Abstract
It has been well established that core binding factor a-1/osteoblast-specific factor-2 (cbfa1/osf2) is a key regulator of osteoblast differentiation and function, however, it is not known whether it can induce bone formation in vitro and in vivo. To investigate the effect of cbfa1/osf2 on bone formation, we used a recombinant adenoviral vector carrying the mouse cbfa1/osf2 gene to transduce primary cultured bone marrow stromal cells (MSCs) of BALB/c mice. We found that Ad-cbfa1/osf2-transduced MSCs produced cbfa1/osf2 protein and differentiated into osteoblast-like cells. The transduced MSCs had increased alkaline phosphatase activity, increased expression of osteocalcin, osteopontin and bone sialoprotein, and increased matrix mineralization in vitro. To observe the induction of bone formation in vivo, MSCs transduced with Ad-cbfa1/osf2 were transplanted into a 5 mm diameter critical-sized skull defect in BALB/c mice, with type I collagen as scaffolding material. Healing of the defect in treatment and control groups was examined grossly and histologically at four weeks. Skull defects transplanted with Ad-cbfa1/osf2-transduced MSCs had an average of 85% osseous closure at four weeks. Control groups in which the defects were not treated (group 1), treated with collagen only (group 2), or treated with collagen and nontransduced MSCs (group 3) showed little or no osseous healing. These studies indicate that cbfa1/osf2 can induce osteoblast differentiation and bone formation both in vitro and in vivo, suggesting that MSCs transduced with the cbfa1/osf2 gene may be useful in treating bone defects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Currently, there are no completely effective treatments for bone defect problems. Conventional therapies of autogenous bone grafts, allograft implants, and prosthetic implants have been used to treat these problems, and can promote reasonable clinical outcome. However, these methods are limited by supply and osteogenic potential. Recent advances in cell and molecular biology have enabled researchers in the bone tissue engineering field to incorporate cell and gene therapies. This new therapy method offers a solution to bone defect problems, and is under active investigation at this time. Several investigators have shown exciting results using ex vivo and in vivo regional gene therapy in animal models [1]. These studies have introduced the genes for bone morphogenic proteins (BMPs), including BMP-2, BMP-4, and BMP-7 (OP-1) [2, 3, 4]. The BMPs are growth factors that have a powerful capacity to elicit new bone formation [5]. However, the regulation of osteoblast differentiation and bone formation requires the cooperation of many factors [6], including transcription factors [7]. Of these, core binding factor (cbfa1/osf2) has been identified as essential for osteoblast differentiation and bone formation.
Cbfa1/osf2 belongs to the runt-domain gene family, and has a DNA binding domain that is homologous to the Drosophila pair-rule gene runt [3]. This transcription factor is highly restricted to osteoblast cells [9]. Molecular and genetic evidence has demonstrated that it activates osteoblast differentiation during embryonic development in mice and humans [10]. Cbfa1/osf2 has been shown to regulate the expression of genes that characterize the osteoblast phenotype, including osteocalcin, osteopontin, type I collagen, bone sialoprotein, and collagenase-3. This regulation occurs when the cbfa1 DNA binding domain (Δcbfa1) [11] interacts with the related DNA sequence element, which is called osteoblast-specific element 2 (OSE2) [12]. In addition, cbfa1/osf2 can induce osteoblast differentiation of nonosteoblastic cells [13]. Patients heterozygous for mutations or deletions of cbfa1/osf2 develop cleidocranial dysplasia (CCD) [14], an autosomal-dominant condition characterized by abnormal skeletal genesis and arrest of osteoblast development [15]. Likewise, heterozygous inactivation of cbfa1/osf2 in mice leads to a CCD phenotype [16]. These data indicate that cbfa1/osf2 is an essential, indispensable regulator of osteoblast differentiation. Furthermore, the skeletons of Δcbfa1-transgenic mice are normal at birth, but show decreased bone formation 3 weeks after birth, leading to osteopenia [17], suggesting that cbfa1/osf2 plays a crucial role in osteoblast function [18].
