Abstract
Many periodontal patients may need orthodontic treatment. Alterations in oral environment particularly the reduction of pH in periodontal patients could affect metal ion release from orthodontic appliances. However, there is no study on metal ion release in periodontal patients. The aim of this preliminary study was to comparatively evaluate, for the first time, salivary levels of nickel and chromium in periodontal patients (versus healthy controls) under orthodontic treatment for 2 months. In this in vivo study, 40 subjects were evaluated. Patient selection and standardization of orthodontic treatment protocols were prospectively designed and performed. Two groups of n = 20 each (control: healthy orthodontic patients, cohort: orthodontic patients with periodontitis) underwent similar protocols of fixed orthodontic treatment for 2 months. After 2 months, salivary nickel and chromium concentrations of the case and cohort groups were measured using inductively coupled plasma mass spectrometry (ICP-MS). The values were compared between the two groups using t test. There were 10 men and 10 women in each group. The mean age of patients was 34.6 ± 3.6 years old. The salivary level of nickel was 338.2 ± 235.5 ng/ml and 182.8 ± 116.5 ng/ml in the cohort and control groups, respectively (P = 0.0118). The salivary level of chromium was 7.4 ± 3.15 ng/ml in the cohort and 6.35 ± 2.39 ng/ml in the control group (P = 0.2214). Salivary level of nickel might be considerably higher in periodontal patients undergoing 2 months of orthodontic treatment compared to orthodontic patients with healthy gingivae.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Release of toxic and carcinogen ions during orthodontic treatment can compromise the biocompatibility of orthodontic appliances [1,2,3,4,5]. Several metals are used in orthodontic alloys, and nickel or chromium is of concern in terms of biocompatibility [1,2,3,4,5,6,7,8]. Stainless steel and NiTi alloys have about 8 wt% and 55 wt% nickel, while their chromium content is about 20 wt% and 0.2 wt%, respectively [6, 9, 10].
Orthodontic appliances are subjected to various disruption forces (such as environmental stresses, foods, thermal alterations and masticatory forces in the oral environment, tooth brushing, saliva flow, acidic drinks, occlusal loadings, frictions between appliances, mechanical stresses, and microorganisms and their enzymatic activity). These factors can remove the protective chromium oxide coating over the alloys. This facilitates corrosion mechanisms such as galvanic corrosion, which happens when different metals are present in the saliva [3,4,5,6, 8, 9, 11,12,13,14,15,16].
The released nickel and chromium ions can have toxic and mutagenic effects and lead to hypersensitivity reactions, asthma, or allergic contact dermatitis [1, 3,4,5,6,7, 9, 11, 13, 17]. Bearing in mind the possible hazard of these metal ions, it seems necessary to evaluate the release or accumulation of nickel and chromium ions, when using orthodontic appliances [1, 2, 4, 5, 8, 10, 17,18,19,20]. In vitro studies (which form most of the literature) are not relevant to clinical conditions because they cannot simulate properly the complex environment of the mouth [1,2,3, 6, 8, 9, 13, 21], and controversy exists over in vivo results [4,5,6, 13]. Therefore, more clinical studies in this regard are needed.
A considerable percentage of orthodontic patients are adults [22], of whom many suffer from periodontitis, particularly the chronic type [23]. In the USA, about 47.2% of adults suffer from periodontitis [24, 25]. The oral environment of periodontal patients has some differences with that of healthy individuals. For instance, the saliva pH is often lower in periodontal patients than that in healthy individuals [26]. Acidic environments (caused by factors such as dietary habits) might enhance corrosion and increase ion release from orthodontic and dental alloys [27,28,29].
Therefore, it may be hypothesized that metal ion release from orthodontic appliances might be greater in periodontal patients (who might have more acidic saliva) compared to healthy controls. This hypothesis may have clinical implications in terms of biocompatibility of orthodontic appliances in patients with periodontal inflammations. However, this hypothesis has not been tested in any previous study. Thus, we aimed to compare salivary concentrations of nickel and chromium ions in patients under fixed orthodontic treatment with periodontitis versus in orthodontic patients with healthy gingivae.
