Abstract
This study was carried out to determine the protective effects of lithium borate (LTB) on blood parameters and histopathological findings in experimentally induced acute cadmium (Cd) toxicity in rats. Twenty-eight male Wistar albino rats were used, weighing 200–220 g, and they were randomly divided into four groups, including one control and the following three experimental groups: a Cd group (0.025 mmol/kg), a LTB group (15 mg/kg/day orally for 5 days), and a LTB + Cd group (15 mg/kg/day orally for 5 days and Cd 0.025 mmol/kg by intraperitoneal injection on the fifth day). All the rats in the study were anesthetized with ketamine at the end of the sixth day, blood was taken from their hearts, and then the rats were decapitated. The values in the control and LTB group were usually close to each other. White blood cell (WBC), neutrophil %, and C-reactive protein (CRP) levels increased in the Cd and LTB + Cd groups while lymphocyte and monocyte levels decreased in a statistically significant manner, in comparison to the other groups. It was determined that the levels of red blood cells (RBCs), hematocrit (Htc), and hemoglobin (Hb) did not change in the groups. The levels of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) in the Cd and LTB + Cd groups significantly increased, in comparison to the other groups, while the glucose, alkaline phosphatase (ALP), albumin (ALB), and total protein (TP) levels decreased. According to histopathological findings in the control and LTB groups, the liver and kidney tissues were found to have normal histological structures. In the Cd group, severe necrotic hemorrhagic hepatitis, mild steatosis, and mononuclear cell infiltration were detected in the liver. In the LTB + Cd group, degeneration and mild mononuclear cell infiltration were found in the liver. Regarding the kidney tissue in the Cd group, severe intertubular hyperemia in both kidney cortex and medulla, as well as degeneration and necrosis in the tubulus epithelium, was observed. In the LTB + Cd group, mild interstitial hyperemia and mononuclear cell infiltration was detected. Resultantly, it can be said that LTB at this dose has non-toxic effects and some beneficial effects for liver and kidney damage caused by acute Cd toxicity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cadmium (Cd) is a toxic heavy metal with carcinogenic and mutagenic effects that has no physiological role in living organisms [1]. It is measurable in food and beverages, because it is very common in nature [2]. Cadmium forms compounds with lead, copper, and zinc, and it is usually observed in the form of cadmium chloride, cadmium oxide, cadmium sulphite, and cadmium sulfate mineral compounds in nature [3]. Cd is activated by iron, calcium, and zinc in living metabolism [4]. Cd is widely used in many industrial areas, and all doses of it are toxic [5, 6]. Cd is known as the most important toxic element within industrial and environmental pollution, and its accumulation in the atmosphere has increased the incidence of volcanic explosions and forest fires [5, 7]. However, it has been reported that 3 to 10 times more of the Cd accumulation in the atmosphere has occurred through anthropogenic pathways than through natural sources [8]. For instance, Cd is one of the major compounds in cigarettes [9].
Heavy metals, such as Cd, accumulate primarily in aquatic environments and are transported to the upper trophic levels via the food chain [10]. Due to Cd’s link with lung cancer, Cd falls into the class of materials having carcinogenic effects [3], and it is classified as a first class carcinogen [11]. Cd is taken into the body through the digestive, respiratory, and integumentary systems. Cd and its compounds are absorbed in the digestive tract from 0.5–12%, according to animal species. Protein, iron, and calcium deficiency increase the intestines’ absorption of Cd. Almost all types of Cd vapors can be absorbed by the lungs. This fact is significant to the presence of Cd in cigarette smoke. Water-soluble Cd salts, such as CdCl2, are strongly absorbed (up to 4%) when in close proximity to the intestinal tract [12]. In Cd toxicity cases, death occurs within a few hours as a result of cardiovascular collapse, metabolic acidosis, and coagulopathic bleeding. Acute Cd toxicity is characterized by high, short-term doses, and chronic Cd toxicity results from low, long-term levels of exposure. Respiratory and urinary system diseases are often preliminary in these types of exposure. In cases of chronic poisoning, coloring of the teeth (yellowish, cystic teeth), respiratory disorders (such as rhinitis, chronic bronchitis, and emphysema), and kidney disorders are usually observed [13]. Boron and its compounds, such as lithium borate (LTB), are very effective in the prevention of oxidative stress due to heavy metals, and these compounds demonstrate antioxidant activity, as indicated in the literature [14, 15]. Boron and boron compounds are preferred in the manufacture of eye irrigation solutions, mouthwashes, irrigant solutions, and medicines, due to their disinfectant, antiseptic, and antiepileptic properties. In addition, shampoo, cologne, perfume, and baby powder often utilize boron compounds in their manufacturing processes. Furthermore, boron neutron capture therapy is known as a method in cancer treatment. The use of boron is preferred as a treatment of brain cancer, because it allows cancer cells to be selectively cleared from the environment, while having a minimal effect on healthy cells. In addition, psychiatry, magnetic resonance devices, and the treatment of osteoporosis, menopause, and allergic diseases also benefit from the use of boron [16]. However, in the studies investigating the effects of LTB, there is not enough evidence to say.
