Abstract
Kaschin-Beck disease (KBD) is an endemic osteoarthritis, and the etiology is closely related with levels of trace elements in the human body. Currently, it is clear that the selenium (Se) status of children in KBD areas is lower than that in non-KBD areas in the Tibetan Plateau, whereas role of other elements are yet unknown. This study aimed to assess some essential trace elements (Se, Mo, Mn, Zn, Fe, Cu, Co, and Sr) in children using scalp hair as a biomarker, and 157 samples from school children aged 8–14 years old were collected from both KBD and non-KBD areas in Shigatse, Tibet. Se and Mo were measured by inductive coupled plasma mass spectrometry, and the other elements were determined by inductive coupled plasma optical emission spectrometry. Compared with the non-KBD areas, Se, Mo, Mn, Fe, Zn, Co, and Sr levels of children in KBD areas were found to be significantly different (P < 0.05); while in linear discriminant analysis, only Se and Zn were found to contribute to the KBD prevalence in the study area. The hair Se level of children in KBD areas ranged from 0.115 to 0.299 mg/kg, while in non-KBD areas it ranged from 0.135 to 0.519 mg/kg. The Zn content of children’s hair was between 83 and 207 mg/kg in KBD areas, while it was 37 and 219 mg/kg in non-KBD areas. Lower Se and higher Zn levels in children in KBD areas was found when compared with non-KBD groups. In addition, Mo levels were found to be different between KBD areas and non-KBD areas on the opposite side of the Yarlung Zangbo River, but no close relationship was shown because there was no difference compared with the non-KBD area on the same side of the river. Our observations suggest that Se deficiency is still an important factor for the occurrence and prevalence of KBD, while the relationship between Zn and KBD needs to be further explored in the Tibetan Plateau.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Kaschin-Beck disease (KBD) is an endemic, chronic degenerative osteoarthritis that most commonly occurs in children aged 5–15 years old [1, 2]. KBD was first described by N. I. Kaschin in 1849, and is mainly distributed throughout North Korea, Russia, and China [3, 4]. Although the etiology remains unclear up to now, several hypotheses were dominating, including selenium (Se) deficiency in the environment, grain contamination by mycotoxin-producing fungi, water contamination by organic materials such as fulvic acid, biological toxin poisoning, and dietary nutrient deficiency [1, 5–7].
In previous reports, trace element imbalances in humans can be shown to result from the deficiency or excess of trace elements in the environment in KBD areas. Se was the most studied element in KBD areas. Since the distribution of KBD areas in China was found to correspond with the Se deficiency distribution, Se deficiency in the external environment (soil, water, plants, and grain foods), and the internal environment of the human body have been extensively studied [3, 8–11]. Moreover, experimental Se supplementation in KBD areas showed a reduction of the incidence of KBD. Although there were some problems remained to be solved, such as the failure to experimentally prove a relationship between Se and KBD in animals, previous studies indicated that Se deficiency is not the initial etiology causing KBD [12], it is also a key factor in the occurrence and development of KBD [3, 13]. Meanwhile, levels of some other essential trace elements (iodine, molybdenum, manganese, iron, and zinc) may be related to the occurrence of the KBD [14, 15].
Recently, with economic development and improvements in living standards, the prevalence of KBD has greatly decreased in most affected areas of China. However, KBD in Tibet is still endemic [16]. To improve the diet of children in the locality, the free education policy and nutrition improvement plan was adopted in Tibet since 2000. Although Se levels in school children have greatly improved [17], levels of the other essential trace elements in children remain unknown. For environmental exposure bio-monitoring of trace elements in the human body, hair is widely used for its advantages of noninvasive sampling and ease of storage [18, 19]; therefore, we used scalp hair as a biomarker. The aims of this study were: (1) to assess the trace element status of school children in KBD and non-KBD areas in Shigatse, Tibet, and (2) to explore the relationship between essential trace element levels in children and KBD distribution in Shigatse, Tibet.