Given that cbfa1/osf2 is indispensable for osteoblast differentiation and function, we investigated induction of bone formation by cbfa1/osf2 in vitro and in vivo. We found that transduction of a cbfa1/osf2 construct into bone marrow stromal cells (MSCs) could induce osteoblast-like differentiation, expression of osteoblast proteins, and bone formation. In addition, we showed that transplantation of cbfa1/osf2-expressing MSCs into a critical-sized skull defect in mice could facilitate the healing of bone defects in vivo. These results suggest that cbfa1/osf2 may be a good candidate for bone formation gene therapy.
Materials and Methods
Materials
The pBS KS− plasmid containing full-length mouse cbfa1/osf2 cDNA was generously provided by Professor Gérard Karsenty (Department of Molecular and Human Genetics, Baylor College of Medicine, Huston, TX). The AdEasy System was provided by Dr. Tong-Chuan He (Howard Hughes Medical Institute, Baltimore, MD). Type I collagen was from Sigma Chemical Co (St. Louis, MO). Restriction endonucleases (XhoI, XbaI, PmeI, PacI and BamHI) were obtained from BioLab Scientific Ltd (New Zealand). TRIZOL Reagent and LF2000 Reagent were obtained from Life Technology (Gaithersburg, MD). Histostain™ SP kit was obtained from Zymed Laboratories Inc (South San Francisco, CA). Polyclonal goat anti-mouse cbfa1/osf2 primary antibody was obtained from Santa Cruz Biotechnology, Inc (Santa Cruz, CA). One Step RT-PCR kit was from Qiagen Inc (Stanford, CA). Dulbecco’s modified Eagle medium (DMEM) and fetal serum was from HyClone (Logan, UT).
Construction of Adenovirus Encoding Mouse Cbfa1/osf2 Gene
For construction of adenovirus, the AdEasy system was used. This system is composed of a replication-defective E1 and E3-gene-deleted adenoviral vector (pAdEasy-1), and a shuttle vector (pShuttle-CMV), and has been previously described [19]. Briefly, the pBS KS− plasmid containing full-length mouse cbfa1/osf2 cDNA was digested with XhoI and XbaI, resulting in a 3000 bp fragment containing the mouse cbfa1/osf2 cDNA. The target fragment was inserted into the pShuttle-CMV vector. The resultant plasmid was linearized by PmeI digestion, and subsequently cotransformed into E. coli BJ5183 cells with the adenoviral backbone vector pAdEasy-1. Recombinants were selected with kanamycin, and recombinants were confirmed by restriction digestion with PacI and BamHI. Finally, the recombinant plasmids were linearized by digestion with PacI and transfected into 293T cells with LF2000 Reagent for adenovirus packaging. Recombinant adenoviruses carrying the cbfa1/osf2 gene (called Ad-cbfa1/osf2) were purified by CsCl gradient centrifugation. Viruses were titered by standard limiting dilution methods.
Primary Cultures of Bone Marrow Stromal Cells
Bone marrow cells were obtained from the femurs and tibias of 6 to 8-week-old female BALB/c mice sacrificed by pentobarbital sodium overdose. Femurs and tibias were removed and soft tissues were detached aseptically. Metaphyses were resected from both ends, the diaphyses were flushed with Hank’s balanced salt solution, and bone marrow cells were collected. A suspension of bone marrow cells was obtained by repeated aspiration of the cell preparation through a 20-gauge needle. The cell suspension was centrifuged at 1000g for 5 minutes, resuspended in growth medium (DMEM supplemented with 10% fetal bovine serum, 2% penicillin/streptomycin), aliquotted into 250 ml tissue culture flasks (for transplantation in vivo), 6-well and 24-well culture plates (for examination in vitro), and cultured at 37°C and 5% CO2. Three days later, nonadherent cells were removed by replacing the medium. To confirm that the differentiation of MSCs into osteoblasts was due to the overexpression of cbfa1/osf2, in our experiments, the growth medium was not supplemented with dexamethasone, ascorbic acid, and β-glycerol phosphate because these reagents themselves may induce the differentiation of MSCs into osteoblasts.