Subjects and Methods
This in vivo study had two phases. Patient recruitment and orthodontic treatment/standardization were planned and performed prospectively, whereas salivary ion measurements followed a historical cohort design. The study participants were selected prospectively from those presenting to the Orthodontics Department and three private orthodontic clinics in 2017. The sample size of this pilot study was predetermined as two groups of 20 patients each, in line with previous literature. Patients with metal restorations, cigarette smokers, tobacco consumers, mouth breathers, those with systemic diseases (diabetes mellitus, renal conditions, respiratory diseases), and patients taking medications affecting salivary biochemistry were all excluded. The study ethics were approved by the research committee of the university (thesis #25542, ethics code: IR.IAU.DENTAL.REC.1395.7). The study protocol was thoroughly explained to patients, and written informed consents were obtained from them prior to participation in the study.
Patients were prospectively examined by an expert periodontist to assess their eligibility for study inclusion. Control patients had to have healthy gingivae (no bleeding on probing) and probing depths <3 mm. Periodontal patients had to have gingival inflammation, attachment losses with probing depths ≥3 mm, and radiographically confirmed bone loss in at least 20% of sites (using panoramic radiography). The numbers of males and females in both groups were prospectively determined as similar or preferably equal in each group.
About 4 to 6 weeks before commencement of orthodontic treatment, all patients in both groups entered the first phase of periodontal treatment (receiving scaling and root planning); also, all orthodontic patients in both groups received oral hygiene instructions and were taught the modified Bass tooth brushing technique. They were re-evaluated 4 to 6 weeks later, and the periodontist had to confirm that the disease was under control in the periodontitis group (by confirming the lack of bleeding on probing and a plaque index of zero) in order for the orthodontic treatment to begin. The periodontitis group underwent this treatment for clinical reasons (in order to ensure the inactivity of periodontal disease during the orthodontic treatment and therefore to ensure minimum gingival/periodontal damage caused by orthodontic forces and tooth movements). The control group underwent the same gingival treatments for standardization in terms of initial extents of plaque and calculus. All patients were requested to use the same toothpaste and mouthwash during the first 2 months of orthodontic treatment.
Fixed orthodontic treatment of both arches was then started for patients in both groups using similar brackets for the purpose of standardization (American Orthodontics; Sheboygan, WI, USA), NiTi wires, and no-mix adhesive (3M ESPE, St. Paul, MN, USA).
Saliva samples were collected from the patients 2 months after the beginning of their fixed orthodontic treatment (at the NiTi wire phase). Saliva was collected from patients in both groups from 9 am to 12 pm because time of the day and time passed since the last meal can change the amount of released ions [27]. Patients were requested not to consume acidic or carbonated beverages and to refrain from eating foods containing sodium chloride for 1 week prior to saliva collection because all these factors can increase the susceptibility of metals to corrosion and change the concentration of metal ions. Patients were also requested to refrain from eating and drinking in the morning prior to saliva collection.
Nickel-free vials were rinsed with distilled water (Merck, Germany) and filled and emptied with acetone to eliminate any moisture. Afterwards, 5 ml of unstimulated saliva samples were collected from each patient in each vial using the spitting method, and the vials were kept in a freezer.
The collected samples were analyzed within 1 week of their collection. The samples were centrifuged at 8000 rpm. Next, 1 ml of the supernatant was collected and transferred to 2 ml microtubes; 50 μl of 20% nitric acid and 50 μl of 1% Triton X-100 were added; the samples were centrifuged again after 60 min in order to deposit proteins. The clear supernatant was transferred to another microtube [30]. The concentrations of nickel and chromium ions were then measured using inductively coupled plasma mass spectrometry (ICP-MS, AA280Z GTA 120; Varian, Mulgrave, Australia) and tabulated. The average ion concentration was recorded in parts per billion (ppb, ng/ml).
Descriptive statistics and 95% confidence intervals (CI) were calculated for ion levels. Data normality was assessed and confirmed using the D’Agostino and Pearson omnibus normality test. Ion levels were compared using the independent samples t test. The level of significance was predetermined as 0.05.
