Abstract
Selenium (Se) is an essential trace element for humans and animals. Appropriate amount of Se in the body can prevent a variety of diseases. However, Se deficiency leads to pathological changes such as skeletal muscle necrosis and pancreatic atrophy in livestock and poultry. Se preparations are widely used in the prevention and treatment of Se-deficient disease, but there is no unified standard of medication, and the safe dose range of Se is narrow. Therefore, it is of great significance to study the pharmacokinetics of low-Se ducklings and to formulate drug administration schemes. In the present study, eighty 1-day-old healthy ducklings were randomly selected, and fed with low-Se diet to 30 days of age (blood Se content ≦ 0.03 μg/mL). After the low Se duckling models were duplicated, blood samples and tissues of livers, pancreases, and thigh muscles were collected at different time points to detect Se content following oral administration of 0.1% sodium selenite (Na2SeO3) at 0.8 mg/kg BW, and the pharmacokinetics parameters were automatically calculated by MCPKP program. The results showed that pharmacokinetics characteristics of Na2SeO3 in blood, livers, and pancreases of ducklings were consistent with the first-order absorption and two-compartment open models; in thigh muscles was consistent with the first-order absorption and one compartment with a lag time open model. The primary kinetic parameters of Na2SeO3 in blood: the half-life of absorption was 5.9026 h, the time of reaching maximum concentration was 23.03 h, and the half-life of elimination was 131.13 h. The absorption of Na2SeO3 in livers was the quickest, pancreases and thigh muscles were in order of becoming slower, and the elimination of Na2SeO3 in thigh muscles was the quickest, livers and pancreases were in order of becoming slower. The administration parameters of multi-dose were calculated according to the kinetic of single-dose: loading dose (D*) was 1.7046 mg/kg BW, maintenance dose (D0) was 0.8 mg/kg BW, and dosing interval (τ) was 120 h. The results of this study can supplement and improve the theoretical system of Se metabolic kinetics, and provide experimental basis for the prevention and treatment of Se deficiency disease by rational drug use.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Selenium (Se) is an indispensable trace element in a variety of organisms, including humans and animals, and plays a vital role in biology and immunity [1]. Appropriate amount of Se in animals can regulate the body’s metabolism, and anti-tumor; anti-aging; antagonize toxic effects of heavy metal, and protects the physiological functions of the body tissues [2,3,4,5]. Se content in animal feed is stipulated in many countries, such as 0.2 mg Se/kg for ducks, 0.1 mg Se/kg for broilers, and 0.1–0.3 mg Se/kg for pigs [6, 7]. However, long-term Se deficiency in feed resulted in many diseases containing exudative diathesis (ED) with greenish and gelatinous edema under the skin and pancreatic atrophy in broilers, microvesicular steatosis of livers in hamsters, mulberry heart disease in pigs, white muscle disease in lambs, etc. [8,9,10,11,12]. Proper Se supplementation can improve the condition or reduce the injury of bodies. Through the analysis of test data from different Se sources, it was proved that in some cases, Se dietary supplementation was beneficial when the background Se level was low [13]. It is generally accepted that 0.15 to 0.30 mg Se/kg diet of feed meet the requirement of animals for Se. The results of Zhan et al. showed that basal diets containing 0.04 mg Se/kg, and the addition of 0.3 mg Se/kg selenomethionine and sodium selenite (Na2SeO3) respectively could meet the need of Se in piglets. However, selenomethionine was more conducive to the deposition of Se in the mother body and could improve the utilization of Se, which provided an effective way to improve the growth performance of piglets from birth to weaning [14].