Infection of Bone Marrow Stromal Cells with Ad-cbfa1/osf2
Infection of bone marrow stromal cells was performed at 80% confluency. Cells were washed with PBS solution, and then incubated with Ad-cbfa1/osf2 (multiplicity of infection = 60) in serum-free DMEM at 37°C. After 4 h, an equal volume of growth medium was added. For examination in vitro, 24 h later, cells were refered with complete medium and the medium was changed every 3 days. For transplantation in vivo, the cells were allowed to recover for 24 h prior to harvesting.
Alkaline Phosphatase (ALP) Assay
An alkaline phosphatase assay was performed as previously described [20]. Confluent mouse MCSs in 24-well plates were transduced with Ad-cbfa1/osf2, Ad-LacZ or left alone. Cells were further incubated for another 7 days, then the cell layers were washed three times with TBS (50 mM Tris, pH 7.4 and 0.15 M NaCl) and stored at −20°C. For the assay, the cell layer from each well was scraped into 0.5 ml of 50 mM Tris, pH 7.4, and sonicated. ALP activity in the sonicate was measured using p-nitrophenyl phosphate (3 mM final concentration) as the substrate in 0.7 M 2-amino-methyl-1-propanol, pH 10.3, and 6.7 mM MgCl2. Protein in the cell layers was measured by Coomassie blue staining. The enzyme activity was expressed as p-nitrophenol produced in nmol/min/mg protein.
Reverse Transcription-Polymerase Chain Reaction Analysis
Expression of cbfa1, osteopontin, osteocalcin, and bone sialoprotein mRNA in MSCs was analyzed by reverse transcription-polymerase chain reaction (RT-PCR). MSCs in 6-well culture plates were transduced with Ad-cbfa1/osf2, Ad-LacZ or left alone. Seven days after transduction, the cells were washed with PBS solution, and total RNA was isolated with the TRIZOL Reagent. RT-PCR was performed with the One Step RT-PCR kit; reverse transcription and PCR were carried out sequentially in the same tube. According to the manufacturer’s instructions, 2µg total RNA was used per RT-PCR reaction in a total volume of 50 µl. A typical thermal cycler program, including steps for both reverse transcription and PCR, was designed according to the One Step RT-PCR kit protocol. Conditions for PCR were optimized, and a condition suitable for all the genes was used. Each cycle consisted of 94°C for 45 sec, 55°C for 30 sec, and 72°C for 45 sec, and 32 cycles were performed. RT-PCR primers are listed in Table 1, and β-actin was reverse transcribed as an internal control. PT-PCR products were electrophoresed through 1.5% agarose gel, stained with ethidium bromide, and photographed.
Immunohistochemical Examination and Matrix Mineralization Analysis In Vitro
The expression of cbfa1/osf2 protein was confirmed by immunohistochemical staining. MSCs were transduced with Ad-cbfa1/osf2, Ad-LacZ, or left alone. Seven days after transduction, cells in 24-well culture plates were washed with PBS and fixed with ice-cold acetone for 5 minutes. Immunohistochemical staining was performed with the Histostain™ SP kit. Briefly, the MSCs were incubated in 1% hydrogen peroxide to quench endogenous peroxidase. Following incubation in blocking solution, cells were incubated in polyclonal goat anti-mouse cbfa1/osf2 primary antibody at a 1:200 dilution, followed by incubation with a biotinylated rabbit anti-goat secondary antibody and a streptavidin-peroxidase amplification reagent. Immunoreactivity was detected with the 3,3′-diaminobenzidine (DAB) peroxidase substrate. The positive cells were stained brown. The percentage of cbfa1/osf2-positive cells was measured under a microscope at 200 X magnification by counting the number of cbfa1/osf2-positive cells and total number of cells in six random fields from each well (total of six wells).
