Abstract
This study aimed to investigate the effects of chromic chloride (CrCl3) on Ca, Mg, Mn, Fe, Cu, and Zn contents in the brain and serum of chicken. Seventy-two chickens were randomly divided into four groups and treated with different doses of CrCl3 via drinking water: 0, 1/8, 1/4, and 1/2 LD50 for 42 days. The contents of the elements were evaluated through inductively coupled plasma mass spectrometry. Results showed that Cr contents in the brain and serum were higher than those in the control groups, although no significant dose-dependent changes (P > 0.05) in brain of the Cr-treated groups were observed at 42 days. As exposure time was prolonged and CrCl3 dosage was increased, Ca contents increased (P < 0.05). Mg and Cu contents in serum decreased; by contrast, Mg and Cu contents initially increased and then decreased in the brain. Fe and Zn contents in the serum increased; conversely, Fe and Zn contents in the brain decreased. CrCl3 exposure did not significantly affect Mn contents at 14 or 28 days, but significantly decreased (P < 0.05) at 42 days. Therefore, excess Cr3+ intake can disrupt absorption and deposition of other trace elements in the brain and serum; the blood–brain barrier may prevent the accumulation of these elements in the brain exposed to CrCl3.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chromium (Cr) is widely distributed in nature; among chromium-containing compounds, trivalent chromium (Cr3+) exhibits the most stable oxidation state, and hexavalent chromium (Cr6+) is a strong oxidant that is easily reduced to Cr3+ in acidic solution [1]. Cr6+ and Cr3+ are both toxic, although Cr6+ is more toxic than Cr3+ [2]. Cr3+ exhibits DNA damage and can induce histopathological changes and oxidative stress in the liver and kidney in chicken and goldfish [3, 4]. However, Cr3+ is ligand-dependent so that it can exist as a stable form of chromium ion in the body [5]. The amount of chromium in the environment has rapidly increased due to the increasing usage of chromium compounds in industrial production which caused industrial waste pollution. Chromium can easily combine with natural and artificial compounds, causing pollution in the environment [6]. Thus, the threats of chromium pollution on public health and in livestock breeding have been considered a major concern. The European Food Safety Authority excluded Cr as an essential element in humans and other animals. However, Cr3+ is also an important trace element often added to livestock feed to improve animal production, growth, and meat quality [7]. However, in the actual process of adding Cr into the fodder, people may add excess trivalent chromium to gain more benefits, adversely causing such an element to be poisonous and dangerous for chicken. [8]. Cr penetrates the blood–brain barrier, but the removal of Cr from the brain proceeds slowly; as a result, Cr accumulates in the brain tissue [9, 10]. Excess amount of Cr causes imbalance of metals in the brain; thus, damage to the nervous system of chickens might exacerbate [11].
Trace elements affect growth and development. These elements are found in enzymes, hormones, and vitamins; these elements also act as important components of bioactive substances that participate in biochemical processes in animals [12]. Although trace elements are needed by living creatures, they could cause toxic effects when these substances are consumed excessively [13]. Manganese (Mn), iron (Fe), zinc (Zn), and Copper (Cu) are trace elements implicated in growth and brain functions. Deficient or excessive amounts of these trace elements result in nervous system disorders [14]. In CrCl3 poisoning, Cr3+ is absorbed by organisms and distributed by the blood throughout the body; as a consequence, Cr3+ accumulates in the brain [10]. An excessive amount of Cr3+ also causes other trace elements to accumulate in the brain because the transport of trace elements into the brain is strictly regulated by the blood–brain barrier system [15]. Serum trace elements directly involved in various metabolic processes in the body are maintained in equilibrium; thus, deficient or excessive amounts of these trace elements result in abnormal electrolyte metabolism, thereby causing changes in the body’s normal physiological activity and biochemical processes [16]. The effects of Cr3+ on trace element contents in the brain and the effects of Cr3+ on transport through the blood–brain barrier have been rarely investigated. The effects of Cr on calcium (Ca), magnesium (Mg), Mn, Fe, Cu, and Zn in the brain and serum of chickens, as well as possible protective effects of blood–brain barrier, which regulates electrolyte metabolism, have also been seldom explored.