The greatest boron exposure for many populations comes through nutrients. There is a direct correlation between the amount of boron taken in daily by humans and their eating habits [17]. Water, especially mineral water, is among the major sources of boron. In a study on drinking water in Germany, it was determined that mineral water contained an average boron concentration of 23.1 μg/L [18]. Another study reported that the average boron content of spring water may range between 0.75 and 4.35 mg/L [19]. In another study conducted on American populations, the average daily boron amounts for males, females, and pregnant women were 1.17, 0.96, and 1.01 mg/day, respectively. This study also stated that vegetarian males take in boron at an average rate of 1.47 mg/day and that vegetarian females take in boron at a rate of 1.29 mg/day [20].
In the literature, some studies on heavy metal toxicities have been carried out regarding the effects of boron and boron compounds in both in vitro and in vivo processes [14,15,16]. However, these studies include limited and insufficient levels, and they do not provide sufficient and detailed research on the effects of LTB on acute Cd toxicity in rats. Therefore, the aim of this study was to investigate the protective effects of LTB on the blood parameters and histopathological findings of the experimental rats’ livers and kidneys that had been exposed to acute Cd toxicity, thereby contributing to the literature.
Materials and Methods
In this study, 28 male Wistar albino rats were used which weighing 200–220 g and randomly divided into four groups as one control and three experimental groups (n =7 per group). Animals were housed in a well-ventilated and air-conditioned area provided with independently adjustable light-dark cycle (12 h light/12 h dark cycle) and temperature regulation systems. Temperature was maintained at 22 ± 2 °C and humidity was kept at 45–70%. The rooms and animal cages were cleaned daily and the animals were provided with fresh food and water ad libitum on a daily basis. In this study, CdCl2.5H2 (Cadmium chloride pentahydrate, CAS No. 7790-78-5, Sigma) was used as the Cd source, and lithium methaboronate dihydrate was used as the source of LTB. All the materials were prepared by dissolving in physiological serum.
-
Group I:
The control group received standard pellet feed, drinking water, and physiological serum by intraperitoneal (IP) injection for 5 days.
-
Group II:
This group received 0.025 mmol/kg of Cd as a single dose by IP injection.
-
Group III:
This group received 15 mg/kg/day of LTB orally for 5 days.
-
Group IV:
This group received 15 mg/kg/day of LTB orally for 5 days and 0.025 mmol/kg of Cd by IP injection on the fifth day.
For Group II, the physiological serum was administered for 5 days; an hour after the physiological serum administration took place on the fifth day, the Cd was applied by IP injection.
In Group IV, LTB was administered orally at 15 mg/kg/day for 5 days. After 1 hour of LTB administration on the fifth day, 0.025 mmol/kg of Cd was administered by IP injection.
All the rats in the study were anesthetized with ketamine at the end of the 6th day and blood was taken from their hearts and then decapitated.
The experimental protocol was approved by the Committee on the Ethics of Animal Experiments at Yuzuncu Yil University (Permit Number: 2014/12).
Blood parameters were determined using rat mode of veterinary the blood cell counter (Abocus Junior Vet-5, Austria) in whole blood. Biochemical parameters from the obtained serum were performed on a Modular PP auto-analyzer (Mindray BS800, China).