Materials and Methods
Site Description and Subject Selection
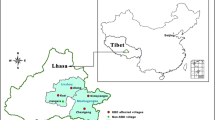
Shigatse is located between the middle section of the Himalayan mountain range and the middle of the Gangdese-Nyainqentanglha hills. The Yarlung Zangbo River, which is the longest plateau river in China, cuts across Shigatse. The river basin is one of the main KBD areas in Tibet, and affected areas are mostly on the north side of the river [16]. The average altitude of Shigatse is over 4000 m. Considering the KBD distribution, geographical landscape, and traffic conditions in Shigatse, a cross-sectional study was conducted in Xietongmen County and Lazi County in October 2015. Xietongmen County lies on the north side of the Yarlung Zangbo River, between longitude 87° 34′-89° 12′ and latitude 29° 18′-30° 26′, at altitudes between 3920 and 6310 m, and Lazi County is located on the south side of the Yarlung Zangbo River, between longitude 87° 24′-88° 21′ and latitude 28° 47′-29° 37′, at altitudes of 3900 to 4280 m. Renqinze village in Xietongmen County is a typical KBD area, with new cases identified in 2009, 2010, and 2015, since Xietongmen was first defined as a severe KBD endemic area in 1999 [20–22]. Three schools were chosen for the hair sample collection in the two counties (Fig. 1). Among them, Renqinze primary school (hereafter indicated by RQs) located in Renqinze village in Xietongmen County represented the KBD area. The other two schools located in non-KBD areas, which were selected as controls, were Tongmen Wanquan primary school (hereafter indicated by TMs) in Tongmen village in Xietongmen County (as an internal contrast to the KBD area) and Chawu Yiwu primary school (hereafter indicated by CWs) in Chawu village in Lazi County (as an external contrast to the KBD area on the north side of the Yarlung Zangbo River). All three schools had implemented the free education policy of Tibet.
The study protocol was approved by the Shigatse Health Bureau of Tibet and parents or legal representatives of all the participants that gave written consent prior to inclusion in the study. Children were randomly selected from various age groups. To guarantee successful hair sample collection, children with dyed hair or short hair (length less than 5 mm) were excluded [9]. Under such selection criteria, 157 children were accepted to the survey, and the description of the groups is presented in Table 1.
Sample Preparation
Approximately 0.5-g hair samples with lengths of 1–3 cm were collected from the nape of the head, as near as possible to the scalp with a pair of stainless steel scissors, and stored in polyethylene bags for later sample preparation. The hair samples were washed three times with neutral detergent, rinsed with distilled water, and dried in an oven at a temperature of 60 °C for 6–8 h. The hair samples were then cut into small pieces (2–3 mm) for trace element determination [11, 23].
Sample Digestion and Analysis
Hair samples (0.1 g) were placed separately in 50 mL beakers and digested in a mixture of concentrated HNO3 and H2O2 (v:v = 2:1) on a hot plate until the solution became clear. During this process, the temperature was maintained at 100 ± 50 °C to prevent volatilizing the element. After cooling, the samples were diluted with deionized water to 10 mL. Se and molybdenum (Mo) concentrations in the hair were determined by inductive coupled plasma mass spectrometry (ICP-MS) [17] (ELAN DRC-e, PerkinElmer Instrument Co, Shelton, CT, USA). Barium (Ba), cobalt (Co), copper (Cu), iron (Fe), manganese (Mn), strontium (Sr), and zinc (Zn) were determined by inductive coupled plasma optical emission spectrometry (ICP-OES) [24] (ICP-OES 5300DV, PerkinElmer Instrument Co, CT, USA). All reagents were of analytical-reagent grade or better, provided by Sino-pharm Chemical Reagent, Beijing, China. The purified water used for all dilutions was of 18.3 MΩ cm−1 purity, obtained using a Milli-Q (Millipore, Bedford, MA, USA) deionization system. For quality assurance and control, blank spikes, certified reference materials (CRMs; human hair powder GBW09101b, Shanghai Institute of Nuclear Research, Shanghai, China), and blind duplicates were used during the analysis. The levels of elements estimated in the CRM were found to be consistent with the values reported in the CRM (Table 2). The recoveries in spiked samples of the nine elements ranged from 88% to 110%, and the relative percentage differences were ≤10% in duplicate samples. The limits of detection for the elements were 0.002–0.017 μg/L, which were determined as three times the standard deviation from seven blank solutions.
