Abstract
Rheumatoid arthritis (RA) is a chronic autoimmune inflammatory disease, which mainly involves the joints. RA is prevalent worldwide with increasing prevalence in elderly people. The mechanism of RA pathogenesis is still undefined, and it is interplaying between genetic susceptibility and environmental factors. Although risk factors for RA are not fully established, various studies have focused on the role of trace elements in association with RA. Trace elements act as co-factors for most of the enzymes, and their deficiency is associated with many untoward effects on human health. The homeostatic alterations in the metabolism of trace elements may partly be due to inflammatory response in RA. The objective of the present study was to determine the serum concentrations and correlation of zinc, copper, and iron in RA patients and healthy controls. The study comprised of 61 RA patients and 61 age- and sex-related healthy individuals of Pakistani population. Serum levels of Zn, Cu, and Fe were measured in all the participants by atomic absorption spectrophotometer. Serum Zn and Fe were significantly reduced in the RA patients than those in the healthy controls. Serum Cu concentrations were found elevated in the RA patients. Correlation studies of trace elements determine that there was negative correlation between Zn and Cu in the RA patients and no correlation in the control group. It is very important to explore the deficiency of essential trace metals in biological samples of the RA patients in different populations which may be helpful for diagnosis and supplementary management of rheumatoid arthritis patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rheumatoid arthritis (RA) is a multisystem disease of obscure etiology that produces symmetrical inflammation of the joints [1]. RA combines chronic inflammation and bone destruction. The emerging evidence indicates that inflammation is a key agent, precipitating the destruction of skeleton [2]. In RA, there is hyperplasia of the synoviocytes, particularly the synovial fibroblasts, which leads to bone and joint damage [3]. Symptoms, including weight loss malaise and fatigue, in addition to articular ones are present [4]. Prevalence of RA in Pakistan is around 0.5 % [5]. The number of females having RA is far greater than that of males throughout the world; with male to female ratio of 1:2–4 [6, 7]. However, basis of such gender-oriented discrimination regarding RA is yet not known [6].
Etiology of RA is still undefined, and it is interplaying between genetic susceptibility and environmental factors like smoking, high birth weight, silica exposure, rheumatoid factor (RF), and anti-citrullinated protein antibody [8, 9]. Although risk factors for RA are not fully established, many studies have focused on the role of trace elements in association with RA. Trace elements in the body are co-factors for most of the enzymes, and their deficiency is associated with many untoward effects on human health [10].
Essential trace elements are required by man in defined amounts per day. Acting as catalytic or structural components of larger molecules, they have definite functions and are vital for life [11]. Zinc, copper, and iron are important dietary chemical required for living organisms.
Zinc (Zn) is an essential element for a number of cellular functions including normal growth, protein metabolism, stability of the membranes, and metallo-enzyme functions. Zn has many influences on the immune system owing to the fact that Zn is indispensible for normal development and function of the cell-mediated innate immunity, neutrophils, and natural killer cells [12, 13]. Zn along with copper plays role as an intracellular scavenger of the reactive oxygen species (ROS) [14] and exhibits a key role in bone mineralization. Deficiency of the Zn has untoward effects on macrophages, phagocytosis, intracellular killing, and production of cytokines. Zn deficiency produces deleterious effects on the growth and functions of the T and B cells.
Copper (Cu) is an important trace element that has a prime role in cell physiology. It acts as a cofactor and a structural component for the enzymes, in addition to acting as an antioxidant or detoxificant for the ROS [15]. Cu is needed for proper mineralization of the cartilage, collagen, and elastin formation. Cu is necessary for cross-linking of the collagen as well as formation of the bony trabeculation structures [16, 17]. Deficiency of Cu in tissues exerts greater harm to the antioxidant enzyme system, rendering the immune system inoperative and decreasing hemoglobin level, thereby leading to increased oxidative stress and inflammatory response [18, 19].