Histopathological Examination
The animals were sacrificed at the end of the six experimentation days by decapitation, and their liver and kidney tissues were quickly removed and processed for histopathology. The liver and kidney tissues were fixed in 10% neutral buffered formaldehyde for 48 h. These tissues were then embedded in paraffin blocks after the routine tissue procedure. These tissues were sectioned at a 5-μm thickness, stained with H&E, and examined under a light microscope (Olympus BX51 optical microscope and Olympus DP25 digital camera, Japan). The tissues were scored as negative (−), slight (+), moderate (++), or severe (+++), according to the histopathologic findings.
Statistical Analysis
Statistical analysis of blood parameters was presented as mean ± standard derivation (X ± SD). SPSS version 20 was used for statistical analysis. ANOVA and Tukey tests were used for comparison between groups. The data were expressed that was considered statistically significant as mean ± SD. p < 0.05.
Results
The results of the blood parameters and histopathological findings for each of the groups are presented in Tables 1, 2, 3 and 4. The values in the control and LTB groups were usually close to each other. The values in the Cd and LTB + Cd groups were outside of the range of change of the healthy rats’ values. The values of the LTB + Cd group were found to be better than the values of the Cd group, and they were closer to the mean values and physiological variation limits. As seen in Tables 1 and 2, the white blood cell (WBC), neutrophil %, and C-reactive protein (CRP) levels increased in the Cd and LTB + Cd groups, while the lymphocyte % and monocyte % levels decreased in a statistically significant manner, in comparison to the other groups. It was determined that the levels of red blood cells, hematocrit, and hemoglobin did not change within the groups.
As seen in Table 2, the levels of aspartate transferase and alanine transferase in the Cd and LTB + Cd groups increased in a statistically significant manner, in comparison to the other groups, while the glucose, alkaline phosphatase, albumin, and total protein levels decreased.
Macroscopic Findings
In the control group, the pathologic findings were not detected macroscopically, whereas in the Cd group, a slight exudate of the abdominal cavity, congestion, and petechial hemorrhage in the liver were detected. The macroscopic examination of the rats in the LTB group did not seen in any pathological finding. In the LTB + Cd group, some slight congestion in the liver was observed.
In the control group, any pathologic findings in the kidneys were not detected macroscopically, whereas in the Cd group, the kidneys were observed to be congested. Macroscopic examination of the kidney tissue in the LTB and LTB + Cd groups did not observed in any pathological finding.
Histopathological Findings
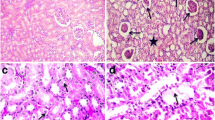
In the control group, when the liver tissues of the rats were examined, the normal histological structure was determined (Fig. 1a). Severe necrotic hemorrhagic hepatitis was detected in the livers of the Cd group (Fig. 1b). More distinct hyperemia, hemorrhage, and mild steatosis were detected in the acinar region, and mononuclear cell infiltration was observed in the portal area. When the liver tissues of the rats in the LTB group were examined, it was determined that the tissues demonstrated the normal histological structure (Fig. 1c). In the LTB + Cd group, mild mononuclear cell infiltration and hyperemia were observed in the portal area. While slight dilation and hyperemia in the sinusoids were detected in the acinar area for this group, the hepatocytes in the liver were not observed to be necrotic (Fig. 1d). The histopathological findings of the liver tissue for the various groups are summarized in Table 3.
Control group: normal histological structure of liver (a), Bar 50 μm. Cd group: severe necrotic hemorrhagic hepatitis in the central region of the liver (b), Bar 100 μm. LTB group: normal histological structure of liver (c), Bar 50 μm. LTB + Cd group: mild mononuclear cell infiltration and hyperemia in the portal region in the liver (d), H&E, Bar 50 μm
The kidney tissues of the rats in the control group were determined to be representative of a normal histological structure (Fig. 2a). In the Cd group, severe intertubular hyperemia was observed in both the medulla and cortex of the kidney tissue, and degeneration and necrosis in the tubulus epithelium were also observed (Fig. 2b). Furthermore, interstitial nephritis and hyaline cylinders in the tubule lumens were detected. The kidney tissues of the rats in the LTB group were determined to represent the normal histological structure (Fig. 2c). In the LTB + Cd group, mild interstitial hyperemia and mononuclear cell infiltration were detected in the medulla and cortex of the kidney; in addition, atrophy and hyperemia were observed in the glomerulus (Fig. 2d). The histopathological findings of the kidney tissue of the all groups are summarized in Table 4.