Results
This study was performed on 40 patients under fixed orthodontic treatment including 20 males (10 in each group) and 20 females (10 per group) with a mean age of 34.6 ± 3.6 years (range 25 to 42 years). None of the patients had any systemic disease or history of medication intake or smoking and had no dental metal restoration.
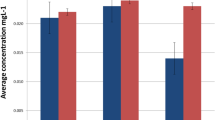
The salivary level of nickel was 156 ppb or 85% higher in the test group than that in the control group. The t test showed a significant difference (P = 0.0118) between nickel values of orthodontic patients with and without periodontitis (Tables 1 and 2). However, the salivary concentration of chromium was only 1.1 ppb or 17.3% higher in the test group compared to that in the control group, and this difference between chromium levels of the groups was not significant (P = 0.2214, Tables 1 and 2).
Discussion
This study showed that the salivary level of nickel but not chromium could be higher in orthodontic patients with periodontal disease compared to that in orthodontic patients with healthy periodontal tissues. No previous study has assessed metal ion release from orthodontic appliances in patients suffering from periodontitis. Increased salivary level of metal ions in periodontal patients under orthodontic treatment can be explained as follows: Lower pH decreases the resistance of alloys to corrosion and enhances the release of metal ions from orthodontic appliances, such that pH reduction from 6.75 to 3.5 can increase the ion release by 100-folds [6, 27, 31]. Although this study did not directly assess the pH, numerous studies have demonstrated that periodontal patients have a lower saliva pH than healthy individuals [26, 32,33,34,35,36]. Huang et al. [37] showed that ion release from metal brackets increases in acidic solutions compared to neutral pH, and the process of ion release accelerates over time [37]. Kuhta et al. [27] reported that acidity has a direct correlation with ion release from orthodontic appliances. Moreover, Takahashi et al. [32, 33] showed growth and proliferation of Porphyromonas gingivalis, Prevotella intermedia, and Fusobacterium nucleatum at acidic pH levels. These microorganisms together with their enzymatic activity and their products might have as well contributed to the increased level of nickel observed in the periodontitis group of this study. In the current study, we standardized the two groups with respect to the aforementioned parameters in order to eliminate or minimize the effects of confounders on the results. Porphyromonas gingivalis, Tannerella forsythia, and Treponema denticola are the major culprits responsible for chronic periodontitis, which destruct the periodontal tissue and cause bone loss [26, 38]. Efficient plaque control accompanied by mechanical plaque and calculus removal can decrease the signs/symptoms of inflammation. Thus, periodontal patients in our study underwent scaling and root planning and received oral hygiene instructions prior to fixed orthodontic treatment in order to improve the orthodontic treatment results. However, this could reduce the inflammation and perhaps reduce the pH contrast between the groups. Nevertheless, saliva was collected 2 months after the beginning of orthodontic treatment; in the presence of orthodontic appliances, this time period is probably sufficient for re-accumulation and colonization of microorganisms resulting in increased inflammation in both groups [18, 39,40,41,42]. Overbrushing and overuse of mouthwashes and toothpastes, due to great emphasis placed on oral hygiene by orthodontists, can be other reasons for the significant increase observed in salivary level of nickel ions in periodontal patients under orthodontic treatment compared to healthy controls, because such chemical solutions might increase metal ion release through reducing the pH and mechanical disruption of the chromium oxide film by brushing can expose the alloy to the corrosion [43, 44]. Also, increased saliva flow in periodontal patients [45] could be another explanation for increasing ion release in periodontal patients.