Statistical Analysis
The Kolmogorov-Smirnov test was performed to verify data normality. As variables were not normally distributed, non-parametric testing was used to compare differences between groups. Linear discriminant analysis (LDA) [18] was used to highlight variables differentiating the groups from KBD areas and non-KBD areas. Differences were considered significant at the level of P < 0.05. SPSS 22.0 (IBM Corp., Armonk, NY, USA) was used to perform all statistical analyses. A location map of the study area was made using ArcGIS 10.0 (Esri, Redlands, CA, USA).
Results and Discussion
The results of hair trace element analysis for RQs, TMs, and CWs are presented in Table 3, Table 4, and Table 5, respectively. Presented values are arithmetic mean (AM), geometric mean (GM), minimal, and maximal value (min and max, respectively), percentiles (P5, P50, and P95), and arithmetic mean values for boys and girls separately (Male-AM and Female-AM). The concentrations of Zn in TMs and CWs samples and Se in samples from all three sites were normally distributed, while the concentrations of other elements in the different sites were not.
Comparative analysis of RQs and the two control areas was performed and showed some similarities: hair Se levels in children were lower in the KBD area (RQs) than those of the two non-KBD areas (P < 0.05), and Co, Fe, Mn, Sr, and Zn levels were higher in the KBD area than those of the other two areas (P < 0.05). The differences in Se content in children between the KBD area and non-KBD areas were similar to that of the previous report of other KBD areas [14, 15, 25]. Se deficiency in children in KBD areas remains a threat to the prevalence of KBD. Besides Se, the fact that levels of some other elements (Co, Fe, Mn, Sr, and Zn) in school children were found to be different between KBD areas and non-KBD areas illustrated that trace elements in children in KBD areas may be in an out-of-balance status.
Trace elements with statistically different median concentrations were subsequently used to calculate LDA between RQs and the other two sites, to evaluate which variables contribute to differentiation of the groups and to better interpret trace element levels in hair of children in KBD areas and non-KBD areas on both sides of the Yarlung Zangbo River (Fig. 2 and Fig. 3). Between RQs and TMs, Se and Zn contributed most to the separation, and between RQs and CWs, Se, Zn, and Mo contributed most to the separation.
As an essential constituent of selenoproteins such as glutathione peroxidase (GPX), Se is known as an antioxidant [26]. In cases of Se deficiency, some types of damage similar to KBD would appear to be due to increased peroxidase levels in cartilage. To control the prevalence of KBD, a pilot project of selenium-iodine salt supplementation was adopted in KBD areas including Renqingze village in Xietongmen County from 2001 to 2003, and children’s hair Se levels improved from 0.074 mg/kg in 2001 to 0.256 mg/kg in 2003 [27]. Since then, no directly Se salt supplementation has been adopted in Renqingze village, the mean hair Se level of school children is rising to 0.218 mg/kg in KBD areas in Renqinze today, which may profit from the outsourced grains (rice and flour purchasing wheat flour from other provinces of China) substituting the local staple grain (tsampa, made of highland barley grains) and the diet improving with the enforcement of the free education policy and nutrition improvement plan in Tibet. Merely, the hair Se levels in school children in KBD area is still lower than those in the non-KBD areas in Shigatse.
Trace elements in the human body mainly come from the daily diet. However, the dietary structure of residents at home in Tibet is simple; grain, mainly barley, accounted for 75.6%, and other food, such as meat, eggs, and dairy products, made up only 22% [28]. The Se content in tsampa, which is made from local barley, was only 0.003 mg/kg and 0.007 mg/kg, respectively, in KBD areas and non-KBD areas [29], and the Se content of rice and wheat flour from other provinces in China was about 0.036 mg/kg and 0.029 mg/kg [30]. Although children consume moderate amounts of Se from rice and wheat flour, which is purchased from other provinces at boarding schools, the children’s diet at home is still dominated by local foods with low Se contents, especially in KBD areas. According to the threshold of children’s hair Se values classified by past research efforts into Se nutrition in China [31], children whose hair Se level is lower than 0.20 mg/kg are deemed Se deficient. In this study, 38.2% of children in RQs were Se deficient in KBD areas, compared to 12.7% in non-KBD areas. Low levels of Se in the local environment are still a limiting factor for Se nutrition levels in children, and much more Se-rich food, such as fish, meat, eggs, and nuts, need to be taken in the diet when children were at home during holidays.