It is well established that pharmacokinetics studies the process of absorption, distribution, metabolism, and elimination of drugs in the body in order to understand the dynamic changes of drugs after entering the body and evaluate the effect of drugs. A recent study demonstrated that PEGylated iron oxide nanoparticles accumulated preferentially in the pancreas of nonobese diabetic mice, thus allowing us to diagnose mice in pre-diabetes [15]. Sun et al. confirmed the specificity of Curcuma comosa extract for the reproductive system of rats according to the longest terminal half-life (t1/2) and the highest drug concentration in the uterus and ovary, and reported that not all oral Curcuma comosa could be absorbed. The absorption limit reached a dose of 250 mg/kg body [16]. There are also numerous studies about the pharmacokinetics of Se. Researchers have found that the biotransformation rate of methylmercury to inorganic mercury in the intestine of the black seabream in the high Se treatment group was 1.5 times higher than that of the low Se treatment group [17]. The bioavailability of Se following oral administration of selenium yeast was studied by Musiol B et al. using Na2SeO3 as control. The results showed that there was no significant difference between pharmacokinetics variables of two preparations including average maximum plasma concentration, the average time reached the maximum plasma concentration and the mean area under the plasma concentration-time curve [18]. The rapid absorption of Se and high Se concentration in blood was found in llamas fed with diet adequate in Se after subcutaneous Se injection (0.1 mg/kg), whereas parenteral Se could not have an effect on blood Se content for a long time [19]. In addition, Blodgett’s study suggested that the biological half-life of Na2SeO3 in sheep was 354 h or 14.7 days [20]. The biosafety range of Se is narrow, and excessive intake of Se also induces diseases in humans and animals. Acute Se exposure affected significantly oxidative stress on earthworms, and resulted in the decrease of superoxide dismutase activity and the enhancement of glutathione reductase activity and lipid peroxidation level [21]. Additionally, exposure to Se for high concentrations inhibited the activity of acetylcholine esterase in fish brain and aggravated the DNA damage in fish liver in a concentration-dependent manner [22].
In summary, Se deficiency affects a variety of animals and human health. Currently, Na2SeO3 is commonly used in the prevention and treatment of Se deficiency disease in various animals, but there is no standard (supplement) dose of Na2SeO3. We established low-Se duckling models, and the necropsy and histopathological changes were observed, and the time course of absorption, distribution, metabolism and elimination of Na2SeO3 in the blood, liver, pancreas, and thigh muscle tissues of ducklings were studied by oral Se supplementation. Pharmacokinetics characteristics of Na2SeO3 in ducklings were clearly understood. Our results not only supplement and perfect the theoretical system of Se pharmacokinetics, and provide experimental basis for the prevention and treatment of Se deficiency in ducklings with scientific and rational medication, but also has important theoretical significance and practical value for biomedicine.
Materials and Methods
Animals and Experimental Design
All procedures involving animals used in our study were approved by the Institutional Animal Care and Use Committee of the Northeast Agricultural University. A total of 80 healthy 1-day-old ducklings (male/female) were purchased from the duck farm of the Heilongjiang Provincial Specialty Research Institute. Ducklings were fed with low Se diet (Se = 0.018 mg/kg) containing corn (63.16%), soybean meal (32%), dicalcium phosphate (1.65%), stone powder (1.13%), soybean oil (0.96%), sodium chloride (0.31%), 98.5% of DL-methionine (0.23%), 70% of L-lysine sulphate (0.18%), 60% of choline chloride (0.1%), 98.5% of L-threonine (0.05%), mixed mineral (0.2%) and mixed vitamin (0.03%). Corn and soybean meal were from the low Se area. Eight replicates with ten ducklings per replicate were performed in the current study. All ducklings were given free access to water and food until blood Se content was lower than or equal to 0.03 μg/mL, and animals displayed typical clinical symptoms of Se deficiency (30 days old). After that, all ducklings were weighed and orally administered with 0.1% Na2SeO3 solution at 0.8 mg/kg BW. At predetermined time points (0, 1, 2, 4, 8, 12, 24, 48, 72, 96, 144, and 192 h) following Na2SeO3 solution administration, 1 mL of blood samples was respectively collected by cardiac puncture from anesthetized ducklings (n = 6 per time point) and stored at − 80 °C until pharmacokinetics analysis. Subsequently, all ducklings were euthanized, and their livers, pancreases, and thigh muscles were isolated. Isolated tissues were dried to constant weight and then pulverized through the mesh filters for detecting Se content.