Control group: the normal histological structure of the kidneys (a), Bar 20 μm. Cd group: severe intertubuler hyperemia in the kidneys, degeneration and necrosis in tubul epithelium, interstitial nephritis (b), Bar 50 μm. LTB group: normal histological structure of the kidneys (c), Bar 20 μm. LTB + Cd group: interstitial and glomerular hyperemia in the kidneys, mild interstitial nephritis (d), H&E, Bar 20 μm
Discussion
Cd can accumulate in the environment, causing serious damages to ecosystem, human, and animal health. The general population is exposed to various concentrations of Cd either voluntarily, such as through supplementation, or involuntarily, such as through the intake of contaminated food and water or through contact with contaminated soil, dust, or air [21]. Cd causes the formation of many free radicals, including superoxide and nitric oxide, and it causes peroxidation of components in the cell membrane, DNA damage, and protein oxidation. The oxidation of membrane lipids results in the degradation of the membrane structure through the cross-linking of the polymerization. Damage to the mitochondrial membrane causes Cd to be released into the mitochondrial cell, leading to DNA damage and caspase-3 activation, which leads to apoptosis and necrosis [22, 23]. Furthermore, it has been reported that Cd toxicity causes both direct and indirect genotoxic effects, such as increased oxidative DNA damage, lipid peroxidation, and apoptosis (through the formation of DNA chain breaks and the inhibition of DNA repair mechanisms). Cd exposure may also be mutagenic and carcinogenic [24, 25].
Boron is considered to be an essential micronutrient, with well-established biological functions and the antioxidant effects of boric acid. Boron is rapidly and completely absorbed by the gastrointestinal tract into the bloodstream [26], and it plays an important role in improving arthritis, plasma lipid profiles, and brain function [27]. A variety of boronated agents with hypolipidemic, anti-inflammatory, or anticancer properties have also been developed [28]. Moreover, boron compounds have minimal potential for genotoxicity in bacteria and cultured mammalian cells [29]. Thus, these compounds are interesting research topics, due to their equivocal and relatively unknown useful effects, roles in the treatment of various diseases, and interactions with other elements.
In this study, as seen in Tables 1 and 2, no anemia was observed in any of the groups, and WBC, neutrophil, and CRP levels increased in both the Cd and LTB + Cd groups; these increases were statistically different, while the decreased lymphocyte and monocyte levels were statistically significant (p < 0.05). These increases in the WBC, neutrophil, and CRP levels, as well as the decreases in the monocyte and lymphocyte levels, may be derived from phagocytic activity. All these activities in the body are caused by the stimulation of the immune system. In general, the hematological parameters of the groups are consistent with other literature [30, 31].
Kidney urea, creatinine, blood urea nitrogen (BUN), and uric acid levels are often measured as indicators of renal function. These measurements may provide additional information about renal function [32]. Decreased glomerular filtration has been suggested to result in the elevation of serum urea, creatinine, and uric acid levels due to the increase in tubular reabsorption [33]. The increase in lactat dehidrogenaza (LDH) in the serum is a clear indicator of the toxic effect on animals. Creatine kinase (CK) and creatine kinase myocardial band (CK-MB) are enzymes that are found in high concentrations within the skeleton, muscles, and heart. Therefore, CK-MB is used as a specific indicator in cases of skeletal muscle injury and cardiac injury. However, it has been reported that an increase in CK-MB isoenzymes may result of the renal insufficiency [34]. In the present study, Table 2 demonstrates that the AST and ALT levels increased in a statistically significant manner in both the Cd and LTB + Cd groups (p < 0.05). However, this increase was less significant in the LTB + Cd group than it was in the Cd group. This may be due to the protective effects of LTB. As shown in Table 2, the glucose, ALP, ALB, and TP levels were decreased by the effect of Cd (p < 0.05). In addition, the total and direct bilirubin levels were increased by the effect of Cd, in comparison to the control group. These changes in the blood parameters may be due to liver and kidney damage. These biochemical parameters are in accordance with the reported values for healthy rats in the literature [30, 31, 35, 36].