In this study, Cr3+ was administered to chickens via drinking water. To investigate the effects of Cr3+ on chicken brain, we evaluated the effects of Cr3+ on Ca, Mg, Mn, Fe, Cu, and Zn contents in the brain and serum. This study may provide basis on the prevention and cure of nervous system toxicity and Cr3+ poisoning among poultry.
Materials and Methods
Chemistry Drugs
Chromium chloride (CrCl3·6H2O, GR) was obtained from Putian Reagent Company (Tai’an, Shandong, China). HNO3 and H2O2 were purchased from Sigma Company (St. Louis, MO, USA). Other reagents were of analytical grade and rinsed with deionized water before use.
Animal Treatment and Experimental Design
Acute toxicity experiments were performed to determine the median lethal dose (LD50) of CrCl3 in chickens in accordance with the Karber method [17]. The results revealed that the LD50 in chicken was 5000 mg/kg body mass [18]. This study was performed as a chronic toxic experiment.
One-day-old clean-grade Hyland chickens were supplied by the Experimental Animal Center of Shandong Agricultural University. All chickens were provided with free access to completed diet for 14 days before the experiment was performed. The chickens were then divided into four groups; the groups were provided with different doses of CrCl3 solution as drinking water (control, 0 mg/kg; low dose, 1/8LD50 = 625 mg/kg; moderate dose, 1/4LD50 = 1250 mg/kg; and high dose, 1/2LD50 = 2500 mg/kg). A total of 18 chickens were included in each group, and each chicken was singly housed in cages based on their groups. All chickens were administered with CrCl3 solution dependent on body mass to receive the proper dosage (Table 1). The water given to each chicken was all finished to make appropriate administrations of Cr3+ intake of measurement. The Cr3+ intake was the three different doses of LD50 multiplied by the date of mean body mass of each group. The chickens were housed in an air-conditioned room. The room temperature was regulated according to the chickens’ body temperature.
The chickens were handled in strict accordance with good animal practice as defined by national and/or local animal welfare bodies. The experimental procedure was approved by the Animal Care and Use Committee of the Shandong Agricultural University and performed in accordance with animal welfare and ethics guidelines.
Samples Collection
Blood sample was separately collected from the heart of the chickens at 14, 28, and 42 days; afterward, the chickens were euthanized through intraperitoneal thiopental (40 mg/kg body mass) injection. The brain was quickly removed and subjected to element assay. After natural sedimentation occurred at room temperature, the serum was collected, stored, and frozen at −70 °C.
Element Detection
Brain tissues were isolated immediately after chickens were euthanized. The samples (approximately 0.20 g of wet mass) were digested with concentrated HNO3 (65 % Merck) and H2O2 (30 % Merck) in a microwave digestion system (MARS-5, CEM) for 30 min to analyze mineral content. The same process was repeated two to three times. All of the digested samples were diluted with deionized water to maintain the analyte within the calibration range. Trace elements (Cr, Ca, Mg, Mn, Fe, Cu, and Zn) were evaluated by using inductively coupled plasma mass spectrometer (ICP-MS; Hewlett-Packard, HP-4500, Avondale, PA, USA) according to the manufacturer’s recommendation. The accuracy of the determined elements was verified through simultaneous analysis of bovine liver BCR®-185R (IRMM) as the standard reference material. Detection limits (LOD, ng/ml) were determined on the basis of blank solution; LODs of Ca, Mg, Fe, Mn, Cu, Zn, and Cr were 0.004, 0.001, 0.58, 0.07, 0.05, 0.05, and 0.04 ng/ml, respectively. The results were then multiplied by the dilution ratio. All measurements of the experiments were independently performed thrice.
Statistical Analysis
Statistical analysis was performed using one-way ANOVA analysis of variance using SPSS 19.0 (SPSS Inc., Chicago, IL, USA) and then followed by a post hoc test using the S-N-K method. All values were expressed as mean ± S.D. P < 0.05 was considered significantly different.