Besides Se, the higher Zn level in children in KBD area also requires some attention. Zn is an essential element for humans, involved in the activity of about 100 enzymes, including RNA polymerase and carbonic anhydrase, and has important functions in growth and development of adolescents [32]. Deficiency or excess in children would both be harmful to health. However, the relationship between Zn and KBD is still controversial. Some reports have shown altered levels in people with KBD, while some show no difference between patients with KBD and healthy people. Although the possibility of higher Zn levels in children influencing occurrence of KBD is unclear, the phenomenon of higher Zn levels in KBD areas needs to be investigated during future study in Tibet.
Mo is also an essential trace element for humans and is a component of many enzymes, such as sulfite oxidase and xanthine oxidase [33]. Although there are some studies showing that Mo content in the environment was much lower in some KBD areas than non-KBD areas [34, 35], Hou reported that they found no correlation between Mo and KBD [36]. In this study, hair Mo levels in children from the non-KBD CWs on the south side was lower than those of children from TMs and RQs on the north side of the river, which supports the likelihood that Mo is not an important influencing factor for KBD. The difference in Mo levels between the two sides of the river may be caused by different environments, since the north side originates in the Gangdese-Nyainqentanglha hills while the south side is in the Himalayan mountain range area [37]. In other words, Mo differences are merely due to the different environments on either side of the Yarlung Zangbo River and have no correction with KBD.
In addition, considering the phenomenon of different disease distributions between the two sides of the Yarlung Zangbo River, it may be in part due to the different levels of trace elements in the environment. Beyond that, geographical factors such as climate, topography, and geomorphology could also affect the spatial KBD distribution [38, 39], and the reason for the difference in KBD distribution between the two sides of the Yarlung Zangbo River requires much more comprehensive studies.
Gender differences in each group were also assessed. For each site, higher Ba, Co, Cu, Fe, Mn, and Sr levels were found in girls than boys. In boys, higher levels of Se were measured for each site, whereas Mo levels were significantly higher in boys than girls in RQs and CWs. Meanwhile, for all the children, no obvious relationships between trace element levels and age or body mass index were found (calculations not shown).
The tendency of gender-related differences of Se levels corroborated reports from some KBD areas and non-KBD areas in Lhasa, Sichuan, and Qinghai [17, 40, 41]. In non-KBD areas of China and Sri Lanka, higher Se in males than females was also found [19, 42]. The similar results in New Zealand and Slovakia also found that serum Se concentration was higher in boys than in girls [43, 44]. For other elements, Co, Fe, Mn, and Sr were reported to have the same tendency in the normal areas [45, 46], and Saranga et al. showed the same tendency for higher Ba, Co, Cu, Fe, Mn, and Sr levels in females than in males [42]. However, in KBD areas, the relevant data was absent. Different food consumption habits and amount of exercise between boys and girls may be possible reasons [19].
Conclusion
The present study has demonstrated that Se status of children is obviously lower in KBD areas than in non-KBD areas in Shigatse, Tibet. Furthermore, levels of other trace elements, such as Co, Fe, Mn, Mo, Sr, and Zn, were found to be significantly different between school children in KBD endemic areas and non-KBD areas. In LDA analysis, only Se and Zn were discovered to be closely related to KBD. In Tibet, Se deficiency remains a threat to the children in KBD areas. Meanwhile, higher Zn levels in children, and their relationship to KBD, need further investigation. Moreover, there are still some limitations, such as lack of investigation of the daily dietary food consumption by children at school and home, and the correspondence between hair element status and dietary trace element intake at home and school will be further studied in Tibet.