Begic et al. [37] reported that the livers of rats that were exposed to Cd toxicity demonstrated necrotic hepatitis, mononuclear cell infiltration, and fat vacuoles in the cytoplasm. However, these findings were detected to be more severe at day 42. In addition, other studies have shown that liver enzymes and oxidative stress markers increased [37, 38]. Furthermore, some studies have reported severe congestion in the liver, as well as degeneration and necrosis in the central region of the liver, whereas the infiltration of lymphocytes and macrophages was observed in the portal region of the liver [39, 40]. Acute Cd intoxications in mice have been reported to cause congestion in the liver, necrosis of hepatocytes, and mononuclear cell infiltration in the portal regions of the liver [41]. These findings are similar to our results regarding the acute Cd toxicity of mononuclear cell infiltration in the portal region, degenerative necrotic heptocytes in the acinar region, and fat vacuoles in the cytoplasm of some hepatocytes in this region of the liver (Table 3).
Cd toxicity studies have reported inflammatory cell infiltration around the glomeruli, glomerular atrophy, congestion in the blood vessels, coagulation necrosis in the renal tubular epithelium, and hyaline cylinders in the renal tubule lumen [39]. In this study, mononuclear cell infiltration in the intertubular region, hyperemia in the intertubular and glomerular vessels, degeneration in the kidney tubule epithelium, and coagulation necrosis and hyaline cylinders in the tubule lumen were all observed (Table 4).
One study that measured the amount of Cd accumulation in tissues reported 17 times more accumulation of Cd in the liver than in the kidneys [42]. However, in our study, we found that the liver damage was more severe than the kidney damage. This conclusion suggests that accumulation and damage do not affect organs at the same level. Also, in accordance with our study, Zhai et al. reported that the damage to the kidney tissues in their study was limited, while the liver was found to be more severely damaged [40]. For this reason, this aspect of the study should be examined in more detail.
Consequently, according to the blood parameters and histopathological findings of this study, it can be said that LTB at this dose has non-toxic effects and some beneficial effects for liver and kidney damage caused by acute Cd toxicity.
References
Kay T, Thomas DG, Brown MW, Cryer A, Shurben D, Solbe JF, Del G, Garvey JS (1986) Cadmium accumulation and protein binding patterns in tissues of rainbow tout, Salmo Gairdneri. Environ Health Perspect 65:133–139
Thompson J, Bannıgan J (2008) Cadmium: Toxic Effects on the Reproductive System and the Embryo. Reprod Toxicol 25:304–315
Méndez-Armenta M, Rios C (2007) Cadmium Neurotoxicity. Environ Toxicol Pharmacol 23(3):350–358
WHO, 1992. Cadmium. Geneva Environmental Health Criteria 134
Cannino G, Ferruggıa E, Luparello C, Rinaldi AM (2009) Cadmium and mitochondria. Mitochondrion 9:377–384
Joe MH, Sun WJ, Seong H, Sang YL, Hyun PS, Ohsuk K, Dong HK (2011) Genome-wide response of Deinococcus radiodurans on cadmium toxicity. Microbiol Biotechnol 21:438–447
Marcano LBC, Carruyo IM, Montiel XM, Morales CB, Soto PM (2009) Effect of cadmium on cellular viability in two species of microalgae (Scenedesmus sp. and Dunaliella viridis). Biol Trace Elem Res 130(1):86–93
