931
Zhu Y (2015) Effect of Foxo3a on DNA damage induced by nanoparticle titanium dioxide by intratracheal administration in rat. Dissertation, University of Hebei medical.39-41.
Tripathi VK, Sivakumar AS, Dhasmana A, Hwang I (2018) Crosstalk between co-cultured 3T3-L1 and C2C12 cells after the exposure of nano-titanium dioxide. J Nanosci Nanotechnol 18(6):3870–3879. https://doi.org/10.1166/jnn.2018.15184
Ghanbary F, Seydi E, Naserzadeh P, Salimi A (2017) Toxicity of nano-titanium dioxide (TiO2-NP) on human monocytes and their mitochondria. Environ Sci Pollut R 25(7):6739–6750. https://doi.org/10.1007/s11356-017-0974-2
Hong F, Yu X, Wu N, Zhang YQ (2017) Progress of in vivo studies on the systemic toxicities induced by titanium dioxide nanoparticles. Toxicol Res (Camb) 6(2):115–133. https://doi.org/10.1039/c6tx00338a
Wu T, Tang M (2018) Review of the effects of manufactured nanoparticles on mammalian target organs. J Appl Toxicol 38(1):25–40. https://doi.org/10.1002/jat.3499
Vasantharaja D, Ramalingam V, Reddy GA (2015) Oral toxic exposure of titanium dioxide nanoparticles on serum biochemical changes in adult male Wistar rats. Int J Nanomedicine 2(1):46–53
Alarifi S, Ali D, Al-Doaiss AA, Ali BA, Ahmed M, Al-Khedhairy AA (2013) Histologic and apoptotic changes induced by titanium dioxide nanoparticles in the livers of rats. Int J Nanomedicine 8:3937–3943. https://doi.org/10.2147/IJN.S47174
Chang X, Zhang Y, Tang M, Wang B (2013) Health effects of exposure to nano-TiO2: a meta-analysis of experimental studies. Nanoscale Res Lett 8(1):51. https://doi.org/10.1186/1556-276X-8-51
Funding
This work was financially supported by the National Natural Science Foundation of China (No. 81560536) and Shihezi University Science and Technology Research and Development Project (No. RCZX201331).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Chunmei Ling is the co-first author
Rights and permissions
About this article
Cite this article
An, H., Ling, C., Xu, M. et al. Oxidative Damage Induced by Nano-titanium Dioxide in Rats and Mice: a Systematic Review and Meta-analysis. Biol Trace Elem Res 194, 184–202 (2020). https://doi.org/10.1007/s12011-019-01761-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-019-01761-z