Abstract
Silicon is essential for bone formation. A low-silicon diet leads to bone defects, and numerous animal models have demonstrated that silicon supplementation increases bone mineral density (BMD) and reduces bone fragility. However, the exact mechanism of this action has not been characterized. In this study, we aimed to determine the role of biological silicon in the induction of osteoblast differentiation and the possible underlying mechanism. We examined whether orthosilicic acid promotes collagen type 1 (COL-1) and osteocalcin synthesis through the bone morphogenetic protein-2 (BMP-2)/Smad1/5/runt-related transcription factor 2 (RUNX2) signaling pathway by investigating its effect in vitro at several concentrations on COL-1 and osteocalcin synthesis in human osteosarcoma cell lines (MG-63 and U2-OS). The expression of relevant proteins was detected by Western blotting following exposure to noggin, an inhibitor of BMP-2. In MG-63 cells, immunofluorescence methods were applied to detect changes in the expression of BMP-2, phosphorylated Smad1/5 (P-Smad1/5), and RUNX2. Furthermore, rat bone mesenchymal stem cells (BMSCs) were used to determine the effect of orthosilicic acid on osteogenic differentiation. Exposure to 10 μM orthosilicic acid markedly increased the expression of BMP-2, P-Smad1/5, RUNX2, COL-1, and osteocalcin in osteosarcoma cell lines. Enhanced ALP activity and the formation of mineralized nodules were also observed under these conditions. Furthermore, preconditioning with noggin inhibited the silicon-induced upregulation of P-Smad1/5, RUNX2, and COL-1 expression. In conclusion, the BMP-2/Smad1/5/RUNX2 signaling pathway participates in the silicon-mediated induction of COL-1 and osteocalcin synthesis, and orthosilicic acid promotes the osteogenic differentiation of rat BMSCs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoporosis is a common metabolic bone disease characterized by low BMD, the deterioration of bone microarchitecture, and increased fracture risk. Osteoporosis is most prevalent in postmenopausal women. Recently, it was estimated that the cost of all osteoporotic fractures amounted to approximately $20 billion in the USA and $30 billion in the European Union [1], and these costs continue to rise.
Silicon is an element that is increasingly appreciated for its potential important roles in improving bone matrix quality and facilitating bone mineralization [2, 3]. In addition, silicon increases BMD and improves bone strength [4–6]. Silicon is abundant in nature, but it can only be absorbed by humans and animals in the form of orthosilicic acid [Si(OH)4]. As the soluble and absorbable form of silica, orthosilicic acid is present in plasma at concentrations ranging from 5 to 20 μM. Silicon is derived mainly from plant-based foods such as cereal grains, and products made from these are also high in silicon, e.g., beer [7, 8].
In animal experiments, BMD, mineral content, and collagen synthesis are significantly decreased in rats with a low-silicon diet [9–11]. Conversely, physiological doses of silicon can promote calcium deposition in bone tissue. Carlisle concluded that silicon associates with calcium during the early stage of bone calcification [12]. In 2008, Spector et al. found that the combination of choline-stabilized orthosilicic acid (ch-OSA) and Ca/Vit D3 has a beneficial effect on bone collagen compared with Ca/Vit D3 alone, thereby suggesting a potential treatment for osteoporosis [13]. Calomme et al. showed that ch-OSA supplementation ameliorates femoral bone loss in the aged ovariectomized (OVX) rat model [14], whereas a study of the effect of sodium silicate on the growth and bone strength of broiler chickens revealed that increased silicon intake can increase BMD and mechanical strength [4]. Researchers reported that supplemental silicon increased plasma and milk silicon concentrations as well as BMD in the third metacarpal in horses [15, 16], implying that silicon has beneficial effects on bone growth and BMD.
Studies have shown that intramuscular injections of silicon in the form of monomethyl trisilanol in postmenopausal women with osteoporosis resulted in significant improvements in femoral BMD and trabecular bone volume compared with a control group [17, 18]. Recent research has confirmed that orthosilicic acid stimulates collagen type 1 (COL-1) synthesis in human osteoblast-like cells at physiological concentrations and enhances osteoblastic differentiation [19].