Histopathological Examination
The fresh tissues of livers, pancreases, and thigh muscles, in the size of 1.5 cm2 × 0.5 cm, were rapidly fixed in 10% neutral formaldehyde, dehydrated, embedded in paraffin and then cut into slices. Sections (5 μm thicknesses) were subjected to hematoxylin and eosin (H&E) staining, dehydrated, dried and finally sealed with neutral resin. Histopathological examinations were performed according to the study of Zheng et al. using an optical microscope (XDS-1B, Olympus, Tokyo, Japan) with a magnification of 400 times [23].
The Determination of Se Concentration in Blood and Tissues
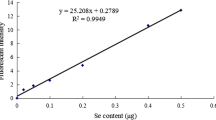
Se concentration in the blood, liver, pancreas, and thigh muscle tissues was determined according to the method of Yang et al. [24]. In brief, Se contained in samples was transferred into selenous acid by digesting with nitric-perchloric acid. The fluorescence of 4, 5-benzopiazselenol complexes generated specifically by the reaction of selenous acid with 2, 3-diaminonaphthalene (DAN), and displayed a brilliant lime-green fluorescence when excited at 366 nm after extraction with aliquots of cyclohexane. Fluorescence spectrophotometry was used to measure the fluorescence with an excitation and emission wavelengths of 366 nm and 520 nm, respectively. Then, Se concentration was calculated by reference to the standard curve.
Calculation of Pharmacokinetics Parameters
Throughout the elapsed time, Se concentration-time data in blood and different tissues of Se deficiency ducklings were fitted automatically by the pharmacokinetics analysis software MCPKP. The pharmacokinetics-related parameters were calculated according to the method of Sun et al. [16].
Statistical Analysis
Statistical analyses of all data were performed using SPSS software (version 17.0, SPSS Inc., Chicago, IL, USA). One-way ANOVA was conducted, and results were considered significant when P < 0.05. All values were represented as mean ± standard deviation (M ± SD). In addition, correlation regression analysis of Se content in blood and tissues during the whole menstrual process was performed by statistical analysis software (SAS).
Results
Growth Performance
Clinical symptoms of Se deficiency occurred in the –Se ducklings as early as 18 days. Thereafter (from 18 to 30 days), the sick ducklings displayed signs of lack of energy, necking, and slow response to irritation. They suffered from loss of appetite, poor growth, fluffy plumage, anemia (gray beak), pale green or milky watery or milky stools, enophthalmos, and weight loss. Ducklings had dyskinesia, joint flexion, claws curling inward, and the outward rotation of attached joints. Sick ducklings sometimes crossed their legs inwards, lean forward as they moved ahead, and had difficulty standing in severe cases.
Pathomorphological Changes
Ducklings of Se deficiency were confirmed to display the symptoms of ED: turquoise discoloration under the skin of the chest and abdomen (Fig. 1A). Yellow staining of the liver (Fig. 1B, arrow a) and pericardial effusion (Fig. 1B, arrow b) were observed. Punctate, mottled, striped, and a flaky hemorrhage, and even large areas of dark purple were also observed in thigh muscle (Fig. 1C). The section of muscular stomach was relatively dry and had varying degrees of grayish-white necrosis (Fig. 1D).
Effect of Se deficiency on the pathomorphological changes of ducklings. Pathological anatomy of Se-deficient ducklings (A–D). Histopathological analysis of liver (E), pancreas (F), skeletal muscle (G), muscular stomach (H), and heart (I) in Se-deficient ducklings at a magnification of 400×. The arrows in black point to the location of the lesion
Hepatic central veins were dilated and hyperemia was observed (Fig. 1E, arrow a). Hepatocytes, with large round vacuoles, underwent granular degeneration and steatosis (Fig. 1E, arrow b). The cytoplasm of hepatocytes almost disappeared and the nucleus of which was squeezed to the edge or disappeared. Acini in pancreas were swollen and acinar epithelial cells showed granular degeneration or vesicular degeneration, even diffused necrosis (Fig. 1F, arrow a) and vacuolization (Fig. 1F, arrow b). Infiltration by eosinophils was observed around the lesion area (Fig. 1F, arrow c). Skeletal muscle cells displayed granular degeneration and local or multifocal of hyaline degeneration (Fig. 1G, arrow a). Skeletal muscle fibers were necrotic (Fig. 1G, arrow b) and capillaries between muscle fibers were expanded with hyperemia and hemorrhage. Capillaries in submucosa layers of muscular stomach were hyperemia and hemorrhage. Myofibril was fused and hyaline degeneration with focal or multifocal area of necrosis (Fig. 1H, arrow a), calcium deposition (purplish red particles, Fig. 1H, arrow b) and eosinophilic infiltration (Fig. 1H, arrow c) were observed in muscular stomach. Cardiomyocytes underwent hyaline degeneration (Fig. 1I, arrow a) and myocardial fibers were swollen (Fig. 1I, arrow b) and disappeared of striation (Fig. 1I, arrow c) accompanied with hemorrhage (Fig. 1I, arrow d).