In order to analyze the mineralization potential, MSCs in 6-well culture plates were transduced with either Ad-cbfa1/osf2 or Ad-LacZ, or left alone. Cells were further incubated for another 14 days in growth medium. The mineralized matrix was stained for calcium by Von Kossa staining. Cells were washed three times with PBS and fixed in ice-cold acetone for 5 minutes. After washing with water three times, cells were soaked in 5% AgNO3 solution for 15 minutes, and then exposed to light for at least 20 minutes. The cells were reduced with 5% sodium hyposulfite for 5 minutes and washed with PBS.
Skull Defect Assay
For the skull defect assay, we used the method described by Lee et al. [21]. Female BALB/c mice (8 mice for each group) were used, as this inbred fine is unable to mount an apparent immune response against each other. The mice were anesthetized with pentobarbital sodium (0.07 g/kg) and placed in a ventral decubitus position on the operating table. After sterilization, the scalp was dissected to the skull and the periosteum was stripped. A 5-mm-diameter full-thickness circular skull defect, which is a nonhealing critical-sized defect, was created at the apex of the skull with a minityp drill, with minimal penetration of the dura. Collagen type I was used as a matrix. The mice were divided into four groups. Group 1 did not receive any treatment with the skull defect. Group 2 received collagen only. Group 3 received collagen mixed with nontransduced MSCs. Group 4 received collagen mixed with Ad-cbfa1/osf2 transduced MSCs. The collagen or collagen-wrapped MSCs were placed into the skull defect (groups 2, 3, 4), and the scalp was dosed with a 4-0 nylon suture. All mice were allowed food and activity ad libitum after the operation. Four weeks after surgery, the mice were sacrificed and the skull specimens were dissected free from soft tissue for digital imaging. The area of the original defect was calculated with a 5-mm circular standard on the digital image, and the area of the closed defect was drawn and calculated with the Image-Jprogram (NIH). The area of the defect filled with new bone divided by the area of the original defect yielded the fraction of skull defect closure. Skull specimens were then fixed in 4% polymerisatum buffered with PBS solution for 24 h.
HE Staining, Alcian Blue Staining, and Von Kossa Staining
The structure of new bone was examined by HE staining. The polymerisatum-fixed skull specimens were decalcified and paraffin-embedded, after which 5 µm sections were cut using a microtome, dewaxed with xylene, and rehydrated through graded alcohols. Then HE staining was performed using the standard methods [22]. Alcian Blue staining was performed to examine if there was cartilage formation in the defect. Paraffin sections were deparaffinized in xylene, hydrated in graduated ethanols, and pretreated with 3% acetic add for 3 min. Sections were then stained with 1% Alcian blue 8GX at pH 2.5 for 30 min, thoroughly rinsed with tap water, and counterstained with HE [23]. To examine the mineralization of bone, the nondecalcified polymerisatum-fixed specimens were embedded in methyl methacrylate and dibutyl phathalate (3:1 v/v). Five micrometer sections were cut using an ultramicrotome (Reichert Jung) and deplasticked with ethylene glycol monoethyl acetate. After rehydration through graded alcohols, the sections were stained with von Kossa staining, as described above, then washed with PBS solution and stained with eosin for viewing.