Runt-related transcription factor 2 (RUNX2) is a transcription factor belonging to the Runx family, which contains three members: RUNX1, RUNX2, and RUNX3. Genetic analysis has revealed that RUNX2, a major target of the bone morphogenetic protein (BMP) pathway, is degraded through a Smurf-mediated ubiquitination pathway. RUNX2 plays an important role in the regulation of bone metabolism and patterning. It has been demonstrated that RUNX2 is the earliest and the most specific marker gene involved in the process of bone formation and is also a key regulator of chondroblast and osteoblast differentiation [20–22].
BMPs are members of the transforming growth factor-β (TGF-β) superfamily. They activate Smad 1/5, and the BMP/Smad signaling pathways play important roles in regulating stem cell differentiation, renewal, apoptosis, migration, proliferation, and the control of embryonic development and postnatal tissue homeostasis. Human and mouse genetic studies have demonstrated that BMPs are of vital importance during osteoblast proliferation, differentiation, and bone formation [23, 24] and are also closely related to tumorigenesis [25]. A deficiency in the BMP/Smad signaling pathways can result in bone-related diseases such as osteoporosis [23].
Taken together, these results suggest the utility of silicon in the treatment of osteoporosis. However, the participation of the BMP-2/Smad1/5/RUNX2 signaling pathway in the silicon-induced regulation of COL-1 and osteocalcin synthesis has not yet been demonstrated. In this study, we aimed to clarify the role of biological silicon in the induction of osteoblast differentiation as well as its mechanism of action.
Materials and Methods
Cell Culture
The osteoblast-like cell lines MG-63 and U2-OS are recognized as models of osteoblasts. They were derived from human osteosarcomas [19, 26–29]. MG-63 and U2-OS cells were grown in DMEM (GIBCO BRL Co. Ltd., USA) supplemented with 10 % fetal bovine serum (FBS, GIBCO BRL Co. Ltd., USA), penicillin (100 IU/ml, Beyotime Biotechnology, Jiangsu, China), and streptomycin (100 μg/ml, Beyotime Biotechnology, Jiangsu, China) in cell culture flasks at 37 °C in a humidified 5 % CO2 incubator. The cells were seeded at a density of 5 × 104 cells/cm2 and grown to confluence.
Rat bone marrow mesenchymal stem cells (BMSCs) were grown in Mesenchymal Stem Cell Medium (MSCM, ScienCell, CA, USA) in cell culture flasks. The cell suspensions were plated on MSCM at a density of 1 × 104 cells/cm2 in 6-well plates with poly-l-lysine and were placed for at least 1 h in a 37 °C incubator. The cells were then incubated at 37 °C in a 5 % CO2 humidified incubator for 1–2 days. When the cells reached 100 % confluence, MSCM was carefully replaced with orthosilicic acid at 0 or 10 μM diluted in Mesenchymal Stem Cell Osteogenic Differentiation Medium (MODM, ScienCell, CA, USA). This medium change was considered differentiation day 1. The medium was replaced with fresh osteogenic differentiation medium every 4–5 days.
All cell lines were purchased from CHI Scientific (Shanghai, China). All plastic ware was obtained from Corning Costar Corp.
Addition and Measurement of Silicon
A 1.0-mM stock solution of orthosilicic acid was prepared from sodium silicate (Sigma-Aldrich Company Ltd., Gillingham, UK) using ultra highly purified water. The Si concentration of the sodium silicate solution was 175 mg/ml. The pH of the 1.0 mM solution was 9.37 ± 0.12. Then, the stock solution was filtered with a strainer (SLGP033RB, EMD Millipore, USA). Aliquots of the sterile stock solution were added to the culture medium at concentrations of 10, 20, and 50 μM. The final concentrations of silicon in the media were measured in duplicate wells by inductively coupled plasma optical emission spectrometry (ICP-OES, Perkin Elmer Inc., USA). At confluence, the two cell lines were washed twice with phosphate-buffered saline (PBS) and changed to serum-free DMEM with either vehicle or soluble Si (10, 20, and 50 μM) [19].