Reference
Cao J, Li S, Shi Z, Yue Y, Sun J, Chen J, Fu Q, Hughes CE, Caterson B (2008) Articular cartilage metabolism in patients with Kashin–Beck disease: an endemic osteoarthropathy in China. Osteoarthr Cartil 16(6):680–688. doi:10.1016/j.joca.2007.09.002
Zou K, Liu G, Wu T, Du L (2009) Selenium for preventing Kashin-Beck osteoarthropathy in children: a meta-analysis. Osteoarthr Cartil 17(2):144–151. doi:10.1016/j.joca.2008.06.011
Zhao ZJ, Li Q, Yang PZ, Wang H, Kong LC, Wang LH, Sun LY (2013) Selenium: a protective factor for Kaschin-Beck disease in Qing-Tibet Plateau. Biol Trace Elem Res 153(1–3):1–4. doi:10.1007/s12011-0 13-9686-8
Peng-Fei GE, Bai SY, Ji-Min XU, Wang WL, Jia JX, Ren YG (2008) Analysis of surveillance results of Keschin-Beck disease in Gansu Province in 2003 and 2004. Endemic Diseases Bulletin 23(1):43–45 (In Chinese)
Suetens C, Moreno-Reyes R, Chasseur C, Mathieu F, Begaux F, Haubruge E, Durand M, Nève J, Vanderpas J (2001) Epidemiological support for a multifactorial aetiology of Kashin-Beck disease in Tibet. Int Orthop 25(3):180–187. doi:10.1007/s002640100247
Sun LY, Li Q, Meng FG, Fu Y, Zhao ZJ, Wang LH (2012) T-2 toxin contamination in grains and selenium concentration in drinking water and grains in Kaschin–Beck disease endemic areas of Qinghai Province. Biol Trace Elem Res 150(1):371–375
Peng A, Yang CL (1991) Examination of the roles of selenium in the Kaschin-Beck disease. Cartilage cell test and model studies. Biol Trace Elem Res 28(1):1–9. doi:10.1007/bf02990457
Tan J, Huang Y (1991) Selenium in geo-ecosystem and its relation to endemic diseases in China. Water Air Soil Pollut 57-58(1):59–68
Li S, Yang L, Wang W, Li Y, Li H, Xirao R (2007) Relationship between selenium concentration in child hair and the distribution of Kashin-Beck disease in Tibet, China. Frontiers of Medicine 1(2):223–225 (In Chinese)
Li SJ, Li W, Hu X, Yang LS, Xirao RD (2008) Distribution of Kashin-Beck disease (KBD) and its relation to selenium content in soil-plant-animal (human being) ecosystem in Tibet. Chin J Ecol
Zhang B, Yang L, Wang W, Li Y, Li H (2011a) Environmental selenium in the Kaschin-Beck disease area, Tibetan Plateau, China. Environmental Geochemistry & Health 33(5):495–501
Wang ZL, Xiong YM, Chen JH, Bi HY, Yang ZT, Tan XW (2003) Study on the relationship between Kaschin-beck disease and selenium ans B19. Chinese J of Endemic 22(1):16–19
Wang Q, Li XX, Li L, Tian JH, Yang KH, Wu TX, Liao YJ (2013) Correlation between selenium and Kaschin-Beck disease: a meta-analysis. Chinese J of Evience-based Medicine 13(12):1421–1430
Zeng LX, Peng XU, Schrauzer GN (1999) Logistic analysis and content of 39 elements in hair of children between Kashin Beck diseased areas and non Kashin Beck disease areas. Chinese Jouranl of Cndemiology 28(6):445–449 (In Chinese)
Liu L, Zhao X, Ming DY, Wen-Jun MA (2014) Analysis of 18 kinds of elements in blood of patients with Kashin-Beck disease in parts of Aba autonomous prefecture, Sichuan. Journal of Environment & Health 31(7):587–589 (In Chinese)
Yang LS, Yao LU, Hai-Rong LI, Yong-Hua LI, Shun-Jiang LI, Wang WY, Tan JA (2006) Features of geographical environment of Kaschin-Beck disease (KBD) affected region in Tibet. Sci Geogr Sin 26(4):466–471 (In Chinese)