Most previous studies [4, 13, 15, 21, 46,47,48,49] showed that salivary nickel and chromium concentrations in orthodontic patients are very low compared to the normal doses already taken daily through diet (100 to 800 μg for nickel and 50 to 280 μg for chromium) [6, 48, 49]. However, this study found high concentrations especially for nickel. This was in line with a few other studies which reported considerably higher concentrations [47, 50]. Various factors can contribute to the controversy, such as differences in sampling methods (such as stimulated versus non-stimulated, or timing of sampling), the extent of galvanic currents, bacterial colonization, salivary compositions, health status (i.e., affliction with systemic diseases such as diabetes mellitus, or smoking), or diet [4, 21, 48, 50,51,52,53]. Noting that about 1.5 l of saliva might be swallowed daily [54, 55], it seems that if the concentrations of salivary metal ions were hypothetically constant throughout the day, the average levels observed in this study could sum up to doses about 274 μg and 9.5 μg daily, respectively, for nickel and chromium in orthodontic patients without periodontitis and doses about 507 μg and 11.2 μg daily, respectively, for nickel and chromium in orthodontic patients with periodontitis. Although the averages were still below toxic doses, nickel doses in some periodontitis patients might reach toxic levels; this might be of clinical significance (in terms of the biocompatibility and toxicity of fixed orthodontic treatment in periodontal patients) if verified in future larger studies. Such elevated concentrations might also worsen the other adverse effects of these ions attributed to chronic release of them, even if at a low dose, namely DNA damage, inflammation, and changes in cellular morphology or metabolism [3, 4, 21]. It is not known yet if metal ion release is constant or changes throughout the day, but it is suggested to peak when the pH drops, e.g., after the meal [3, 4, 21, 49]. However, the main health concerns with these highly sensitizing trace elements might be hypersensitivity and periodontal damage (which can be of a greater concern to patients already having periodontitis) [4, 50, 52, 56,57,58]. These issues especially the periodontal damage happened in some cases can have clinical implications in terms of biocompatibility of orthodontic appliances, especially for patients already suffering from periodontitis, in whom an additional periodontal damage could be even more undesirable.
There were some limitations in this study, most of which were common to all earlier in vivo research and discussed extensively in some of them [4, 8, 20]; these include the lack of total control over many variables in the complex oral environment and the probably complicated pattern of metal ion release that cannot be reflected by a single and brief period of sampling [3, 4, 8, 20, 48]. Another limitation of this pilot study was the lack of sample size calculations which might have contributed to the lack of a significant contrast between the chromium levels of both groups; also, measuring the baseline concentrations was out of the budget for this pilot study. Due to budget constraints of this preliminary study, it was not possible to measure baseline ion levels in this retrospective cohort design. Future studies should adopt more comprehensive setups such as clinical trials and measure baseline values as well (in larger samples) in order to be able to also assess the changes in each group over time. Another limitation was the lack of assessment of salivary pH in this pilot research. We only assumed (based on previous studies) that the cohort group with periodontitis would have more acidic pH levels. However, the pH should have been measured directly, and the correlations between the pH and metal ion leach should have been established. Furthermore, future studies are recommended to assess the correlations between pH with the salivary level of ions in periodontal patients under orthodontic treatment. It should also be researched whether rinsing with alkaline mouthwashes can aid periodontal patients under orthodontic treatment in terms of reducing their relatively high nickel release.
Conclusions
Within the limitations of this preliminary study, it was found for the first time that salivary nickel levels would be considerably higher in periodontal patients under 2 months of orthodontic treatment compared to orthodontic patients with healthy gingivae. Results pertaining to chromium values were inconclusive. Given the significance of biocompatibility of orthodontic appliances in periodontal patients, future studies with more comprehensive designs and larger samples are warranted to assess this further.