Cell Counting Kit-8 Assays
To evaluate cytotoxicity, the two cell lines were seeded in 96-well cell culture cluster plates (Corning Inc., Corning NY, USA) at a concentration of 1 × 104 cells/well in volumes of 100 μl and grown overnight. The cells were incubated with different concentrations of orthosilicic acid (0, 10, 20, 30, 40, 50, and 60 μM). After 72 h, Cell Counting Kit-8 reagents (BestBio, Shanghai, China) were added to each well and then incubated for another 2 h at 37 °C in the dark. The absorbency was measured at 450 nm using an xMark Microplate Absorbance Spectrophotometer (Bio-Rad, CA, USA). The cell survival rate was then calculated according to the instructions. There were three wells per silicate treatment and the experiment was repeated three times.
Western Blot Assays
Standard Western blotting methods were performed. Briefly, MG-63 and U2-OS cells were cultivated in 6-well plates and stimulated with different concentrations of orthosilicic acid (0, 10, 20, and 50 μM). After 72 h [19], the cells were harvested and pooled in RIPA Lysis Buffer (Beyotime Biotechnology, Jiangsu, China). The cell lysates were centrifuged at 14,000 rpm for 20 min at 4 °C, and the supernatants were collected. Corresponding amounts of cell lysate were separated by SDS polyacrylamide gel electrophoresis (Bio-Rad, CA, USA) after determining protein concentration (BCA Protein Assay Kit, Beyotime Biotechnology, Jiangsu, China). The separated proteins were electrotransferred to PVDF membranes (EMD Millipore, USA) and blocked in 5 % skim milk in TBST; then, the membranes were incubated overnight at 4 °C with primary antibodies against BMP-2 (1:1000), RUNX2 (1:1000), COL-1 (1:1000), and osteocalcin (1:500) (Abcam, UK), respectively. Next, the membranes were incubated with peroxidase-conjugated AffiniPure goat anti-mouse or rabbit IgG (H + L) (1:5000, ZSGB-BIO, Beijing, China) for 1 h at room temperature. The membranes were scanned, and the results were normalized for β-actin (1:1000, ZSGB-BIO, Beijing, China) as a control. The results for P-Smad1/5 (1:1000, Cell Signaling Technology, Boston, MA, USA) were normalized by Smad1/5 (1:1000, Abcam, UK). The signal intensities were recorded and quantified using a ChemiDoc Touch Gel Imaging System and Image Lab Touch Software (Bio-Rad, CA, USA). The experiments were performed on three separate occasions.
Alkaline Phosphatase Assay
MG-63 cells were grown in 6-well plates and incubated with orthosilicic acid (0, 10, 20, and 50 μM) for 7 days. Intracellular alkaline phosphatase (ALP) activity was measured using an ALP assay kit (Jiancheng Bioengineering Institute, Nanjing, China). The cells were washed twice with PBS and were then lysed using 1 % Triton X-100. A total of 30 μl of each cell lysate was added to the reaction buffers in 96-well plates and incubated for 15 min at 37 °C; then, the samples were mixed with 150 μl of the color development reagent. The absorbance was measured at 520 nm using an xMark Microplate Absorbance Spectrophotometer (Bio-Rad, CA, USA). There were three wells per silicate treatment and the experiment was repeated three times.
Immunofluorescence Assay
For immunofluorescence assay, MG-63 cells were grown on coverslips and incubated with orthosilicic acid (0 and 10 μM) for 72 h. The cells were then incubated with primary antibodies against BMP-2 (1:200, Abcam, UK), P-Smad1/5 (1:50, Cell Signaling Technology, Boston, MA, USA), and RUNX2 (1:100, Abcam, UK) overnight at 4 °C. The cells were washed thrice with PBS and then reacted with anti-rabbit IgG conjugated with DyLight 488 (1:200, Abcam, UK) for 1 h at room temperature. After washing with PBS, the cells were imaged via immunofluorescence microscopy (OLYMPUS, Tokyo, Japan). The experiments were performed on three separate occasions.
Induction of Osteogenic Differentiation
Rat BMSCs cells (passages 3–5) were seeded in 6-well culture cluster plate (Corning Inc., Corning NY, USA) at a concentration of 2 × 103 cells/well. After stimulation with different concentrations of orthosilicic acid (0 and 10 μM) for 3 weeks, calcium deposition was evaluated by alizarin red S staining (Genmed Scientifics Inc., USA, Shanghai, China). Take three samples from each group. One microscopic field of each sample was selected at random and imaged via invented microscope (OLYMPUS, Tokyo, Japan). The alizarin red S amount was determined by absorbance measurement at 570 nm using an ELISA reader (Bio-Rad, CA, USA). The differences of the two groups were quantified and compared.