Iron (Fe) is crucial for energy metabolism, electron transport chain, and transport of oxygen. Fe has potential for the formation of free radicals. It is considered as a powerful pro-oxidant and is required for generation of ROS. It is obvious that Fe-related oxidative damage is closely associated with diseases [20].
In Pakistani population, few reports for the essential trace elements are described for their protective role in an inverse correlation with toxic elements in rheumatoid arthritis patients [21]. Previously, in another study, important trace elements like Zn, Cu, and Fe were significantly reduced in rheumatoid arthritis patients from Sindh province [22]. The risk factor like cigarette smoking is also associated with low level of Zn and higher exposure of toxic elements in Pakistani and Irish population [23].
The objective of the present study was to measure the serum concentrations of essential trace elements (Zn, Cu, and Fe) in the RA patients from Punjab province of Pakistan to find out any alterations in levels of these trace elements in association with RA.
Sample Population and Blood Collection
A total of 122 subjects of both genders were included in this study. Sixty-one were patients of rheumatoid arthritis (RA), and 61 age- and sex-matched were healthy controls. The patients were recruited from out-patient department of orthopedic, District Head Quarter Teaching Hospital, Dera Ghazi Khan, Punjab, Pakistan while the healthy controls were taken randomly from the same region. Diagnostic criteria for RA was according to the internationally accepted criteria, i.e., American College of Rheumatology/European League Against Rheumatism. Classification criteria for RA (score-based algorithm: add score of categories A–D; a score of ≥6/10 is needed for classification of a patient as having definite RA (ACR/EULAR, 2010)).
-
A.
Joint involvement (score 0–5)
-
B.
Serology either RF or ACPA (at least one test result is needed for classification) (score 0–3)
-
C.
Acute phase reactants either CRP or ESR (at least one test result is needed for classification) (score 0–1)
-
D.
Duration of symptoms (score 0–1)
The following parameters were considered for inclusion of the subjects: (1) gender both sex, (2) age ranging from 20 to 65 years, and (3) both sero-positive and sero-negative cases of rheumatoid arthritis. On the other hand, cases of other autoimmune connective tissue diseases, other chronic inflammatory conditions, and hyperuricemic conditions were not included in this study. The patients with RA who were using medications like anti-inflammatory, anti depressants, corticosteroids, and oral use of multivitamins and minerals were also excluded from the study. For the control group, subjects of both genders with age ranging from 20 to 65 years were included while subjects having any history of autoimmune inflammatory diseases, recent history of any infection, or history of anemia were excluded from the study.
After informed consent, a 5 ml of blood sample was withdrawn aseptically from each subject for determination of Zn, Cu, and Fe without venous stasis and frothing. The samples were left to clot for 15–30 min at 37 °C and then were centrifuged at 3000 rpm for 10 min. Serum was separated and carefully shifted into properly labeled 1-ml nitric acid-treated aliquots. The samples were then stored at −20 °C to be used afterwards for analysis. Centrifugation and freezing of the samples was completed within 2 h. Samples were neither lipemic nor haemolyzed.
Measurement of Trace Elements on Atomic Absorption Spectrophotometer
The measuring principle is based on absorption of primary radiation by the analyte atom in their ground state. The measured absorbance signal is directly proportional to the concentration of respective element in the samples.
Zn, Cu, and Fe were measured on atomic absorption spectrophotometer (Hitachi Z2000) with polarized Zeeman atomic absorption spectrophotometer flame. Samples were digested with 10 % nitric acid and incubated overnight in microwave temperature. Next morning, the samples were centrifuged to remove proteins and debris. The clear supernatant was diluted ten times in 10 % nitric acid for analysis on atomic absorption. Zn, Cu, and Fe standard stock solutions (1000 mg/l, Merck) were used to establish calibration curves with different calculated concentrations of standards. After successful calibration of the instrument, normal and low levels of Bio-Rad controls of Zn, Cu, and Fe were ran with each batch of the analysis to ensure reliability and reproducibility of the results according to the protocols described elsewhere [24]. The analytical conditions for the analysis of trace elements are given in Table 1.