Results
Cr Content in the Brain and Serum of Chicken
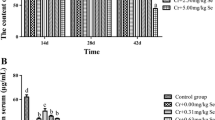
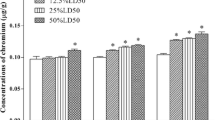
After the chickens were exposed to CrCl3 for 14, 28, and 42 days, Cr contents (P < 0.05) in the brain and in the serum were higher than those in the control group. In the brain, Cr content was significantly different (P < 0.05) among the three Cr-exposed groups at 14 days; Cr content did not significantly differ between high- and moderate-dose groups at 28 days, although Cr content in these two groups were significantly increased (P < 0.05) compared with that in the low-dose group, whereas Cr content did not significantly change among the three Cr-exposed groups at 42 days (P > 0.05). In the serum, Cr content was significantly increased (P > 0.05) among the three Cr-exposed groups at 14 and 42 d but did not differ at 28 days compared with that of the control group. Cr accumulated more slowly in the brain than in the serum (Table 2).
Elemental Contents in the Brain and Serum of Chickens
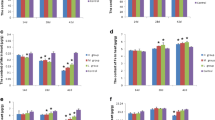
Longer exposure to a higher CrCl3 dose significantly increased (P < 0.05) the amount of Ca both in the brain and in the serum. No significant change was observed in Ca content of the serum at 14 days; Ca content was significantly increased compared with that of the control group at 28 and 42 days. Compared with the control group, only the high-dose group showed significant changes in Ca content in the brain (P < 0.05).
Mg contents were generally reduced, although the reduction was not dose and time dependent. Mg content in the serum was higher than that in the control group at 14 days; by contrast, Mg content was lower in the serum than that in the control group at 28 and 42 days; Mg content decreased significantly (P < 0.05) with time. In the brain, Mg content was significantly higher (P < 0.05) than that of the control group at 14 and 28 days; conversely, Mg content was lower than that of the control group at 42 days.
Exposure to CrCl3 did not significantly affect (P > 0.05) Mn contents of the serum at 14 and 28 days, although Mn contents significantly decreased (P < 0.05) compared with those of the control at 42 days. In the brain, Mn content decreased significantly (P < 0.05) as CrCl3 dosage and exposure time increased, especially at 42 days.
The effects of CrCl3 on Fe content showed opposite trends in the serum and in the brain. In the serum, Fe content was significantly increased (P < 0.05) with longer exposure time and higher CrCl3 dose compared with that of the control group; by contrast, Fe content was significantly decreased (P < 0.05) in the brain.
Cu content in the serum did not significantly change (P > 0.05) among the three CrCl3-exposed groups at 14 and 28 days; Cu contents were significantly lower (P < 0.05) than those of the control groups. Cu content in the high-dose group significantly decreased (P < 0.05) at 42 days compared with that in the three other groups with longer exposure to a higher CrCl3 dose. In the brain, Cu content significantly decreased (P < 0.05) at 14 days compared with that of the control group; Cu content increased at 28 days. Cu content decreased at 42 days.
Longer exposure to CrCl3 resulted in a slightly higher Zn content in the serum compared with that in the control group at 14 days; by contrast, Zn contents significantly decreased (P < 0.05) at 28 and 42 days compared with those in the control group. Zn content did not exhibit significant dose-dependent changes (P > 0.05) among the three Cr-exposed groups. In the brain, Zn content in the three Cr-exposed groups was significantly lower (P < 0.05) than that of the control group, although no significant (P > 0.05) differences were observed among the groups at 14 and 28 days. By contrast, Zn content in the high-dose group significantly decreased (P < 0.05) at 42 days compared with that of the control group and the two other groups. Thus, changes in Zn content are time and dose dependent (Figs. 1 and 2).
Discussion
Under normal physiological condition, various trace elements in the body are not isolated; instead, these substances interact and limit one another to achieve equilibrium in systems [19, 20]. In this study, long-term exposure of chickens to high Cr3+ dose via drinking water significantly increased Cr content in the serum and in the brain.
Cr poisoning can increase Ca content in the serum and brain of chickens; Ca content was significantly higher when exposed to a higher CrCl3 dose. Ca content could increase when the body is injured, and this finding is consistent with the experimental results obtained in our study. Changes of Mg content initially increased and then decreased; Mg content did not significantly differ at different doses. [21] indicated that Mg deficiency may increase intracellular Ca content; thus, the high Ca content may have resulted from the decrease in Mg. Excessive Cr significantly reduced Mn and Zn contents in the serum and in the brain; Mg and Zn contents were further reduced as exposure to higher CrCl3 dose was prolonged. Fe and Cu contents increased in the serum; by contrast, Fe and Cu contents decreased in the brain. The changes of Cu showed no linear time and dose relationship. It was speculated that such changes of Cu might be related to the blood–brain barrier. These unusual results induced us great interest in continuing to explore the specific mechanism on Cu and Cr3+ in the brain. Increased Cr content in the body alters absorption, distribution, and excretion of other trace elements; thus, these elements accumulate in the central nervous system. Reduced Mn and Zn contents also resulted from the increased Cu and Fe contents; these trace elements compete against one another in terms of absorption and transport.