Statistical Analyses
All statistical analyses were performed using SPSS 19.0 software package (SPSS Inc., Chicago, IL, USA). Mean values were calculated and presented with an error bar representing the values ± SD. The data were analyzed by one-way ANOVA with Tukey’s multiple comparison test, and P < 0.05 was considered significant.
Results
Silicon Concentration in the Culture Medium
The baseline silicon concentration in the serum-free DMEM in the plastic containers was 1.43 ± 0.5 μM. Following the addition of 10, 20, 30, 40, 50, and 60 μM orthosilicic acid, the final concentrations measured in triplicate were 10.7 ± 2.1, 19.6 ± 2.2, 30.5 ± 1.7, 39.4 ± 1.9, 50.2 ± 1.8, and 61.1 ± 2.1 μM silicon, respectively. The baseline silicon concentration in the MODM in the plastic containers was 1.78 ± 0.8 μM. Following addition of 10 μM orthosilicic acid, the final concentration measured in triplicate was 12.1 ± 1.4 μM silicon. After incubated at 37 °C in a 5 % CO2 humidified incubator for 72 h, the final concentrations measured in triplicate were as follows. The serum-free DMEM was 1.54 ± 0 μM. Following the addition of 10, 20, 30, 40, 50, and 60 μM orthosilicic acid, measured in triplicate were 13.4 ± 1.8, 23.6 ± 1.7, 34.5 ± 2.4, 43.4 ± 2.2, 55.2 ± 2.6, and 66.1 ± 2.9 μM silicon. The MODM was 1.97 ± 1.1 μM. Following addition of 10 μM orthosilicic acid, measured in triplicate was 15.4 ± 1.7 μM silicon.
The Effect of Soluble Silicon on BMP-2/Smad1/5/RUNX2 Protein Expression in MG-63 and U2-OS Cells
As shown in Fig. 1, the expression of BMP-2, P-Smad1/5, and RUNX2 generally increased in the two cell lines investigated following treatment with orthosilicic acid at 10 μM compared with those in the control group. The expression of BMP-2 (Fig. 1a, f) and P-Smad1/5 (Fig. 1g) increased following orthosilicic acid treatment at 10 or 20 μM compared with the control group (# P < 0.01 vs. control; *P < 0.01 vs. Si20 + Si50). A significant increase in the expression of P-Smad1/5 (Fig. 1b) and RUNX2 (Fig. 1 c, h) protein was observed only for the 10 μM treatment (# P < 0.01 vs. control; *P < 0.01 vs. Si20 + Si50). However, the addition of orthosilicic acid at a supraphysiological concentration (50 μM) did not significantly increase the expression of any of the assessed proteins. The percentage increase following orthosilicic acid treatment refers to Table 1.
The Effect of Soluble Silicon on COL-1 and Osteocalcin Synthesis in MG-63 and U2-OS Cells
As shown in Fig. 1, the protein expression of COL-1 and osteocalcin increased in the two cell lines investigated following treatment with orthosilicic acid at 10 μM compared with the control group (# P < 0.01 vs. control; *P < 0.01 vs. Si20 + Si50). The expression of COL-1 (Fig. 1i) was increased under orthosilicic acid treatment at 10 and 20 μM. Osteocalcin expression (Fig. 1j) was increased following the 10, 20, and 50 μM treatments compared with the control group (# P < 0.01 vs. control; *P < 0.01 vs. Si20 + Si50). The increased osteocalcin expression with higher concentrations of orthosilicic acid was only seen in the U2-OS cells. The percentage increase following orthosilicic acid treatment refers to Table 1.