Patra RC, Amıya K, Swarup D (2011) Oxidative stress in lead and cadmium toxicity and its amelioration. Vet Med Int 9:4061
Aydogdu N, Kanter M, Erbas H, Kaymak K (2007) Kadmiyuma Bağlı Karaciger Hasarında Taurin, Melatonin ve Asetilsisteinin Nitrik Oksit, Lipit Peroksidasyonu ve Bazı Antioksidanlar Üzerindeki Etkileri. Erciyes Med J 29(2):89–96
Simon O, Ribeyre F, Boudou A (2000) Comparative experimental study of cadmium and methylmercury trophic transfers between the asiatic clam Corbicula fluminea and the crayfish Astacus astacus. Arch Environ Contam Toxicol 38:317–326
International Agency for Research on Cancer (1986) Tobacco smoking (IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans, Vol. 38). International Agency for Research on Cancer (IARCPress), Lyon
Baldwin DR, Marshall WJ (1999) Heavy metal poisoning and it’s laboratory Investigation. Ann Clin Biochem 36:267–300
Gökalp O, Özer MK, Koyu A, Çiçek E, Sütçü R, Koçak A, Özdem S, Aktürk O (2005) Ratlarda Kadmiyumun Pankreasa Etkileri. SDÜ Tıp Fak Derg 12(3):27–30
Türkez H, Geyikoglu F, Tatar A, Keles MS, Kaplan I (2012) The effects of some boron compounds against heavy metal toxicity in human blood. Exp Toxicol Pathol 64(1):93–101
Çelikezen FÇ, Turkez H, Başak T, İzgi MS (2014) DNA damaging and biochemical effects of potassium tetraborate. EXCLI J 13:446–450
Oto G, Yıldırım S, Dede S, Ozdemir H, Yener Z, Usta A, Taspinar M (2017) Therapeutic potential of boric acid and borax: dietary approaches for cancer prevention. Fresenius Environ Bull 26(3):2260–2268
Naghii MR, Saman S (1996) The effect of boron supplementation on the distribution of boron in selected tissues and on testosterone synthesis in rats. J Nutr Biochem 7:507–512
Becker K, Müssig-Zufika M, Hoffmann K, Krause C, Meyer E, Nöllke P, Schulz C, Seiwert M (1997) Umwelt-survey 1990/92 band V: Trinkwasser. Deskription der Spurenelementgehalte im Haushaltsund Wasserwerks Trinkwasser der Bevölkerung in der Bundesrepublik Deutschland
Moore JA (1997) Expert scientific committee, an assessment of boric acid and borax using the IEHR evaluative process for assessing human developmental and reproductive toxicity of agents. Reprod Toxicol 11(1):123–160
Rainey CJ, Nyquist LA, Christensen RE, Strong PL, Culver BD (1999) Daily boron intake from the American diet. J Am Diet Assoc 99:335–340
Meeker JD, Rossano MG, Protas B, Diamond MP, Puscheck E, Daly D, Paneth N, Wirth JJ (2008) Cadmium, lead, and other metals in relation to semen quality: human evidence for molybdenum as a male reproductive toxicant. Environ Health Perspect 116:1473–1479
Bridges CC, Zalups RK (2005) Molecular and ionic mimicry and the transport of toxic metals. Toxicol Appl Pharmacol 204(3):274–308
Bertin G, Averbeck D (2006) Cadmium: cellular effects, modifications of biomolecules, modulation of DNA repair and genotoxic consequences (review). Biochimie 88(11):1549–1559
Mourón SA, Golıjow CD, Dulout FN (2001) DNA damage by cadmium and arsenic salts assessed by the single cell gel electrophoresis assay. Mutat Res 498:47–55
Sarkar A, Ravindran G, Krishnamurthy V (2013) A brief review on the effect of cadmium toxicity: from cellular to organ level. Int J Biotechnol Res 3(1):17–36
Usuda KKK, Orita Y, Dote T, Iguchi K, Nishiura H, Tominaga M, Tagawa T, Goto E, Shirai Y (1998) Serum and urinary boron levels in rats after single administration of sodium tetraborate. Arch Toxicol 72:468–474