Chen Z, Li H, Yang L, Wang W, Li Y, Gong H, Guo M, Nima C, Zhao S, Wang J, Ye B, Danzeng S, Deji Y (2015a) Hair selenium levels of school children in Kashin-Beck disease endemic areas in Tibet, China. Biol Trace Elem Res 168(1):25–32. doi:10.1007/s12011-015-0333-4
Dongarra G, Varrica D, Tamburo E, D'Andrea D (2012) Trace elements in scalp hair of children living in differing environmental contexts in Sicily (Italy). Environ Toxicol Pharmacol 34(2):160–169. doi:10.1016/j.etap.2012.03.005
Li S, Banuelos GS, Wu L, Shi W (2014) The changing selenium nutritional status of Chinese residents. Nutrients 6(3):1103–1114. doi:10.3390/nu6031103
Li QW (2000) Investigative report on the prevalence condition of Kashin-Beck diserase (KBD) in Tibet. Chinese Jouranl of Cndemiology 19(1):41–43 (In Chinese)
Gong H, BAIMA, Zhao S, ZHAXI, Lang J (2010) Inetigation of Kaschin-Beck disease in Shigatse area in Tibet in 2008. China Journal Endem Disase 25(1):41–42 (In Chinese)
Gong H, Zhao S, DeYang, Guo M, NiMa, Li QW (2016) A case of III Kashin-Beck disease was found in Tibet. China J Endemiol (1):58–58 (In Chinese)
Gao J, Liu Y, Huang Y, Lin Z-Q, Bañuelos GS, Lam MH-W, Yin X (2011) Daily selenium intake in a moderate selenium deficiency area of Suzhou, China. Food Chem 126(3):1088–1093. doi:10.1016/j.foodchem.2010.11.137
Guise R (2011) Development and application of inductively coupled plasma atomic emission spectrometry. Chinese Journal of Inorganic Analytical Chemistry 01(4):15–18 (In Chinese)
Bai C, Lu S, Wang Z (1995) Determinations of hair copper, Zinc, Manganese and iron contents in children from a Kashin-Beck disease (KBD) area and non-KBDAreas with normal and low hair selenium contents. Endem Dis Bull (1):4–6 (In Chinese)
Rayman MP (2000) The importance of selenium to human health. Lancet 356(9225):233–241. doi:10.1016/s0140-6736(00)02490-9
Gong HQ, Zhasang, Baima, Gesang (2004) Study of the effect of idoine selenium salt prevention of prevention on Kaschin-Beck disease (KBD) in Xietongmen county in Tibet. Endemic Diseases Bulletin 19(1):61–62 (In Chinese)
Qu H, Nima T, Wei Z, Mei X (2015) Soil selenium (Se) status and the production on Se-enriched hulless barley in the Tibet Autonomous Region. Sci Agric Sin 48(18):3645–3653 (In Chinese)
Zhaxi DZ, Gong HQ, Geang DJ, Baima CW (2003) Result of selenium content in Kaschin-Beck disease (KBD) areas. Chinese Jouranl of Endemiology 18(3):177–177 (In Chinese)
Chen Z, Li HR, Yang LS, Gong HQ, Li YH, Guo M, Wang WY, Nima CJ, Zhao SC (2015b) Staple food consumption and related selenium intake among residents in Kaschin-Beck disease endemic areas of Lhasa municipality, China. China J Public Health 31(7):915–918 (In Chinese)
Tan J (1990) Chemico-geography of some life elements and endemic diseases with an emphasis on China, Environmental life elements and health. Science Press, Beijing, pp 145–157
Fraga CG (2005) Relevance, essentiality and toxicity of trace elements in human health. Mol Asp Med 26(4–5):235–244
Chan S, Gerson B, Subramaniam S (1998) The role of copper, molybdenum, selenium, and zinc in nutrition and health. Clin Lab Med 18(4):673–685
Fang W, Wu P, Hu R, Huang Z (2003) Environmental Se–Mo–B deficiency and its possible effects on crops and Keshan–Beck disease (KBD) in the Chousang area, Yao County, Shaanxi Province, China. Environ Geochem Health 25(2):267–280