Statistical Analysis
The data was entered and analyzed using SPSS 1 (Statistical Package for Social Sciences) version 8.0. Mean ± SD were given for quantitative variables of Cu, Zn, and Fe concentrations. Student t test was applied to observe difference between quantitative variables (patients with rheumatoid arthritis and healthy subjects). A value of p ≤ 0.05 was considered as statistically significant. Pearson correlation was applied between the patients with RA and healthy controls.
Results
In the present study, a total of 122 subjects were recruited for the investigation. Clinical history and physical examination of 61 RA patients included family history of rheumatoid arthritis, duration of the disease, history of pain (symmetrical or localized), and joint involvement (single or multiple), and laboratory reports about rheumatoid factor (RF) were taken into consideration at the time of recruitment. All data were recorded on a prescribed proforma. Other features noted in the affected joints of these patients were redness and swelling. Socio-demographic data obtained from both the study groups (RA group and control group) included age, gender, education level, occupational history, and height and weight.
Mean age of the rheumatoid arthritis patients was 44.33 ± 15.68 years (mean ± SD), while in the control group, it was 43.3 ± 12.5 years. Mean height (cm) and weight (kg) of the patients and controls were (63.4 ± 3.8 and 57.6 ± 9.6) and (63.3 ± 3.7 and 58.1 ± 9.3), respectively. The duration of the disease was 61.77 ± 52.71 months (mean ± SD) in the patient group. RA was more prevalent in middle and old age group, and persistence of the disease was more than 30 months at an average (Table 2).
In this study, RA was more prevalent in females with approximate male to female ratio of 1:4. Rheumatoid factor (RF) was positive in 80 % of the patients while it was negative in 20 % of the patients. Family history was positive in 10 % of the patients with RA. Most of the patients were uneducated (33 (54.1 %)), primary (16 (26.2 %)), matriculation (10 (16.4 %)), and above matriculation (02 (3.3 %)). All females (48 (79 %)) included in this study were house wives while males were farmers and laborers (13 (21 %)). On caste-based distribution of disease, the most affected cases were in Maliks (34 %), Balochs (34 %), followed by Arains (25 %), Jattois (5 %), and Pathans (1.6 %). History of disease duration was noted, and most of the patients were having a chronic history of the disease which was more than 1 year in 47 (77 %), while only 14 (23 %) patients represented less than 1-year history of the disease. All the patients were having symmetrical and multiple joint involvement, and no one showed asymmetrical joint involvement (Table 3).
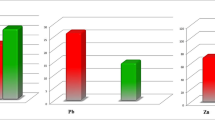
Serum concentrations of trace elements showed that the average serum Zn levels in the RA patients were 85.6 ± 32.3 μg/dl (mean ± SD), while average Zn levels in the controls were 95.9 ± 16.4 μg/dl. Minimum Zn levels were 23 μg/dl, and maximum Zn levels were 120 μg/dl. It was significantly lower (p value = 0.028) in the RA patients as compared to that in the healthy subjects. For Cu, the mean serum levels in the patients with RA were 130.3 ± 42.8 μg/dl with a range of 71–250 μg/dl, while it was lower in the control group (102.7 ± 22.5 μg/dl). The difference was noted statistically significant (p value = <0.001), with the patients having higher values than the controls. Fe levels were (76.8 ± 38.5 μg/dl) (mean ± SD) reduced in the patient group while the mean level (113.3 ± 25.7 μg/dl) was in the control group. Serum Fe was statistically significant (p value = 0.001) in the RA patients with reduced levels as compared to that in the healthy subjects (Fig. 1).
Diagram showing the comparison of the serum levels of Fe, Zn, and Cu between the patients and controls. Values in the patients are significantly different when compared with respective controls according to the “t” test (n = 61). p values of these trace elements were 0.001, 0.028, and 0.001, respectively
A correlation analysis was done for the RA patient group and the normal control group, for serum concentrations of the trace metals (Zn, Cu, and Fe). No correlation was seen in the controls between the Cu and Zn levels in the serum, while significant negative correlation (p < 0.000) was observed between these elements (Zn and Cu) in the RA patient group (Fig. 2). On the other hand, no correlation was seen between Cu and Fe and Zn and Fe in both the control group and the patient group of RA patients.