Trace element contents in the blood and in various organs indicate their physiological roles in the body; these factors may also show an organism’s exposure to environmental pollution [22]. Trace elements are involved in physiological processes in the brain; any deficiency or excess in these elements results in nervous system disorders [23]. Once these elements penetrate the body, exogenous metals may directly or indirectly interfere with the normal distribution of biologically important metals; as a result, imbalance among trace elements occur and may disrupt the body’s physiological activities [24]. Cr commonly exists in the environment and may be absorbed by the body through the digestive tract; Cr interferes with the amount and distribution of essential trace elements, causing toxicity to animals [25].
In this study, the chickens presented anorexia symptoms, excitement or depressed on the spirit in the high-dose group. Cr poisoning affected the contents of other trace elements, and the pattern of these changes in the serum differs from that in the brain. For instance, Fe content significantly increased in the serum but decreased in the brain. The distribution of essential elements in the brain is also affected by the transport of Cr into the brain through the blood–brain barrier. The blood–brain barrier is a regulatory interface found between capillaries and nerve tissues in the brain and in the spinal cord [26]. The blood–brain barrier is an active protective barrier rather than a simple interface because this part selectively pumps excessive or harmful substances out of the brain to maintain a constant internal environment [19]. Thus, excessive amounts of Cr may damage the blood–brain barrier; as a consequence, Cr in the blood migrates to the brain tissue and accumulates in the brain. The damage caused by Cr indicates not only the accumulation of Cr but also the effect of Cr on the distribution of other essential elements in the brain. The obtained concentrations of the trace elements may be considered as baseline data for further brain research.
Conclusion
Excess Cr3+ intake may disrupt the absorption of trace elements in the serum and in the brain, although changes in trace element contents in the brain differ from those in the serum. Thus, the blood–brain barrier may prevent trace element absorption; however, the underlying mechanism should be further elucidated. Trace element absorption in the brain should also be explored.
References
Sundaram B, Aggarwal A, Sandhir R (2013) Chromium picolinate attenuates hyperglycemia-induced oxidative stress in streptozotocin-induced diabetic rats. J Trace Elem Med Biol 27:117–121
Matos RC, Vieira C, Morais S, Pereira Mde L, Pedrosa J (2009) Nephrotoxicity effects of the wood preservative chromium copper arsenate on mice: histopathological and quantitative approaches. J Trace Elem Med Biol 23:224–230
Stearns DM, Wise JP, Patierno SR, Wetterhahn KE (1995) Chromium(III) picolinate produces chromosome damage in Chinese hamster ovary cells. Faseb Journal Official Publication of the Federation of American Soci Exp Biol 9:1643–1649
Lushchak OV, Kubrak OI, Lozinsky OV, Storey JM, Storey KB, Lushchak VI (2009) Chromium(III) induces oxidative stress in goldfish liver and kidney. Aquat Toxicol 93:45–52
Tang HY, Xiao QG, Xu HB, Zhang Y (2015) Hypoglycemic activity and acute oral toxicity of chromium methionine complexes in mice. J Trace Elem Med Biol 29:136–144
Liu M, Liu Y, Cheng Z, Liu J, Chai T (2015) Effects of chromic chloride on chick embryo fibroblast viability. Toxicol Rep 2:555–562
Vincent JB (2004) Recent developments in the biochemistry of chromium (III). Biol Trace Elem Res 99:1–16
Fan WT, Zhao XN, Cheng J, Liu YH, Liu JZ (2015) Oxidative stress and hepatocellular injury induced by oral administration of Cr(3+) in chicken. J Biochem Mol Toxic 29:280–287