Zhang B, Yang L, Wang W, Li Y, Li H (2010) Quantification and comparison of soil elements in the Tibetan Plateau Kaschin-Beck disease area: a case study in Zamtang County, Sichuan Province, China. Biol Trace Elem Res 138(1):69–78
Hou SF, Wang WZ (1994) Study on the stablity of the relationship between elements and Kaschin-Beck disease. Geographical Reaserch 4:28–35 (In Chinese)
Zhu S (2012) River landform and geology environment evolution in the Yarlung Zangbo River valley. Chinese academy of geological science (In Chinese)
Yong-Hui AN, Xu-Feng LI, Jin HE, Jia XF, Liang LI (2010) Distribution characteristics of Kaschin-Beck disease in relation to geological environment of Zoige County. Geol China 37(3):587–593 (In Chinese)
Li Q (2010) Tentative study on causes of geographical distribution of Keshan disease and Kaschin-Beck disease. Foreign Medical Scenice Section of Medgeography 31(3):139–142
Xiao-Yan XU, Gao Q, Chen YQ, Yang XZ, Wang DC (2012) The selenium nutritional states of the children in Kaschin-Beck disease area in Gangmuda Village, Rangtang County, Sichuan. Journal of China West Normal University 33(1):78–82 (In Chinese)
Zhang Q, Wang H, Duo-Long HE, Hai-Kun WU, Zhang XL, Hui-Zhen YU (2011b) Selenium levels inside and outside Qinghai Province Kaschin-Beck disease district environment from 2007 to 2009. Chin J Control Endem Dis (2): 119–1221 (In Chinese)
Diyabalanage S, Fonseka S, Dasanayake DMSNB, Chandrajith R (2016) Environmental exposures of trace elements assessed using keratinized matrices from patients with chronic kidney diseases of uncertain etiology (CKDu) in Sri Lanka. Journal of Trace Elements in Medicine & Biology Organ of the Society for Minerals & Trace Elements 39:62
Brtková A, Magálová T, Béderová A, Babinská K, Barteková S (1999) Serum selenium levels in healthy Slovak children and adolescents. Biol Trace Elem Res 67(1):49–54
Thomson CD, Mclachlan SK, Parnell WR, Wilson N, Wohlers M, Scragg R (2007) Serum selenium concentrations and dietary selenium intake of New Zealand children aged 5–14 years. Br J Nutr 97(2):357–364
Drobyshev EJ, Solovyev ND, Ivanenko NB, Kombarova MY, Ganeev AA (2017) Trace element biomonitoring in hair of school children from a polluted area by sector field inductively coupled plasma mass spectrometry. J Trace Elem Med Biol 39:14–20. doi:10.1016/j.jtemb.2016.07.004
Varrica D, Tamburo E, Dongarra G, Sposito F (2014) Trace elements in scalp hair of children chronically exposed to volcanic activity (Mt. Etna, Italy). Sci Total Environ 470-471:117–126. doi:10.1016/j.scitotenv.2013.09.058
Acknowledgments
We gratefully acknowledge fundings from the National 12th Five-Year Plan scientific and technological issues of China (No.2013BAC04B03) and the National Natural Science Foundation of China (No. 41671500; No. 41171081). We also thank the local government of Tibet Centers for Disease Control and Prevention and Shigatse Centers for Disease Control and Prevention, Tibet Autonomous Region for their help during the field investigations and sampling, which were carried out in October 2015.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Guo, Y., Li, H., Yang, L. et al. Trace Element Levels in Scalp Hair of School Children in Shigatse, Tibet, an Endemic Area for Kaschin-Beck Disease (KBD). Biol Trace Elem Res 180, 15–22 (2017). https://doi.org/10.1007/s12011-017-0988-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-017-0988-0