Pharmacokinetics Characteristics of Na2SeO3 in the Blood of Se Deficiency Ducklings
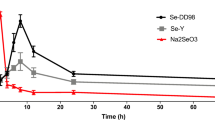
The actual and theoretical values of Se content and drug time curves in blood at different time points after oral administration of Na2SeO3 were shown in Table 1 and Fig. 2, respectively. The pharmacokinetics parameters of Na2SeO3 in the blood of low Se ducklings after oral administration were shown in Table 2. And the pharmacokinetics characteristic of Na2SeO3 was in accord with the two compartment open model of first-order absorption. The kinetic equation for the change of blood drug concentration over time was calculated: C = − 0.0439e−2.6925t + 0.1085e−0.0053t − 0.0646e−0.1174t. The results showed that Na2SeO3 concentration reached a peak of 0.0917 μg/mL at 23.03 h in the blood of Se deficiency ducklings, and the absorption half-life (T1/2Ka) of Na2SeO3 was 5.90 h. At 24 h, the drug concentration in blood began to be eliminated slowly, dropped to 0.0401 μg/mL at 192 h, and the elimination half-life (T1/2β) of Na2SeO3 was 131.13 h.
Pharmacokinetics Characteristics of Na2SeO3 in Different Tissues of Se Deficiency Ducklings
The actual and theoretical values of Se content in livers, pancreases, and thigh muscles of low Se ducklings after oral administration of Na2SeO3 were shown in Table 3. Derived pharmacokinetics parameters of different tissues were listed in Table 4. The Na2SeO3 concentration-time data in different tissues were automatically fitted by MCPKP software. The pharmacokinetics characteristic of Na2SeO3 in livers and pancreases was in accord with the two compartment open model of first-order absorption, and thigh muscles was consistent with the one compartment open model of first-order absorption with a lagtime. The kinetic equations for the changes of drug in livers, pancreases, and thigh muscles were respectively calculated as follows: C = 1.1418e−0.1835t + 1.6211e−0.0050t − 2.7629e−1.7003t, C = 0.2395e−0.0558t + 0.2617e−0.0019t − 0.5012e−0.5197t, C = 0.1146 (e−0.0152t − e−0.0785t). Our data confirmed that Na2SeO3 was absorbed the quickest in livers and reached the peak concentration at 2.02 h, followed by pancreases at 6.30 h. The absorption of Na2SeO3 in thigh muscles was the slowest, with a lag time of 0.67 h at the beginning, then reached the peak concentration at 26.60 h. However, the elimination of Se in tissues was slower. The tissue which eliminated Se the fastest was thigh muscle with elimination half-life of 45.55 h, followed by liver and pancreas with a half-life of 139.62 h and 375.59 h, respectively.
Correlation Regression Analysis of Se Concentration in Blood and Tissues of Se Deficiency Ducklings
As shown in Table 5, throughout the elapsed time, Se concentration in blood was correlated significantly with that in thigh muscles (P < 0.01), but was not related to the concentration of Se in livers and pancreases.
Multi-Dose Administration Parameters and Dosage Regimen of Na2SeO3 in Se Deficiency Ducklings
The parameters of multiple doses were calculated for clinical treatment based on the kinetic parameters of the single dose oral administration. And the interval of oral Na2SeO3 in Se deficiency ducklings was set as 120 h. As for the multi-dose administration, parameters were listed as follows: the mean steady state concentration (C) was 0.1664 μg/mL, the maximum steady state concentration (C∞)max was 0.2216 μg/mL, the minimum steady state concentration (C∞)min was 0.1227 μg/mL, cumulative coefficient (R) was 2.1308, the loading dose (D*) was 1.7046 mg/kg BW, and the maintenance dose (D0) was 0.8 mg/kg BW.