The Influence of Noggin, a BMP-2 Antagonist, on the Expression of the BMP-2/Smad1/5/RUNX2 Signaling Pathway
As shown in Fig. 2, after 10 μM orthosilicic acid treatment, the expression of P-Smad1/5 in the two cell lines increased significantly compared with that in the control group. We first confirmed that treatment with 500 ng/ml noggin for 2 h could significantly block the silicon-induced upregulation of P-Smad1/5. Similar effects were observed for RUNX2 and COL-1 in the two cell lines. This phenomenon suggests that BMP-2 is located upstream of Smad1/5 in mediating the silicon-induced activation of Smad1/5 (*P < 0.01 vs. control; & P < 0.01 vs. the Si group).
The Effect of Soluble Silicon on Cell Survival
As shown in Fig. 3a, b, after being incubated with different concentrations of orthosilicic acid for 72 h, the cell survival rate did not significantly decline compared to that of the control group, indicating that orthosilicic acid has no toxicity to the cells (*P > 0.05 vs. control; # P > 0.05 vs. control).
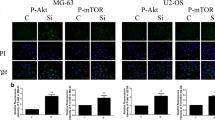
a, b After incubated with different concentrations of orthosilicic acid for 72 h, the cell survival rate did not significantly decline compared to that of the control group (* P > 0.05 vs. control; # P > 0.05 vs. control). c After the MG-63 cells were treated with 10 μM orthosilicic acid for 72 h, the numbers of BMP-2, P-Smad1/5, and RUNX2 fluorescent cells increased compared to those of the control cells
The Effect of Soluble Silicon on the BMP-2/Smad1/5/RUNX2 Signaling Pathway, as Determined by Immunofluorescence
To further investigate whether orthosilicic acid is capable of stimulating COL-1 and osteocalcin synthesis through the aforementioned signaling pathway, BMP-2, P-Smad1/5, and RUNX2 were examined by immunofluorescence. As shown in Fig. 3c, treatment with 10 μM orthosilicic acid resulted in an increase in the number of BMP-2, P-Smad1/5, and RUNX2 fluorescent cells compared with the control cells.
The Effect of Soluble Silicon on ALP Activity
Colorimetric methods showed that silicon promoted ALP expression in MG-63 cells. The activity of ALP in the experimental groups (with the exception of Si50) was markedly higher than that of the control group. Orthosilicic acid at 10 μM had the most significant effect (Fig. 4a; # P < 0.01 vs. control; *P < 0.01 vs. Si20 + Si50). The percentage increase following orthosilicic acid treatment refers to Table 1.
a Exposure to 10 μM orthosilicic acid for 7 days markedly increased ALP activity in MG-63 cells (# P < 0.01 vs. control; *P < 0. 01 vs. Si20 + Si50). b, c Rat BMSCs were incubated in osteogenic differentiation medium for 3 weeks. Alizarin red staining indicates that more calcium deposits were observed throughout the culture (10 μM Si) compared with the control cells (# P < 0.05 vs. control)
The Effect of Soluble Silicon on Osteogenic Differentiation
Rat BMSCs were cultured for 3 weeks with 0 or 10 μM orthosilicic acid, and osteogenic differentiation was then assessed by alizarin red S staining. As shown in Fig. 4b, c, differentiated BMSCs and osteoblasts treated with 10 μM orthosilicic acid formed more bone nodules than the control cells (# P < 0.05 vs. control). The percentage increase following orthosilicic acid treatment refers to Table 1.
Discussion
Nutrition, exercise, and lifestyle, which benefit the management of osteoporosis, greatly affect bone health and its maintenance [30, 31]. In addition, several molecules have been found to aid in the prevention and treatment of osteoporosis, such as calcium and vitamin D. Silicon has recently received attention in this context due to its essential role in bone formation and maintenance. With varying amounts of bioavailability, silicon is available in some nutritional supplements [32]. Choline-stabilized orthosilicic acid is an intermediate absorbable form of silicon while supplemental monomethyl silanetriol is advanced. Generally, the smaller molecules, or monomeric forms, are better absorbed than the larger, highly polymerized, or oligomeric forms [32]. For European and North American populations, the average daily dietary intake of silicon is 20–50 mg [33]. Compared to all other regions of the world, China and India have the lowest prevalence of hip fractures and the highest average daily dietary intakes of silicon, 140–200 mg [34, 35].