Results
Gene Expression In MSCs Transduced with pAd-cbfa1/osf2
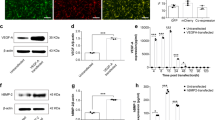
Immunohistochemical staining (Fig. 1) showed the expression of cbfa1/osf2 protein in most Ad-cbfa1/osf2 transduced MSCs, with a positive percentage of 80 ± 7.6%. The Ad-LacZ transduced MSCs and nontransduced MSCs did not show staining. RT-PCR was used to examine the gene expression of cbfa1/osf2, osteocalcin, osteopotin, and bone sialoProtein (Fig. 2). The mRNA expression of Cbfa1/osf2, osteocalcin and bone sialoprotein were detected in Ad-cbfa1/osf2 transduced MSCs, but not in Ad-LacZ transduced MSCs and nontransduced MSCs. Expression of osteopontin mRNA was detect in Ad-cbfa1/osf2 transduced MSCs, Ad-LacZ transduced MSCs and nontransduced MSCs with different levels. Expression of osteopontin in Ad-cbfa1/osf2 transduced MSCs is much higher than that in Ad-LacZ transduced MSCs and nontransduced MSCs. These results suggested that osteoblast differentiation was triggered by transduction of Ad-cbfa1/osf2.
Representative immunohistochemical staining of primary cultured MSCs. Primary cultured MSCs were transduced with Ad-cbfa1/osf2, Ad-LacZ or left alone. Seven days after transduction, expression of cbfa1/osf2 was detected by immunohistochemical staining. No expression of cbfa1/osf2 was detected in Ad-LacZ transduced MSCs (A) and parent MSCs (B). In Ad-cbfa1/osf2 transduced MSCs (C), there was notable expression of cbfa1/osf2. The experiment was repeated three times, each in triplicate (×100).
A. Analysis of expression of mRNA for cbfa1/osf2, osteopontin, osteocalcin, bone sialoprotein by RT-PCR. Four kinds of mRNA were expressed in Ad-cbfa1/osf2 transduced MSCs(c). In Ad-LacZ transduced MSCs(b) and nontransduced MSCs(a), expression of mRNA for osteopontin was detected as well, but the expression is much lower than that in Ad-cbfa1/osf2 transducedMSCs. Expression of mRNA for osteocalcin and bone sialoprotein was detected only in Ad-cbfa1/osf2 transduced MSCs. Experiments were repeated independently three times. B.Detection of alkaline phosphatase (ALP) activity. Primary cultured MSCs in 24-well culture plates were transduced with Ad-cbfa1/osf2, Ad-LacZ or left alone. Seven days after transduction, Ad-cbfa1/osf2 transduced MSCs exhibited higher ALP activity than Ad-LacZ transduced cells and parent cells(P < 0.01 vs both of groups). Values are means ± SD, n = 6. Experiments were repeated three times. C. Representative von Kossa staining of primary cultured MSCs. Primary cultured MSCs were transduced with Ad-cbfa1/osf2, Ad-LacZ or left alone. After additional growth for 2 weeks in growth medium, the cells were stained for mineralization by von Kossa staining. Individual mineralized nodules with irregular shapes were present in Ad-cbfa1/osf2 transduced MSCs (c,d). No detectable mineralized nodules were present in Ad-LacZ transduced MSCs (b) and nontransduced MSCs (a). Experiments were repeated three times (a,b,c ×40; d×200).
ALP Assay and Examination of Mineralization
Since ALP has been implicated as a marker of osteoblast differentiation [24], the ALP activity of transduced and nontransduced MSCs was measured (Fig. 2). We found that the ALP activity of Ad-cbfa1/osf2 transduced MSCs was about 40 nmol/min/mg protein, which was much higher than that of Ad-LacZ transduced MSCs (about 12 nmol/min/mg protein) and nontransduced MSCs (15 nmol/min/mg protein). Next, von Kossa staining was performed to allow visualization of bone mineralization (Fig. 2). We found that the matrix of the MSCs transduced with Ad-cbfa1/osf2 became quite mineralized by 14 days after transduction, whereas the matrix of Ad-LacZ transduced MSCs and nontransduced MSCs was little mineralized at that time. These results suggest that pAd-cbfa1/osf2 can induce the differentiation of MSCs to osteoblasts that are able to mineralize.