Discussion
Although the pharmacokinetics of Na2SeO3 in different animals have been studied, the biosafety dose of Se in some animals is still unclear because of the narrow safe dose range of Se, and the different species, physiological, and pathological state of animals. In order to explore the rational scheme of Se supplementation for Se deficiency ducklings, the pharmacokinetics parameters of Na2SeO3 orally administered in vivo were calculated based on the successful replication of low Se duckling models. The results indicated that characteristics of pharmacokinetics of Na2SeO3 in blood, livers, and pancreases of ducklings were consistent with the two compartment open model with first-order absorption; thigh muscles were in accordance with the one compartment open model with first-order absorption with a lagtime and the parameters of multi-dose were calculated for clinic treatment based on kinetic parameters of the single dose as follows: D* was 1.7046 mg/kg BW, D0 was 0.8 mg/kg BW, and dosing internal (τ) was 120 h.
It is generally believed that Se content in animal feeds below 0.05 mg/kg may cause diseases, and below 0.02 mg/kg will definitely induce the disease of Se deficiency [25]. Moreover, Se deficiency could induce various typical pathological changes in variety of animals. For example, the common lesion in pigs and rats presented liver necrosis and the poultry showed ED and pancreatic atrophy [26,27,28,29]. In our study, the clinical manifestations of ducklings fed a Se-deficient diet were mental wilting, loss of appetite, poor growth, pale green or milky loose stools, ataxia, joint flexion, and standing instability. The necropsy of ducklings showed greenish edema under the skin of chest and abdomen, varying degrees of bleeding in thigh muscles, the increase of pericardial fluid, and degeneration and necrosis of the muscular stomach. Se content in blood was measured to be 0.025 μg/mL. Our experiments confirmed that duckling models of Se deficiency could be successfully replicated by feeding with diet in which Se content was 0.018 mg/kg.
The study of pharmacokinetics in blood/tissue of livestock and poultry is important for determining the dose and dose regime of clinical medications [30]. Our previous studies investigated that in the blood of healthy ducklings, the T1/2Kα of Na2SeO3 was 0.86 h, the time of reaching maximum concentration (Tmax) was 2.79 h, the T1/2β was 37.95 h, the area under the curve (ACU) was 17.02 mg/L h, and the volume of distribution (Vd) was 27.85 L/kg. Compared with pharmacokinetics parameters in healthy ducklings, except for the similar ACU (19.93 mg/L h), T1/2Kα (5.90 h), Tmax (23.03 h), and T1/2β (131.13 h) of Na2SeO3 were significantly prolonged, and Vd (7.60 L/kg) of Na2SeO3 were decreased in the blood of low Se ducklings. It could be concluded that the absorption, distribution, and elimination time of Na2SeO3 in the tissues of different species and Se levels are not the same. Se was distributed in various tissues and organs through blood circulation after absorption by the intestine. However, Se in the body was first to supply the tissues and organs that need it, rather than being transported until reaching peak concentrations in the blood. The pharmacokinetics parameters calculated from the blood level of rats indicated that Se was rapidly absorbed, distributed into internal organs and eliminated from the blood [31]. Besides, Guenter et al. found that livers responded more quickly to the body’s intake of Se than blood [32]. In the present study, our data showed that the absorption of Na2SeO3 in the livers of low Se ducklings was the fastest, and the T1/2Kα and Tmax of Na2SeO3 in the livers were significantly earlier than that in blood, pancreases, and thigh