Recent studies have shown that supplementation with bioavailable silicon increases BMD in animals and humans [5, 17, 18]. Silicon interacts closely with glycosaminoglycan and plays an important role in the formation of cross-links between collagen and proteoglycans [2, 36, 37]. Because silicon crystal has a piezoresistive effect when subjected to stress, it is commonly used in microscopic pressure transducers. The collagen matrix of immature bone thus has the ability to generate an electrical potential because it exhibits piezoelectric properties. When subjected to strain, compression is generated in electronegative areas in which bone mineralization occurs. These findings suggest a role for silicon in the electrochemical process of mineralization. Hampson reported that the cellular mechanism by which silicon affects COL-1 synthesis may be related to a possible role in the regulation of prolyl hydroxylase activity [19].
The BMP-2/Smad signaling pathway plays an important role in the differentiation of BMSCs into osteoblasts. In addition, the function of this signaling pathway in the process of bone extracellular matrix synthesis and secretion is of vital importance. After BMP recognition by cell surface receptors, intracellular Smad1 and Smad5 are activated. The phosphorylated forms of Smad1/5 then combine with Smad4 to form complexes and connect with nexin DNA sequences, resulting in downstream gene transcription to direct cell differentiation [38].
The experiments here have revealed that the proliferation of the osteosarcoma cells can be significantly inhibited by exogenous noggin proteins in vitro, and the proliferation of the osteosarcoma cells was inhibited in a dose- and time-dependent fashion. The regulation of BMPs is influenced by a variety of cytokines, including noggin, which is the first confirmed BMP antagonist. After combination with BMPs, a ring-like structure is formed that prevents BMPs from combining with other receptors [39, 40]. Noggin inhibits BMP signaling by blocking the binding sites of both types of receptors (type I and type II), mimicking their modes of binding [41].
Although recent research has confirmed that orthosilicic acid stimulates COL-1 synthesis in human osteoblast-like cells at physiological concentrations and enhances osteoblastic differentiation [19], the exact mechanism underlying this process has not been identified. Whether the BMP-2/Smad1/5/RUNX2 signaling pathway participates in the Si-induced regulation of COL-1 and osteocalcin synthesis remains to be demonstrated.
Our results suggested that during COL-1 synthesis in MG-63 and U2-OS cells, silicon increases the expression of BMP-2, RUNX2, and osteocalcin as well as the phosphorylation of Smad1/5. Noggin inhibits the increase in phosphorylated Smad1/5, and a similar effect was observed for RUNX2 and COL-1 in the two cell lines, which suggests that BMP-2 is located upstream of Smad1/5 in mediating the silicon-induced activation of Smad1/5. In our experiments, we also found the optimal concentration of orthosilicic acid is 10 μM. Exposure at this condition of orthosilicic acid, the number of P-Smad1/5, RUNX2, and COL-1 fluorescent positive cells was significantly increased. The enhanced ALP activity and the formation of mineralized nodules indicated that orthosilicic acid in this concentration indeed promote osteogenic expression and differentiation. With silicon supplementation, serum measurements of bone turnover are inconsistent, but markers of bone matrix formation are consistently increased. This may indicate that silicon improves mineralization without affecting the rate of bone formation or bone loss. There may also be an effect on collagen that improved bone strength independent of BMD [13]. The exact mechanism for this action has not been identified. Two experimental studies in rats reported that when excessively high and prolonged levels of dietary silicon were added to the diet, it resulted in a small reduction in bone strength [6, 42]. In our studies, the addition of orthosilicic acid at a supraphysiological concentration (50 μM) did not significantly increase the expression of any of the assessed proteins, which is consistent with the former studies. Excessive silicon may decrease intestinal absorption of calcium and magnesium [42].
In conclusion, we consider that silicon stimulates COL-1 and osteocalcin synthesis in human osteoblast-like cells through the BMP-2/Smad1/5/RUNX2 signaling pathway. Our in vitro experiments suggest that a similar mechanism may exist in vivo. The impact of orthosilicic acid on bone metabolism in humans also needs to be studied further, as it could yield strategies for the treatment of osteogenic bone disorders.