Skull Defect Healing Induced by pAd-cbfa1/osf2 Transduced MSCs
We examined the healing of the skull defects by gross image analysis and histological staining. Image analysis (Fig. 3) showed that group 1 (untreated defect) and group 2 (defect treated with collagen alone) had little osseous healing at four weeks. Group 3 (defect treated with collagen and nontransduced MSCs) showed slight healing at four weeks (average 18%). Finally, group 4 (defect treated with collagen and Ad-cbfa1/osf2 transduced MSCs) showed average 85% osseous healing by 4 weeks. In all groups, where no osseous healing had occurred, the detect was closed by soft tissue membrane.
Percent osseous closure of the skull defect in the four groups. Group 1 = untreated defect, Group 2 = defect treated with collagen alone, Group 3 = defect treated with collagen and nontransduced cells, Group 4 = defect treated with collagen and Ad-cbfa1/osf2 transduced cell. Six animals were used for each group. The percent osseous closure of the skull defect in group 4 was much higherthan the control groups (P < 0.05). Values are means ± SD. Experiment were repeated three time.
Histological specimens were stained with HE and Alcian Blue to further visualize healing (Fig. 4). Typical specimens from groups 1 and 2 showed no evidence of osseous healing. Specimens from group 3 showed slight formation of new bone, while typical specimens from group 4 showed nearly complete osseous closure of the defect. Collagen (groups 2, 3, and 4) was completely absorbed by 4 weeks. No cartilage was observed in the skull defects where there was new bone formed (groups 3 and 4). The results of von Kossa staining (Fig. 5), which was performed to identify mineralized bone, were similar to those of the HE staining. There was little or no mineralized bone in the defect in any of the control groups (1, 2, and 3). However, in the experimental group (group 4), most of the defect was closed by mineralized bone.
Representative images of gross specimens (A, B, C, D). A = group 1 (defect not treated), B = group 2 (defect treated with ;collagen alone), C=group 3 (defect treated with collagen and nontransduced cells), D = group 4 (defect treated with collagen and Ad-cbfa1/osf2 transduced cells), respectively. In A, B, and C, the defects (arrows) were closed with soft tissue membrane. Only a small defect was left in D, E, F, G, and H show representative HE staining of coronal sections through the skull defect area of group1, 2, 3, and 4, respectively. Histological evaluation showed no bone formation in group 1 and 2, minimal bone formation (arrow) in group 3 and much new bone formation (arrows) in group 4. I and J show representative Alcian Blue staining of coronal section through the skull defect area of groups 3 and 4, respectively; there was no notable cartilage formation. Six animals were used for each group. Experiments were repeated three times (E, F, G, H, I, J × 20).
(A), (B), (C), and (D) shows representative Von Kossa staining of coronal nondecalcified sections from group 1 (defect not treated), group 2 (defect treated with collagen alone), group 3 (defect treated with collagen and nontransduced cells), and group 4 (defect treated with collagen and Ad-cbfa1/osf2 transduced cells), respectively. Six animals were used for each group. Experiments were repeated three times. (A) and (B) shows no new bone formation. (C) shows minimal newly formed bone (arrow). (D) indicates that robust bone was formed (arrows), which almost closes the defect. (×20).
Discussion
Analyses of many cell lineages such as the myoblast, the adipocyte, or the osteoclast lineage have all shown that cell differentiation is triggered by cell-specific transcription factors acting as gene expression switches [25]. In vitro experiments and animal studies have shown that cbfa1/osf2 is a specific transcriptional activator and a molecular switch of osteoblast differentiation. Recent data suggest that the genetic mechanisms that regulate fetal skeletogenesis also regulate adult skeletal regeneration, pointing to important regulators of osteoblast differentiation and ossification. The regenerative capacity of adult bone may depend upon the re-induction of the molecular pathways that mediate osteogenesis during fetal development [26]. Cbfa1/osf2 gene, essential for bone development, is re-induced during adult bone repair [27] suggesting that cbfa1/osf2 may be an important potential target for gene therapy leading to bone regeneration.