muscles. The T1/2β of Na2SeO3 in thigh muscles was the shortest at 45.55 h, followed by blood and livers, and was the longest in pancreases. Selenium protein P functions as the main carrier of Se transport in the body, which is synthesized in the liver [33]. After Se supplementation, the concentration of Se in the liver reached the peak in a short time, indicating that the liver plays an important role in the transport and utilization of Se in the body. Se in muscle exists in the form of selenoaminoacid [34], so there is a lag period in its absorption, and the elimination of Se in skeletal muscle is the fastest, which may be one of the reasons why Se deficiency is most likely to induce skeletal muscle damage in clinical. The pancreas is the main target organ for Se deficiency in poultry [28], and the slowest elimination of Se in the pancreas may be related to the preferential use of Se in the body. Our results also revealed that Se content in blood had a remarkable correlation with that in thigh muscles (P < 0.01), and had no correlation with Se content in livers and pancreases. Pharmacokinetics analysis could help to understand the correlation of Se content among different tissues, in addition, objectively evaluate the metabolic process of Se in tissues. Moreover, multi-dose parameters for clinical treatment can also be calculated through pharmacokinetics parameters. Lehr et al. described the pharmacokinetics parameters of oral meloxicam in cats and presented dosage regimes for the treatment of pain following an initial dose of 0.1 mg/kg and maintained at 0.05 mg/kg after 1 day [35]. Pharmacokinetic studies of posaconazole in adults indicated that the recommended dose of posaconazole solid tablet formulation (> 0.5 mg/L) could provide adequate target therapy for a wide range of patients at high risk for invasive fungal disease [36]. In this test, we also derived the multi-dose administration parameters of oral Na2SeO3 in the Se-deficient ducks according to the single-dose pharmacokinetics parameters, which provided a theoretical basis for the clinical treatment of Se deficiency ducks.
In summary, our work is the first to study the pharmacokinetics of Na2SeO3 in the blood and different tissues of low Se ducklings by oral administration, and to formulate a dosage regimen for clinical treatment. This study is of great significance for a comprehensive understanding of Se metabolism, detection of Se nutrition status, prevention of Se deficiency disease and formulation of Se medication standards.
References
Khoso PA, Zhang Y, Yin H, Teng X, Li S (2018) Selenium deficiency affects immune function by influencing selenoprotein and cytokine expression in chicken spleen. Biol Trace Elem Res. https://doi.org/10.1007/s12011-018-1396-9
Wang Y, Chen P, Zhao G, Sun K, Li D, Wan X, Zhang J (2015) Inverse relationship between elemental selenium nanoparticle size and inhibition of cancer cell growth in vitro and in vivo. Food Chem Toxicol 85:71–77. https://doi.org/10.1016/j.fct.2015.08.006
Gong T, Torres DJ, Berry MJ, Pitts MW (2018) Hypothalamic redox balance and leptin signaling - emerging role of selenoproteins. Free Radic Biol Med 127:172–181. https://doi.org/10.1016/j.freeradbiomed.2018.02.038
Sun X, Cui Y, Wang Q, Tang S, Cao X, Luo H, He Z, Hu X, Nie X, Yang Y, Wang T (2018) Proteogenomic analyses revealed favorable metabolism pattern alterations in rotifer Brachionus plicatilis fed with selenium-rich chlorella. J Agric Food Chem 66(26):6699–6707. https://doi.org/10.1021/acs.jafc.8b00139
Zheng S, Song H, Gao H, Liu C, Zhang Z, Fu J (2016) The antagonistic effect of selenium on lead-induced inflammatory factors and heat shock protein mRNA level in chicken cartilage tissue. Biol Trace Elem Res 173(1):177–184. https://doi.org/10.1007/s12011-016-0630-6