References
Sambrook P, Cooper C (2006) Osteoporosis. Lancet 367:2010–2018
Carlisle EM (1981) Silicon: a requirement in bone formation independent of vitamin D1. Calcif Tissue Int 33:27–34
Sahin K, Onderci M, Sahin N, Balci TA, Gursu MF, Juturu V, Kucuk O (2006) Dietary arginine silicate inositol complex improves bone mineralization in quail. Poult Sci 85:486–492
Merkley JW, Miller ER (1983) The effect of sodium fluoride and sodium silicate on growth and bone strength of broilers. Poult Sci 62:798–804
Bae YJ, Kim JY, Choi MK, Chung YS, Kim MH (2008) Short-term administration of water-soluble silicon improves mineral density of the femur and tibia in ovariectomized rats. Biol Trace Elem Res 124:157–163
Kim MH, Bae YJ, Choi MK, Chung YS (2009) Silicon supplementation improves the bone mineral density of calcium-deficient ovariectomized rats by reducing bone resorption. Biol Trace Elem Res 128:239–247
Casey TR, Bamforth CW (2010) Silicon in beer and brewing. J Sci Food Agric 90:784–788
Tucker KL, Jugdaohsingh R, Powell JJ, Qiao N, Hannan MT, Sripanyakorn S, Cupples LA, Kiel DP (2009) Effects of beer, wine, and liquor intakes on bone mineral density in older men and women. Am J Clin Nutr 89:1188–1196
Seaborn CD, Nielsen FH (1994) Effects of germanium and silicon on bone mineralization. Biol Trace Elem Res 42:151–164
Seaborn CD, Nielsen FH (2002) Silicon deprivation decreases collagen formation in wounds and bone, and ornithine transaminase enzyme activity in liver. Biol Trace Elem Res 89:251–261
Seaborn CD, Nielsen FH (2002) Dietary silicon and arginine affect mineral element composition of rat femur and vertebra. Biol Trace Elem Res 89:239–250
Carlisle EM (1970) Silicon: a possible factor in bone calcification. Science 167:279–280
Spector TD, Calomme MR, Anderson SH, Clement G, Bevan L, Demeester N, Swaminathan R, Jugdaohsingh R, Berghe DA, Powell JJ (2008) Choline-stabilized orthosilicic acid supplementation as an adjunct to calcium/vitamin D3 stimulates markers of bone formation in osteopenic females: a randomized, placebo-controlled trial. BMC Musculoskelet Disord 9:85
Calomme M, Geusens P, Demeester N, Behets GJ, D'Haese P, Sindambiwe JB, Van Hoof V, Vanden Berghe D (2006) Partial prevention of long-term femoral bone loss in aged ovariectomized rats supplemented with choline-stabilized orthosilicic acid. Calcif Tissue Int 78:227–232
Nielsen BDPG, Morris EL, Odom TW, Senor DM, Reynolds JA, Smith WB, Martin MT, Bird EH (1993) Training distance to failure in young racing quarter horses fed sodium zeolite A. J Equine Vet Sci 13:562–567
Lang KJ, Nielsen BD, Waite KL, Hill GM, Orth MW (2001) Supplemental silicon increases plasma and milk silicon concentrations in horses. J Anim Sci 79:2627–2633
Eisinger J, Clairet D (1993) Effects of silicon, fluoride, etidronate and magnesium on bone mineral density: a retrospective study. Magnes Res 6:247–249
Schiano A, Eisinger F, Detolle P, Laponche AM, Brisou B, Eisinger J (1979) Silicon, bone tissue and immunity. Rev Rhum Mal Osteoartic 46:483–486
Reffitt DM, Ogston N, Jugdaohsingh R, Cheung HF, Evans BA, Thompson RP, Powell JJ, Hampson GN (2003) Orthosilicic acid stimulates collagen type 1 synthesis and osteoblastic differentiation in human osteoblast-like cells in vitro. Bone 32:127–135
Jeon EJ, Lee KY, Choi NS, et al. (2006) Bone morphogenetic protein-2 stimulates Runx2 acetylation. J Biol Chem 281:16502–16511
Zhang X, Aubin JE, Inman RD (2003) Molecular and cellular biology of new bone formation: insights into the ankylosis of ankylosing spondylitis. Curr Opin Rheumatol 15:387–393