For osteoinductive regional gene therapy, ex vivo (harvesting, manipulation, and reimplantation) techniques offer the most potential target cells. Several specific types of cell have been suggested for this work, including bone marrow stromal cells (MSCs), myoblasts, or skin fibroblasts [28]. MSCs are an attractive option as they can be easily expanded in cell culture, and they can differentiate into osteoblasts, chondroblasts, and fibroblasts [29]. In our study, primary cultured MSCs from BALB/c mice were used. Since this inbred mouse line shows almost no immune response among individuals, there was little chance that the mice would reject the transplanted MSCs. For transaction of the cultured MSCs with cbfa1/osf2, we used the pAdEasy1 vector, a replication-defective, E1 and E3-deleted adenoviral vector containing a CMV promoter. Unlike retroviruses and adeno-associated viruses, adenoviruses do not integrate into the target cell’s genome. Adenoviral vectors are efficient, can be produced in high titers, achieve high levels of expression following transduction, and can transfer genes to both replicating and nonreplicating cells. The duration of expression is generally weeks to months, which is an appropriate timeframe for bone healing. Therefore, we used this system to analyze the effect of cbfa1/osf2 on bone formation in vitro and in vivo.
Previous studies have reported that cbfa1 could induce the expression of principal osteoblast-specific genes encoding for osteocalcin, osteopontin, and bone sialoprotein in non-osteoblastic cells, and cbfa1/osf2 binding sites were found in their promoter regions of these genes [12, 30]. Overexpression of cbfa1/osf2 in nonosteogenic cells such as C3H10T1/2 and skin fibroblasts induced them to express osteoblast-related genes [31]. Furthermore, it has been shown that disruption of cbfa1/osf2 by antisense oligonucleotides in osteoblast cultures inhibited expression of osteoblastic markers and formation of mineralized nodules [32]. In our in vitro studies, transduction with Ad-cbfa1/osf2 induced the MSCs to express osteoblastic proteins (ALP, osteocalcin, osteopontin, and bone sialoprotein), and to increase the formation of mineralized nodules in cultures.
For our in vivo experiments, MSCs transduced with the cbfa1/osf2 gene were transplanted into murine skull defects. Although parent MSCs have been shown to maintain osteogenic potential after transplantation in vivo, with extended culture they display a tendency to lose their multipotentiality, proliferation potential, and in vivo bone-forming efficiency [33]. The amount of bone formed in the non-transduced MSC control was not sufficient for bone repair within the 4-week time frame examined. In contrast, 4 weeks after transplantation of Ad-cbfa1/osf2 transduced MSCs, the test group showed an average defect osseous repair of 85%. This indicates that transduced MSC transplants expressing cbfa1/osf2 are capable of bone formation. However, our healing was not at the 95–100% level described by Lee et al. [21] in their report of Ad-BMP-2 transduced muscle-derived cells healing similar skull defects in the same time frame. This may be due to the fact that cbfa1/osf2 is a transcription factor and therefore works within the host cell. In contrast, BMP-2 can be secreted into the extracellular matrix and affect the surrounding cells. However, our results suggest that cbfa1/osf2 is an effective inducer of bone formation that may be useful in healing adult bone defects.
Although MSCs can differentiate into both osteoblasts and chondrocytes, in this study we found no cartilage formation after transplantation of pAd-cbfa1/osf2 transduced MSCs into the skull defect. This might due to the following factors. First, cbfa1/osf2 is an osteoblast-specific transcription factor that induces MSCs to differentiate directly into the osteoblast phenotype without an intermediate chondrocyte step. Second, bone formation of calvarium occurs with intramembranous ossification, suggesting that bone formation within the defect might be affected by local microenvironment. Third, skull has an abundant blood supply with high oxygen tension, whereas low oxygen tension is known to effectively induce cartilage formation [34].