Scatter diagrams showing correlation of trace elements (Zn and Cu) between the RA patients and controls. Strong negative correlation was found between serum Cu and Zn levels in the patients with rheumatoid arthritis. The r 2 value was 0.716 which showed positivity of the correlation, and p value was <0.000 which showed that correlation is statistically significant. This shows that as Cu levels increase the in patients, serum Zn level decreases. In the control group, there was no significant difference in trace element levels with r 2 value of 0.008
Discussion
In present study, a total of 122 individuals comprising of rheumatoid arthritis cases and health controls were studied in population of Punjab, Pakistan. The subjects were included in the study after fulfilling the standard criteria. Mean age of the patients with RA was 44.33 ± 15.68 (mean ± SD) years while that of the control group was 43.3 ± 12.5 years. Duration of the disease noted in this study was 61.77 ± 52.71 (mean ± SD) months. According to the results of the present study, RA was more prevalent during middle and old age, and in females than males with a ratio of 4:1. This type of gender discrimination has also been described earlier by other researchers, indicating that two thirds of the RA patients were females [25, 26]. However, another study indicated that male to female ratio in autoimmune diseases, like RA, was 1:9 [27]. In the present study, the rheumatoid factor (RF) was positive in 80 % of the patients with RA which is in agreement with Visser et al. (2002), which documented that 2/3 of the patients with RA were RF-positive [28].
In the present study, it was observed that most of the patients with RA were of low socioeconomic status (SES) and were not well educated. A Swedish study indicated that risk of RA was greater in individuals having low SES as compared to that in individuals of high SES [29]. It was shown that not only prevalence of RA was greater in individuals of low SES but they also showed worse disease outcomes in contrast to RA patients who were of high SES [30].
In our study, serum Cu levels in the patients with RA were higher than those in the controls. Similar results for Cu concentrations in the serum of patients with RA were seen in several other studies [15, 31–35]. In contrary, a previous study from Pakistan reported reduced concentrations of Cu in different biological samples of RA patients [22, 35]. The possibility for higher levels of Cu in RA patients could be the use of Cu utensils for cooking in this geographic area that may produce higher Cu concentrations. Also, since Cu and Zn are both divalent metals, their dynamics are such that when one metal increases in concentration, the other decreases (antagonist) in order to keep the osmotic pressure of the extracellular fluid in normal limits. A study reported that 30–50 % elevation in serum Cu concentration during the course of an acute phase reaction is initiated by interleukin-1 (IL-1) release and depends mainly on increased synthesis of ceruloplasmin [36]. It was shown also that ceruloplasmin increases during inflammatory process owing to the fact that generation of the cytokines (both, IL-1 and IL-6) has stimulatory effects on hepatocytes to increase ceruloplasmin level in the blood serum [17, 37]. It is further reported that ceruloplasmin neutralizes oxygen free radicals in arthritis, thus mitigating the inflammatory process to become chronic, and this fact might provide possible explanation for both the increased serum Cu and Cp levels in RA patients [15, 38].
In this study, levels of serum Zn were significantly lower in the RA patients as compared to those in the healthy control which are comparable to the previous studies [22, 32, 39–41]. Contrary to the above studies, higher Zn levels were observed in the patients with RA than those in the healthy controls (80.1 ± 12.5 and 72.0 ± 8.0) but the correlation was not significant [42]. Low concentrations of the Zn seen in RA may be due to malabsorption of this metal in RA [43], or it could be because of the pro-inflammatory cytokines, which induce the expression of the Zn importer, ZIP-14, and metallothionein (MT) in the hepatocytes, that in turn, facilitates Zn uptake and storage by the liver [44]. Previously, it was reported that acute phase response to stress decreases concentration of the Zn in plasma due to redistribution of the Zn into cellular compartments [45].