Devirian TA, Volpe SL (2003) The physiological effects of dietary boron. Crit Rev Food Sci Nutr 43:219–231
Barranco WT, Kim DH, Stella SL Jr, Eckhert CD (2008) Boric acid inhibits stored Ca(2+) release in DU-145 prostate cancer cells. Cell Biol Toxicol 25:309–320
Türkez H (2008) Effects of boric acid and borax on titanium dioxide genotoxicity. J Appl Toxicol 28:658–664
Comba B, Çınar A, Comba A, Gencer YG (2016) Sıçanlarda ACTH uygulamasının böbrek fonksiyon testleri elektrolitler ve hematolojik parametreler üzerine etkileri. Ank Üniv Vet Fak Derg 63:229–233
Comba B, Mis L, Comba A, Çınar A, Tas A (2014) Deneysel Olarak Diabet Oluşturulmuş Ratlarda Yara İyileşmesinde Sildenafil Sitratın Bazı Hematolojik Parametrelere ve Mineral Maddelere Etkisi. Atatürk Üniv Vet Bil Derg 9(3):180–186
Guyton AC, Hall JE (2007) Tıbbi Fizyoloji, 11. Nobel Tıp Kitabevleri, Baskı
Noyan A (2011) Yaşamda ve Hekimlikte Fizyoloji, 10. Palme Yayıncılık, Baskı
O'brien PJ, Smith DEC, Knechtel TJ, Marchak MA, Pruimboom-Brees I, Brees DJ, Spratt DP, Archer FJ, Butler P, Potter AN, Provost JP, Richard J, Snyder PA, Reagan WJ (2006) Cardiac troponin I is a sensitive, specific biomarker of cardiac injury in laboratory animals. Lab Anim 40(2):153–171
Gencer YG, Çınar A, Comba B (2015) Stresin Ratlarda Bazı Karaciger Enzimleri AST ALT ALP Üzerine Etkilerinin Arastırılması. Atatürk Üniv Vet Bil Derg 10(1):21–26
Mert N (1997) Veteriner Klinik Biyokimya. Uludağ Universitesi Güclendirme Vakfı Yayını, Ceylan Matbaacılık, Bursa
Begic A, Djuric A, Ninkovic M, Stevanovic I, Djurdjevic D, Pavlovic M, Jelic K, Pantelic A, Zebic G, Dejanovic B, Stanojevic I, Vojvodic D, Milosavljevic P, Djukic M, Saso L (2017) Disulfiram moderately restores impaired hepatic redox status of rats subchronically exposed to cadmium. J Enzyme Inhib Med Chem 32(1):478–489
Lebedová J, Bláhová L, Večeřa Z, Mikuška P, Dočekal B, Buchtová M, Míšek I, Dumková J, Hampl A, Hilscherová K (2016) Impact of acute and chronic inhalation exposure to CdO nanoparticles on mice. Environ Sci Pollut Res 23(23):24047–24060
El-Boshy M, Ashshi A, Gaith M, Qusty N, Bokhary T, AlTaweel N, Abdelhady M (2017) Studies on the protective effect of the artichoke (Cynara scolymus) leaf extract against cadmium toxicity-induced oxidative stress, hepatorenal damage, and immunosuppressive and hematological disorders in rats. Environ Sci Pollut Res 24(13):12372–12383
Zhai Q, Wang G, Zhao J, Liu X, Tian F, Zhang H, Chen W (2013) Protective effects of lactobacillus plantarum CCFM8610 against acute cadmium toxicity in mice. Appl Environ Microbiol 79(5):1508–1515
Wang M, Wang J, Sun H, Han S, Feng S, Shi L, Meng P, Li J, Huang P, Sun Z (2016) Time-dependent toxicity of cadmium telluride quantum dots on liver and kidneys in mice: histopathological changes with elevated free cadmium ions and hydroxyl radicals. Int J Nanomedicine 11:2319
Baker JR, Edwards RJ, Lasker JM, Moore MR, Satarug S (2005) Renal and hepatic accumulation of cadmium and lead in the expression of CYP4F2 and CYP2E1. Toxicol Lett 159(2):182–191
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yildirim, S., Celikezen, F.C., Oto, G. et al. An Investigation of Protective Effects of Litium Borate on Blood and Histopathological Parameters in Acute Cadmium-Induced Rats. Biol Trace Elem Res 182, 287–294 (2018). https://doi.org/10.1007/s12011-017-1089-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-017-1089-9