References
Mikulewicz M, Chojnacka K (2010) Trace metal release from orthodontic appliances by in vivo studies: a systematic literature review. Biol Trace Elem Res 137:127–138
Mikulewicz M, Chojnacka K (2011) Release of metal ions from orthodontic appliances by in vitro studies: a systematic literature review. Biol Trace Elem Res 139:241–256
Hafez HS, Selim EM, Kamel Eid FH, Tawfik WA, Al-Ashkar EA, Mostafa YA (2011) Cytotoxicity, genotoxicity, and metal release in patients with fixed orthodontic appliances: a longitudinal in-vivo study. Am J Orthod Dentofac Orthop 140:298–308
Amini F, Rakhshan V, Mesgarzadeh N (2012) Effects of long-term fixed orthodontic treatment on salivary nickel and chromium levels: a 1-year prospective cohort study. Biol Trace Elem Res 150:15–20
Amini F, Rakhshan V, Sadeghi P (2012) Effect of fixed orthodontic therapy on urinary nickel levels: a long-term retrospective cohort study. Biol Trace Elem Res 150:31–36
House K, Sernetz F, Dymock D, Sandy JR, Ireland AJ (2008) Corrosion of orthodontic appliances--should we care? Am J Orthod Dentofac Orthop 133:584–592
Faccioni F, Franceschetti P, Cerpelloni M, Fracasso ME (2003) In vivo study on metal release from fixed orthodontic appliances and DNA damage in oral mucosa cells. Am J Orthod Dentofac Orthop 124:687–693 discussion 693-684
Amini F, Harandi S, Mollaei M, Rakhshan V (2015) Effects of fixed orthodontic treatment using conventional versus metal-injection molding brackets on salivary nickel and chromium levels: a double-blind randomized clinical trial. Eur J Orthod 37:522–530
Eliades T, Athanasiou AE (2002) In vivo aging of orthodontic alloys: implications for corrosion potential, nickel release, and biocompatibility. Angle Orthod 72:222–237
Amini F, Rakhshan V, Pousti M, Rahimi H, Shariati M, Aghamohamadi B (2012) Variations in surface roughness of seven orthodontic archwires: an SEM-profilometry study. Korean J Orthod 42:129–137
Hwang CJ, Shin JS, Cha JY (2001) Metal release from simulated fixed orthodontic appliances. Am J Orthod Dentofac Orthop 120:383–391
Mikulewicz M, Chojnacka K (2011) Cytocompatibility of medical biomaterials containing nickel by osteoblasts: a systematic literature review. Biol Trace Elem Res 142:865–889
Matos de Souza R, Macedo de Menezes L (2008) Nickel, chromium and iron levels in the saliva of patients with simulated fixed orthodontic appliances. Angle Orthod 78:345–350
Freitas MP, Oshima HM, Menezes LM (2011) Release of toxic ions from silver solder used in orthodontics: an in-situ evaluation. Am J Orthod Dentofac Orthop 140:177–181
Agaoglu G, Arun T, Izgi B, Yarat A (2001) Nickel and chromium levels in the saliva and serum of patients with fixed orthodontic appliances. Angle Orthod 71:375–379
Macedo de Menezes L, Cardoso Abdo Quintão C (2010) The release of ions from metallic orthodontic appliances. Semin Orthod 16:282–292
Amini F, Mollaei M, Harandi S, Rakhshan V (2015) Effects of fixed orthodontic treatment on hair nickel and chromium levels: a 6-month prospective preliminary study. Biol Trace Elem Res 164:12–17
Amini F, Shariati M, Sobouti F, Rakhshan V (2016) Effects of fixed orthodontic treatment on nickel and chromium levels in gingival crevicular fluid as a novel systemic biomarker of trace elements: a longitudinal study. Am J Orthod Dentofac Orthop 149:666–672
Khaneh Masjedi M, Haghighat Jahromi N, Niknam O, Hormozi E, Rakhshan V (2017) Effects of fixed orthodontic treatment using conventional (two-piece) versus metal injection moulding brackets on hair nickel and chromium levels: a double-blind randomized clinical trial. Eur J Orthod 39:17–24
Khaneh Masjedi M, Niknam O, Haghighat Jahromi N, Javidi P, Rakhshan V (2016) Effects of fixed orthodontic treatment using conventional, copper-included, and epoxy-coated nickel-titanium archwires on salivary nickel levels: a double-blind randomized clinical trial. Biol Trace Elem Res 174:27–31