For iron concentration, serum levels were significantly lower (p = 0.001) in the patients than those in the controls. The results of the present study are comparable to the precious data [22, 32, 46]. It has been reported that approximately 30–60 % of the patients with RA are anemic. Iron deficiency anemia (IDA) is one of the main factors in patients with RA. While anemia of inflammation (AI) is the major cause of lower Fe in RA, this anemia usually shows no improvement on iron therapy because of IL-6-mediated suppression of the bone marrow [47].
Another observation noted in this study was a statistically negative correlation between serum Zn and Cu levels (p value = 0.000 and r 2 = 0.716) in the patient group. Although significant alterations in serum levels of Zn, Cu, and Fe in association with RA had been documented in previous studies, no study to date has reported a significant correlation within the RA group between serum Zn and Cu levels. This change might be due to the relatively greater decreased in the levels of serum Zn while increased in the levels of serum Cu in the RA patients, because of the active disease. Powanda and Biesel (1982) are of the view that specific alterations in trace element metabolism are part of the acute phase reaction and usually are seen as reductions in the serum levels of the Zn and elevations in serum Cu levels. Nutritional history of the patients can also be a factor in this connection [48].
Conclusion
In this study, serum trace elements including Zn, Cu, and Fe are significantly altered in the patients with rheumatoid arthritis as compared to those in the healthy controls. Correlation was not observed among these trace elements in the RA patients while negative correlation was noted in Zn and Cu levels. These results present direction to clinicians and other professional exploring deficiency of essential trace metals in biological samples of RA patients which may be helpful for diagnosis and supplementary management of rheumatoid arthritis.
References
Doan T, Massarotti E (2005) Rheumatoid arthritis: an overview of new and emerging therapies. J Clin Pharmacol 45:751–762
Schett G (2006) Rheumatoid arthritis: inflammation and bone loss. Wien Med Wochenschr 156(1–2):34–41
Neumann E, Lefèvre S, Zimmermann B, Gay S, Müller-Ladner U (2010) Rheumatoid arthritis progression mediated by activated synovial fibroblasts. Trends Mol Med 16:458–468
Lipsky PE (2007) Why does rheumatoid arthritis involve the joints? N Engl J Med 356:2419–2420
Akhter E, Bilal S, Kiani A, Haque U (2011) Prevalence of arthritis in India and Pakistan: a review. Rheumatol Int 31(7):849–855
Khurana R, Berney SM (2005) Clinical aspects of rheumatoid arthritis. Pathophysiology 12:153–165
Silman AJ, Hochberg MC (2001) Epidemiology of the rheumatic diseases, 2nd edn. Oxford University Press, Oxford
Turk SA, van Beers-Tas MH, van Schaardenburg D (2014) Prediction of future rheumatoid arthritis. Rheum Dis Clin North Am 40(4):753–770
Chang K, Yang SM, Kim SH, Han KH, Park SJ, Shin JI (2014) Smoking and rheumatoid arthritis. Int J Mol Sci 15(12):22279–22295
Yazar M, Sarban S, Kocyigit A, Isikan UE (2005) Synovial fluid and plasma selenium, copper, zinc, and iron concentrations in patients with rheumatoid arthritis and osteo arthritis. Biol Trace Elem Res 106(2):123–132
Mertz W (1981) The essential trace elements. Science 213(4514):1332–1338
Overbeck S, Rink L, Haase H (2008) Modulating the immune response by oral zinc supplementation: a single approach for multiple diseases. Arch Immunol Ther Exp (Warsz) 56:15–30