National Research Council (1998) Nutrient requirements of poultry, 10th edn. National Academy Press, Washington, DC
National Research Council (1998) Nutrient requirements of swine, 10th edn. National Academy Press, Washington, DC
Zheng SF, Bao RK, Zhang QJ, Wang SC, Lin HJ (2018) Endogenous hydrogen sulfide promotes apoptosis via mitochondrial pathways in the livers of broilers with selenium deficiency exudative diathesis disease. Biol Trace Elem Res 186:249–257. https://doi.org/10.1007/s12011-018-1292-3
Xu J, Wang L, Tang J, Jia G, Liu G, Chen X, Cai J, Shang H, Zhao H (2017) Pancreatic atrophy caused by dietary selenium deficiency induces hypoinsulinemic hyperglycemia via global down-regulation of selenoprotein encoding genes in broilers. PLoS One 12(8):e0182079. https://doi.org/10.1371/journal.pone.0182079
Del Bas JM, Rodriguez B, Puiggros F, Marine S, Rodriguez MA, Morina D, Armengol L, Caimari A, Arola L (2017) Hepatic accumulation of S-adenosylmethionine in hamsters with non-alcoholic-fatty liver disease associated to metabolic syndrome under selenium and vitamin E deficiency. Clin Sci. https://doi.org/10.1042/CS20171039
Oropeza-Moe M, Wisloff H, Bernhoft A (2015) Selenium deficiency associated porcine and human cardiomyopathies. J Trace Elem Med Biol 31:148–156. https://doi.org/10.1016/j.jtemb.2014.09.011
Sheppard AD, Blom L, Grant AB (1984) Levels of selenium in blood and tissues associated with some selenium deficiency diseases in New Zealand sheep. N Z Vet J 32(6):91–95. https://doi.org/10.1080/00480169.1984.35076
Surai PF, Fisinin VI (2015) Selenium in pig nutrition and reproduction: boars and semen quality-a review. Asian Australas J Anim Sci 28(5):730–746. https://doi.org/10.5713/ajas.14.0593
Zhan X, Qie Y, Wang M, Li X, Zhao R (2011) Selenomethionine: an effective selenium source for sow to improve se distribution, antioxidant status, and growth performance of pig offspring. Biol Trace Elem Res 142(3):481–491. https://doi.org/10.1007/s12011-010-8817-8
Dubreil C, Sainte Catherine O, Lalatonne Y, Journe C, Ou P, van Endert P, Motte L (2018) Tolerogenic iron oxide nanoparticles in type 1 diabetes: biodistribution and pharmacokinetics studies in nonobese diabetic mice. Small 14:e1802053. https://doi.org/10.1002/smll.201802053
Su J, Sripanidkulchai K, Suksamrarn A, Hu Y, Piyachuturawat P, Sripanidkulchai B (2012) Pharmacokinetics and organ distribution of diarylheptanoid phytoestrogens from Curcuma comosa in rats. J Nat Med 66(3):468–475. https://doi.org/10.1007/s11418-011-0607-x
Wang X, Wang WX (2017) Selenium induces the demethylation of mercury in marine fish. Environ Pollut 231(Pt 2):1543–1551. https://doi.org/10.1016/j.envpol.2017.09.014
Szulc-Musiol B, Gadomska-Nowak M, Danch A, Ryszka F (2004) Pharmacokinetics of selenium following oral administration selenium preparation in rabbits. Boll Chim Farm 143(2):62–64
Waldridge BM, Duran SH, Ravis WR, Paxton R, Herdt TH, Pugh DG (2004) Pharmacokinetics of subcutaneous selenium in adult llamas. Vet Ther 5(4):272–278
Blodgett DJ, Bevill RF (1987) Pharmacokinetics of selenium administered parenterally at toxic doses in sheep. Am J Vet Res 48(3):530–534
Ecimovic S, Velki M, Vukovic R, Stolfa Camagajevac I, Petek A, Bosnjakovic R, Grgic M, Engelmann P, Bodo K, Filipovic-Marijic V, Ivankovic D, Erk M, Mijosek T, Loncaric Z (2018) Acute toxicity of selenate and selenite and their impacts on oxidative status, efflux pump activity, cellular and genetic parameters in earthworm Eisenia andrei. Chemosphere 212:307–318. https://doi.org/10.1016/j.chemosphere.2018.08.095
Gobi N, Vaseeharan B, Rekha R, Vijayakumar S, Faggio C (2018) Bioaccumulation, cytotoxicity and oxidative stress of the acute exposure selenium in Oreochromis mossambicus. Ecotoxicol Environ Saf 162:147–159. https://doi.org/10.1016/j.ecoenv.2018.06.070