Eliades T, Trapalis C, Eliades G, Katsavrias E (2003) Salivary metal levels of orthodontic patients: a novel methodological and analytical approach. Eur J Orthod 25:103–106
McMorrow S (2015) Adult orthodontics: internet information and a national survey [PhD Thesis]. University College Cork, National University of Ireland, Cork, Ireland
American Academy of Periodontology Task Force Report on the Update to the 1999 Classification of periodontal diseases and conditions (2015) J Periodontol 86:835–838
Eke PI, Dye BA, Wei L, Thornton-Evans GO, Genco RJ (2012) Prevalence of periodontitis in adults in the United States: 2009 and 2010. J Dent Res 91:914–920
Socransky SS (1977) Microbiology of periodontal disease—present status and future considerations. J Periodontol 48:497–504
Baliga S, Muglikar S, Kale R (2013) Salivary pH: a diagnostic biomarker. J Indian Soc Periodontol 17:461
Kuhta M, Pavlin D, Slaj M, Varga S, Lapter-Varga M (2009) Type of archwire and level of acidity: effects on the release of metal ions from orthodontic appliances. Angle Orthod 79:102–110
Basir L, Meshki R, Behbudi A, Rakhshan V (2018) Effects of restoring the primary dentition with stainless-steel crowns on children’s salivary nickel and chromium levels, and the associations with saliva pH: a preliminary before-after clinical trial. Biol Trace Elem Res
Wolowiec P, Chojnacka K, Loster BW, Mikulewicz M (2017) Do dietary habits influence trace elements release from fixed orthodontic appliances? Biol Trace Elem Res 180:214–222
Olmedo P, Pla A, Hernandez AF, Lopez-Guarnido O, Rodrigo L, Gil F (2010) Validation of a method to quantify chromium, cadmium, manganese, nickel and lead in human whole blood, urine, saliva and hair samples by electrothermal atomic absorption spectrometry. Anal Chim Acta 659:60–67
Huang HH, Chiu YH, Lee TH, Wu SC, Yang HW, Su KH, Hsu CC (2003) Ion release from NiTi orthodontic wires in artificial saliva with various acidities. Biomaterials 24:3585–3592
Takahashi N, Schachtele CF (1990) Effect of pH on the growth and proteolytic activity of Porphyromonas gingivalis and Bacteroides intermedius. J Dent Res 69:1266–1269
Takahashl N, Saito K, Schachtele CF, Yamada T (1997) Acid tolerance and acid-neutralizing activity of Porphyromonas gingivalis, Prevotella intermedia and Fusobacterium nucleatum. Oral Microbiol Immunol 12:323–328
Fujikawa K, Numasaki H, Kobayashi M, Sugano N, Tomura S, Murai S (1989) pH determination in human crevicular fluids. Examination of the pH meter and evaluation of the correlation between pH level and clinical findings or the microflora in each periodontal pocket. Nihon Shishubyo Gakkai Kaishi (Journal of the Japanese Society of Periodontology) 31:241–248
Galgut P (2001) The relevance of pH to gingivitis and periodontitis. Journal of the International Academy of Periodontology 3:61–67
Seethalakshmi C (2016) Correlation of salivary pH, incidence of dental caries and periodontal status in diabetes mellitus patients: a cross-sectional study. J Clin Diagn Res
Huang T-H, Yen C-C, Kao C-T (2001) Comparison of ion release from new and recycled orthodontic brackets. Am J Orthod Dentofac Orthop 120:68–75
Socransky SS, Haffajee AD, Cugini MA, Smith C, Kent RL (1998) Microbial complexes in subgingival plaque. J Clin Periodontol 25:134–144
Sobouti F, Rakhshan V, Heydari M, Keikavusi S, Dadgar S, Shariati M (2018) Effects of fixed orthodontic treatment and two new mouth rinses on gingival health: a prospective cohort followed by a single-blind placebo-controlled randomized clinical trial. Int Orthod 16:12–30
Freitas AOA, Marquezan M, Nojima MCG, Alviano DS, Maia LC (2014) The influence of orthodontic fixed appliances on the oral microbiota: a systematic review. Dental Press J Orthod 19:46–55