Prasad AS (2008) Zinc in human health: effect of zinc on immune cells. Mol Med 14:353–357
Choy EH, Panayi GS (2001) Cytokine pathways and joint inflammation in rheumatoid arthritis. N Engl J Med 344:907–916
Strecker D, Mierzecki A, Radomska K (2013) Copper levels in patients with rheumatoid arthritis. Ann Agric Environ Med 20(2):312–316
Kagan HM, Li W (2003) Lysyl oxidase: properties, specifity, and biological roles inside and outside of the cell. J Cell Biochem 88(4):660–672
Schümann K, Classen HG, Dieter HH, König J, Multhaup G, Rükgauer M, Summer KH, Bernhardt J, Biesalski HK (2002) Hohenheim consensus workshop: copper. Eur J Clin Nutr 56:469–483
Zago MP, Oteiza PI (2001) The antioxidant properties of zinc: interactions with iron and antioxidants. Free Radic Biol Med 31:266–274
Chen H, Huang G, Su T, Gao H, Attieh ZK, McKie AT, Anderson GJ, Vulpe CD (2006) Decreased hephaestin activity in the intestine of copper-deficient mice causes systemic iron deficiency. J Nutr 136:1236–1241
Swerlick RA, Korman NJ (2004) UVA and NF-B activity: ironing out the details. J Invest Dermatol 122:xi–xi
Afridi HI, Kazi TG, Kazi N, Talpur FN, Shah F, Naeemullah FS, Arain SS, Brahman KD (2013) Evaluation of status of arsenic, cadmium, lead and zinc levels in biological samples of normal and arthritis patients of age groups (46–60) and (61–75) years. Clin Lab 59(1–2):143–153
Afridi HI, Kazi TG, Kazi N, Shah F (2012) Evaluation of status of zinc, copper, and iron levels in biological samples of normal and arthritis patients in age groups 46–60 and 61–75 years. Clin Lab 58(7–8):705–717
Afridi HI, Kazi TG, Brabazon D, Naher S (2012) Interaction between zinc, cadmium, and lead in scalp hair samples of Pakistani and Irish smokers rheumatoid arthritis subjects in relation to controls. Biol Trace Elem Res 148(2):139–147
Nisa FU, Mumtaz A, Ullah MI, Atif M, Sami W (2014) Determination of serum zinc and magnesium levels in patients with hypothyroidism. Trace Elem Electroly 31:43–47
Doran MF, Pond GR, Crowson CS, O'Fallon WM, Gabriel SE (2002) Trends in incidence and mortality in rheumatoid arthritis in Rochester, Minnesota, over a forty-year period. Arthritis Rheum 46(3):625–631
Charles J, Britt H, Pan Y (2013) Rheumatoid arthritis. Aust Fam Physician 42(11):765
Cooper GS, Bynum ML, Somers EC (2009) Recent insights in the epidemiology of autoimmune diseases: improved prevalence estimates and understanding of clustering of diseases. J Autoimmun 33:197–207
Visser H, le Cessie S, Vos K, Breedveld FC, Hazes JM (2002) How to diagnose rheumatoid arthritis early: a prediction model for persistent (erosive) arthritis. Arthritis Rheum 46(2):357–365
Harrison MJ, Farragher TM, Clarke AM, Manning SC, Bunn DK, Symmons DP (2009) Association of functional outcome with both personal and area-level socioeconomic inequalities in patients with inflammatory polyarthritis. Arthritis Rheum 61:1297–1304
Camacho EM, Verstappen SM, Symmons DP (2012) Association between socioeconomic status, learned helplessness, and disease outcome in patients with inflammatory polyarthritis. Arthritis Care Res 64(8):1225–1232
Brewer GJ (2003) Copper in medicine. Curr Opin Chem Biol 7:207–212
Mayadah H, AL-Jamma S, Saad M, Alhasani, Mowafak K, Hassan (2010) Serum concentration of zinc copper, manganese and iron in rheumatoid arthritis. Raf J Sci 21(2):19–28
Söderlin MK, Petersson IF, Geborek P (2012) The effect of smoking on response and drugsurvival in rheumatoid arthritis patients treated with their first anti-TNF drug. Scand J Rheumatol 41(1):1–9