Zheng S, Jin X, Chen M, Shi Q, Zhang H, Xu S (2018) Hydrogen sulfide exposure induces jejunum injury via CYP450s/ROS pathway in broilers. Chemosphere 214:25–34. https://doi.org/10.1016/j.chemosphere.2018.09.002
Yang J, Zhang Y, Hamid S, Cai J, Liu Q, Li H, Zhao R, Wang H, Xu S, Zhang Z (2017) Interplay between autophagy and apoptosis in selenium deficient cardiomyocytes in chicken. J Inorg Biochem 170:17–25. https://doi.org/10.1016/j.jinorgbio.2017.02.006
Yao HD, Wu Q, Zhang ZW, Zhang JL, Li S, Huang JQ, Ren FZ, Xu SW, Wang XL, Lei XG (2013) Gene expression of endoplasmic reticulum resident selenoproteins correlates with apoptosis in various muscles of se-deficient chicks. J Nutr 143(5):613–619. https://doi.org/10.3945/jn.112.172395
Niyo Y, Glock RD, Ramsey FK, Ewan RC (1977) Effects of intramuscular injections of selenium and vitamin E on selenium-vitamin E deficiency in young pigs. Am J Vet Res 38(10):1479–1484
Fraga CG, Arias RF, Llesuy SF, Koch OR, Boveris A (1987) AEffect of vitamin E- and selenium-deficiency on rat liver chemiluminescence. Biochem J 242(2):383–386
Zhao X, Yao H, Fan R, Zhang Z, Xu S (2014) Selenium deficiency influences nitric oxide and selenoproteins in pancreas of chickens. Biol Trace Elem Res 161(3):341–349. https://doi.org/10.1007/s12011-014-0139-9
Huang JQ, Li DL, Zhao H, Sun LH, Xia XJ, Wang KN, Luo X, Lei XG (2011) The selenium deficiency disease exudative diathesis in chicks is associated with downregulation of seven common selenoprotein genes in liver and muscle. J Nutr 141(9):1605–1610. https://doi.org/10.3945/jn.111.145722
Haraya K, Tachibana T, Nezu J (2017) Quantitative prediction of therapeutic antibody pharmacokinetics after intravenous and subcutaneous injection in human. Drug Metab Pharmacokinet 32(4):208–217. https://doi.org/10.1016/j.dmpk.2017.05.002
Jastrzebski Z, Czyzewska-Szafran H, Remiszewska M, Fijalek Z, Fitak BA, Suchocki P (1997) Pharmacokinetics of selol, a new agent containing selenium, in rats. Drugs Exp Clin Res 23(1):7–11
Guenter W, Bragg DB (1977) Response of broiler chick to dietary selenium. Poult Sci 56(6):2031–2038
Saito Y, Takahashi K (2002) Characterization of selenoprotein P as a selenium supply protein. Eur J Biochem 269(22):5746–5751. https://doi.org/10.1046/j.1432-1033.2002.03298.x
Zhao Z, Barcus M, Kim J, Lum KL, Mills C, Lei XG (2016) High dietary selenium intake alters lipid metabolism and protein synthesis in liver and muscle of pigs. J Nutr 146(9):1625–1633. https://doi.org/10.3945/jn.116.229955
Lehr T, Narbe R, Jons O, Kloft C, Staab A (2010) Population pharmacokinetic modelling and simulation of single and multiple dose administration of meloxicam in cats. J Vet Pharmacol Ther 33(3):277–286. https://doi.org/10.1111/j.1365-2885.2009.01134.x
van Iersel M, Rossenu S, de Greef R, Waskin H (2018) A population pharmacokinetic model for a solid Oral tablet formulation of posaconazole. Antimicrob Agents Chemother 62(7). https://doi.org/10.1128/AAC.02465-17
Funding
This study was supported by the International (Regional) Cooperation and Exchange Projects of the National Natural Science Foundation of China (31320103920).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
All other Authors have read the manuscript and have agreed to submit it in its current form for consideration for publication in the Journal.
Rights and permissions
About this article
Cite this article
Zheng, S., Xing, H., Zhang, Q. et al. Pharmacokinetics of Sodium Selenite Administered Orally in Blood and Tissues of Selenium-Deficient Ducklings. Biol Trace Elem Res 190, 509–516 (2019). https://doi.org/10.1007/s12011-018-1567-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-018-1567-8