Zheng H, Guo Z, Ma Q, Jia H, Dang G (2004) Cbfa1/osf2 transduced bone marrow stromal cells facilitate bone formation in vitro and in vivo. Calcif Tissue Int 74:194–203
Chen D, Zhao M, Mundy GR (2004) Bone morphogenetic proteins. Growth Factors 22:233–241
Wang X, Harris RE, Bayston LJ, Ashe HL (2008) Type IV collagens regulate BMP signalling in Drosophila. Nature 455:72–77
Hardwick JC, Kodach LL, Offerhaus GJ, van den Brink GR (2008) Bone morphogenetic protein signalling in colorectal cancer. Nat Rev Cancer 8:806–812
Franceschi RT, Young J (1990) Regulation of alkaline phosphatase by 1,25-dihydroxyvitamin D3 and ascorbic acid in bone-derived cells. J Bone Miner Res 5:1157–1167
Lajeunesse D, Frondoza C, Schoffield B, Sacktor B (1990) Osteocalcin secretion by the human osteosarcoma cell line MG-63. J Bone Miner Res 5:915–922
Orimo H, Goseki-Sone M, Hosoi T, Shimada T (2008) Functional assay of the mutant tissue-nonspecific alkaline phosphatase gene using U2OS osteoblast-like cells. Mol Genet Metab 94:375–381
Ryu B, Li Y, Qian ZJ, Kim MM, Kim SK (2009) Differentiation of human osteosarcoma cells by isolated phlorotannins is subtly linked to COX-2, iNOS, MMPs, and MAPK signaling: implication for chronic articular disease. Chem Biol Interact 179:192–201
Body JJ, Bergmann P, Boonen S, et al. (2011) Non-pharmacological management of osteoporosis: a consensus of the Belgian Bone Club. Osteoporos Int 22:2769–2788
Office of the Surgeon G (2004) Reports of the Surgeon General. Bone Health and Osteoporosis: A Report of the Surgeon General. Office of the Surgeon General (US), Rockville (MD)
Sripanyakorn S, Jugdaohsingh R, Dissayabutr W, Anderson SH, Thompson RP, Powell JJ (2009) The comparative absorption of silicon from different foods and food supplements. Br J Nutr 102:825–834
Jugdaohsingh R (2007) Silicon and bone health. J Nutr Health Aging 11:99–110
Anasuya A, Bapurao S, Paranjape PK (1996) Fluoride and silicon intake in normal and endemic fluorotic areas. J Trace Elem Med Biol 10:149–155
Chen F, Cole P, Wen L, Mi Z, Trapido EJ (1994) Estimates of trace element intakes in Chinese farmers. J Nutr 124:196–201
Carlisle EM (1976) In vivo requirement for silicon in articular cartilage and connective tissue formation in the chick. J Nutr 106:478–484
Schwarz K (1973) A bound form of silicon in glycosaminoglycans and polyuronides. Proc Natl Acad Sci U S A 70:1608–1612
Panchision DM, Pickel JM, Studer L, Lee SH, Turner PA, Hazel TG, McKay RD (2001) Sequential actions of BMP receptors control neural precursor cell production and fate. Genes Dev 15:2094–2110
Krause C, Guzman A, Knaus P (2011) Noggin. Int J Biochem Cell Biol 43:478–481
Smith WC, Harland RM (1992) Expression cloning of noggin, a new dorsalizing factor localized to the Spemann organizer in Xenopus embryos. Cell 70:829–840
Groppe J, Greenwald J, Wiater E, et al. (2003) Structural basis of BMP signaling inhibition by noggin, a novel twelve-membered cystine knot protein. J Bone Joint Surg Am 85-A(Suppl 3):52–58
Kayongo-Male H, Julson JL (2008) Effects of high levels of dietary silicon on bone development of growing rats and turkeys fed semi-purified diets. Biol Trace Elem Res 123:191–201
Acknowledgments
This study was supported by a grant from the Department of Science and Technology of Shandong Province (2014GSF118097).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Dong, M., Jiao, G., Liu, H. et al. Biological Silicon Stimulates Collagen Type 1 and Osteocalcin Synthesis in Human Osteoblast-Like Cells Through the BMP-2/Smad/RUNX2 Signaling Pathway. Biol Trace Elem Res 173, 306–315 (2016). https://doi.org/10.1007/s12011-016-0686-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-016-0686-3