Lushchak OV, Kubrak OI, Torous IM, Nazarchuk TY, Storey KB, Lushchak VI (2009) Trivalent chromium induces oxidative stress in goldfish brain. Chemosphere 75:56–62
Cheng J, Fan W, Zhao X, Liu Y, Cheng Z, Liu Y, Liu J (2016) Oxidative stress and histological alterations of chicken brain induced by oral administration of Chromium(III). Biol Trace Elem Res. doi:10.1007/s12011-016-0640-4
Shimamura T, Iijima S, Hirayama M, Iwashita M, Akiyama S, Takaku Y, Yumoto S (2013) Age-related effects of major and trace element concentrations in rat liver and their mutual relationships. J Trace Elem Med Biol 27:286–294
Haenlein GFW, Anke M (2011) Mineral and trace element research in goats: a review. Small Ruminant Res 95:2–19
Wagner A, Boman J (2003) Biomonitoring of trace elements in muscle and liver tissue of freshwater fish. Spectrochim Acta B 58:2215–2226
Takeda A (2003) Manganese action in brain function. Brain Res Rev 41:79–87
Höck A, Demmel U, Schicha H, Kasperek K, Feinendegen L (1975) Trace element concentration in human brain. Activation analysis of cobalt, iron, rubidium, selenium, zinc, chromium, silver, cesium, antimony and scandium. Brain J Neurol 98:49–64
Sturrock AM, Hunter E, Milton JA, Trueman CN (2013) Analysis methods and reference concentrations of 12 minor and trace elements in fish blood plasma. J Trace Elem Med Biol 27:273–285
Bittenbender H, Howell GS Jr (1974) Adaptation of the Spearman-Karber method for estimating the T50 of cold stressed flower buds. Journal of the American Soc Hortic Sci 99:187–190
Cao J (2004) Effects of chromium (III) on humoral immune response, fecundity of chicks and bioavailability, residues, toxicity following oral administration. Wuhan Huazhong Agricultural University. (in chinese)
Ward RJ, Zucca FA, Duyn JH, Crichton RR, Zecca L (2014) The role of iron in brain ageing and neurodegenerative disorders. Lancet Neurol 13:1045–1060
Silva E, Viana ZCV, Souza NFA, Korn MGA, Santos VLCS (2016) Assessment of essential elements and chemical contaminants in thirteen fish species from the Bay Aratu, Bahia, Brasil. Braz J Biol. doi: http://dx.doi.org/10.1590/1519-6984.02415
Crossgrove JS, Yokel RA (2005) Manganese distribution across the blood–brain barrier. Neurotoxicology 26:297–307
Esposito M, Cavallo S, Chiaravalle E, Miedico O, Pellicanò R, Rosato G, Sarnelli P, Baldi L (2016) Trace elements in free-range hen eggs in the Campania region (Italy) analyzed by inductively coupled plasma mass spectrometry (ICP-MS). Environ Monit Assess 188:326
Anderson RA, Polansky MM, Bryden NA (1984) Acute effects on chromium, copper, zinc, and selected clinical variables in urine and serum of male runners. Biol Trace Elem Res 6:327–336
Dallago BSL et al (2016) Tissue accumulation and urinary excretion of Cr in chromium picolinate (CrPic)-supplemented lambs. J Trace Elem Med Biol 35:30–35
Liu Y, Zhao X, Zhang X, Zhao X, Liu Y, Liu J (2015) Effects of oral administration of CrCl3 on the contents of Ca, Mg, Mn, Fe, Cu, and Zn in the liver, kidney, and heart of chicken. Biol Trace Elem Res. doi:10.1007/s12011-015-0559-1
Klinge PM, Messier A, Heile A, Nouri M, Johanson CE, Duncan JA, Brinker T, Silverberg GD (2007) Opposite changes in cerebellar vs. cortical blood brain barrier (BBB) expression in aged and hydrocephalic rats. Cerebrospinal Fluid Res 4:S4
Acknowledgments
This work was supported by the the National Key R&D Program (2016YFD0501208), the Shandong Modern Agricultural Technology & Industry System (No. SDAIT-11-04).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Additional information
Yanhan Liu and Pan Hao contributed equally to this work.
Rights and permissions
About this article
Cite this article
Liu, Y., Hao, P., Zhang, X. et al. Effects of Excess Cr3+ on Trace Element Contents in the Brain and Serum in Chicken. Biol Trace Elem Res 177, 180–186 (2017). https://doi.org/10.1007/s12011-016-0875-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-016-0875-0