Önal S, Nazıroğlu M, Çolak M, Bulut V, Flores-Arce MF (2011) Effects of different medical treatments on serum copper, selenium and zinc levels in patients with rheumatoid arthritis. Biol Trace Elem Res 142:447–455
Sahebari M, Ayati R, Mirzaei H, Sahebkar A, Hejazi S, Saghafi M, Saadati N, Ferns GA, Ghayour-Mobarhan M. (2015) Serum trace element concentrations in rheumatoid arthritis. Biol Trace Elem Res
Mazzetti I, Grigolo B, Borzì RM, Meliconi R, Facchini A (1996) Serum copper and zinc superoxide dismutase levels in patients with rheumatoid arthritis. Int J Clin Lab Res 26:245
Taneja SK, Mandal R (2009) Assessment of mineral status (Zn, Cu, Mg and Mn) in rheumatoid arthritis patients in Chandigarh, India. Rheumatol Rep 1(1):e5
Louro MO, Cocho JA, Mera A, Tutor JC (2000) Immunochemical and enzymatic study of ceruloplasmin in rheumatoid arthritis. J Trace Elem Med Biol 14(3):174–178
Colak M, Bingol NK, Ayhan O, Avci S, Bulut V (2001) Serum copper, zinc and selenium levels in rheumatoid arthritis. Romatizma Cilt 16(2):66–71
Farid YZ, Mohammed AK, Mohammed IS (2005) Serum copper and zinc levels and copper/zinc ratio in patients with rheumatoid arthritis. Iraqi J Med Sci 4(1):49–56
Ala S, Shokrzadeh M, Pur Shoja AM, Saeedi Saravi SS (2009) Zinc and copper plasma concentrations in rheumatoid arthritis patients from a selected population in Iran. Pak J Biol Sci 12(14):1041–1044
Mierzecki A, Strecker D, Radomska K (2011) A pilot study on zinc levels in patients with rheumatoid arthritis. Biol Trace Elem Res 143:854–862
Zoli A, Altomonte L, Caricchio R, Galossi A, Mirone L, Ruffini MP, Magaró M (1998) Serum zinc and copper in active rheumatoid arthritis: correlation with interleukin 1 beta and tumor necrosis factor alpha. Clin Rheumatol 17(5):378–382
Liuzzi JP, Lichten LA, Rivera S, Blanchard RK, Aydemir TB, Knutson MD, Ganz T, Cousins RJ (2005) Interleukin-6 regulates the zinc transporter Zip14 in liver and contributes to the hypozincemia of the acute-phase response. Proc Natl Acad Sci U S A 02:6843–6848
Kim S, Watanabe K, Shirahata T, Watarai M (2004) Zinc uptake system (znuA locus) of Brucellaabortus is essential for intracellular survival and virulence in mice. J Vet Med Sci 66:1059–1063
Huda MA, Al-Zubaidi MA (2012) Evaluation of trace elements in iraqi patients with rheumatoid arthritis by using atomic absorption spectrophotometer. Iraqi J Pharm Sci 21(2):77–84
Nikolaisen C, Figenschau Y, Nossent JC (2008) Anaemia in early RA is associated with interleukin 6-mediated bone marrow suppression, but has no effect on disease course or mortality. J Rheumatol 35(3):380–386
Powanda MC, Biesel WR (1982) Hypothesis: leukocyte endogenous mediator/endogenous pyrogen/lymphocyte-activating factor modulates the development of nonspecific and specific immunity and affects nutritional status. Am J Clin Nutr 35:23–29
Acknowledgments
We are grateful to University of Health Sciences, Lahore, Pakistan, for facilitating this study. We also acknowledge the patients and volunteers who participated in this research.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Compliance of Ethical Standards
The study was approved by the ethical review committee of the University of Health Sciences, Lahore, Pakistan.
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Ullah, Z., Ullah, M.I., Hussain, S. et al. Determination of Serum Trace Elements (Zn, Cu, and Fe) in Pakistani Patients with Rheumatoid Arthritis. Biol Trace Elem Res 175, 10–16 (2017). https://doi.org/10.1007/s12011-016-0746-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-016-0746-8