Paolantonio M, Festa F, di Placido G, D'Attilio M, Catamo G, Piccolomini R (1999) Site-specific subgingival colonization by Actinobacillus actinomycetemcomitans in orthodontic patients. Am J Orthod Dentofac Orthop 115:423–428
Rakhshan H, Rakhshan V (2015) Effects of the initial stage of active fixed orthodontic treatment and sex on dental plaque accumulation: a preliminary prospective cohort study. Saudi J Dent Res 6:86–90
Huang TH (2004) Metal ion release from new and recycled stainless steel brackets. Eur J Orthod 26:171–177
Danaei SM, Safavi A, Roeinpeikar SM, Oshagh M, Iranpour S, Omidkhoda M (2011) Ion release from orthodontic brackets in 3 mouthwashes: an in-vitro study. Am J Orthod Dentofac Orthop 139:730–734
Rajesh KS, Zareena HS, Arun Kumar MS (2015) Assessment of salivary calcium, phosphate, magnesium, pH, and flow rate in healthy subjects, periodontitis, and dental caries. Contemp Clin Dent 6:461–465
Petoumenou E, Arndt M, Keilig L, Reimann S, Hoederath H, Eliades T, Jager A, Bourauel C (2009) Nickel concentration in the saliva of patients with nickel-titanium orthodontic appliances. Am J Orthod Dentofac Orthop 135:59–65
Kerosuo H, Moe G, Hensten-Pettersen A (1997) Salivary nickel and chromium in subjects with different types of fixed orthodontic appliances. Am J Orthod Dentofac Orthop 111:595–598
Kocadereli L, Atac PA, Kale PS, Ozer D (2000) Salivary nickel and chromium in patients with fixed orthodontic appliances. Angle Orthod 70:431–434
Fors R, Persson M (2006) Nickel in dental plaque and saliva in patients with and without orthodontic appliances. Eur J Orthod 28:292–297
Singh DP, Sehgal V, Pradhan KL, Chandna A, Gupta R (2008) Estimation of nickel and chromium in saliva of patients with fixed orthodontic appliances. World J Orthod 9:196–202
International Programme on Chemical Safety (1991) 108. Nickel. In: Environmental health criteria. World Health Organization, Geneva, pp 16–17
Menezes LM, Quintao CA, Bolognese AM (2007) Urinary excretion levels of nickel in orthodontic patients. Am J Orthod Dentofac Orthop 131:635–638
Gjerdet NR, Erichsen ES, Remlo HE, Evjen G (1991) Nickel and iron in saliva of patients with fixed orthodontic appliances. Acta Odontol Scand 49:73–78
Edgar W, O'Mullane D, Dawes C (2004) Saliva and oral health. British Dental Association London,
Dawes C (1972) Circadian rhythms in human salivary flow rate and composition. J Physiol 220:529–545
Pazzini CA, Junior GO, Marques LS, Pereira CV, Pereira LJ (2009) Prevalence of nickel allergy and longitudinal evaluation of periodontal abnormalities in orthodontic allergic patients. Angle Orthod 79:922–927
Genelhu MC, Marigo M, Alves-Oliveira LF, Malaquias LC, Gomez RS (2005) Characterization of nickel-induced allergic contact stomatitis associated with fixed orthodontic appliances. Am J Orthod Dentofac Orthop 128:378–381
Bishara SE, Barrett RD, Selim MI (1993) Biodegradation of orthodontic appliances. Part II. Changes in the blood level of nickel. Am J Orthod Dentofac Orthop 103:115–119
Funding
The study was funded by the authors and their institution.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study ethics were approved by the research committee of the university (thesis #25542, ethics code: IR.IAU.DENTAL.REC.1395.7). The study protocol was thoroughly explained to patients, and written informed consents were obtained from them prior to participation in the study.
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Amini, F., Asadi, E., Hakimpour, D. et al. Salivary Nickel and Chromium Levels in Orthodontic Patients with and Without Periodontitis: a Preliminary Historical Cohort Study. Biol Trace Elem Res 191, 10–15 (2019). https://doi.org/10.1007/s12011-018-1582-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-018-1582-9