Here we have reported the successful transduction of MSCs with vectors containing cbfa1/osf2, and the analysis of osteoblast differentiation and skull defect healing by these cells. We have shown that cbfa1/osf2 can facilitate bone formation in vitro and in vivo, and suggested that cbfa1/osf2 may be a valuable candidate for bone regeneration gene therapy. However, further studies will be needed to optimize the carriers and target cells, as well as to increase the expression of cbfa1/osf2 before this important transcription factor will be of clinical use in treating nonhealing bone defects, bone fractures, spine fusions, and even osteoporosis.
References
WF Anderson (2001) ArticleTitleExcitement in gene therapy. Hum Gene Ther 12 IssueID12 1483–1484 Occurrence Handle10.1089/10430340152480212 Occurrence Handle1:CAS:528:DC%2BD3MXmt1Skur0%3D Occurrence Handle11506691
DS Musgrave R Pruchnic P Bosch BH Ziran J Whalen J Huard (2002) ArticleTitleHuman skeletal muscle cells in ex vivo gene therapy to deliver bone morphogenetic protein-2. J Bone Joint Surg Br 84 IssueID1 120–127
KN Kishimoto Y Watanabe H Nakamura S Kokubun (2002) ArticleTitleEctopic bone formation by electroporatic transfer of bone morphogenetic protein-4 gene. Bone 31 IssueID2 340–347 Occurrence Handle10.1016/S8756-3282(02)00825-6 Occurrence Handle1:CAS:528:DC%2BD38XlvVWgtbc%3D Occurrence Handle12151088
RT Franceschi D Wang PH Krebsbach RB Rutherford (2000) ArticleTitleGene therapy for bone formation: In vitro and in vivo osteogenic activity of an adenovirus expressing BMP7. J Cell Biochem 78 IssueID3 476–486 Occurrence Handle10.1002/1097-4644(20000901)78:3<476::AID-JCB12>3.0.CO;2-5 Occurrence Handle1:CAS:528:DC%2BD3cXltlShtLg%3D Occurrence Handle10861845
MR Urist (1997) ArticleTitleBone morphogenetic protein: the motecularization of skeletal system development. J Bone Miner Res 12 IssueID3 343–346
A Yamaguchi K Toshihisa S Tatsuo (2000) ArticleTitleRegulation of osteoblast differentiation mediated by bone morphogenetic proteins, hedgehogs, and cbfa1. Endocr Re 21 IssueID4 393–411 Occurrence Handle1:CAS:528:DC%2BD3cXmtlSktLY%3D
G Karsenty (2000) ArticleTitleBone formation and factors affecting this process. Matrix Biol 19 IssueID2 85–89 Occurrence Handle1:CAS:528:DC%2BD3cXkt1aqurY%3D Occurrence Handle10842091
E Ogawa M Maruyama H Kagoshima M Inuzuka J Lu M Satake K Shigesada Y Ito (1993) ArticleTitlePEBP2/PEA2 represents a family of transcription factors homologous to the products of the Drosophila runt gene and the human AML1 gene. Proc Natl Acad Sd USA 90 IssueID14 6859–6863 Occurrence Handle1:CAS:528:DyaK3sXlvFKqtrY%3D
ZS Xiao R Thomas TK Hinson LD Quaries (1998) ArticleTitleGenomic structure and isoform expression of the mouse, rat and human Cbfa1/osf2 transcription factor. Gene 214 IssueID1-2 187–197 Occurrence Handle10.1016/S0378-1119(98)00227-3 Occurrence Handle1:CAS:528:DyaK1cXltlKksLw%3D Occurrence Handle9651525
G Karsenty (2000) ArticleTitleRole of Cbfa1 in osteoblast differentiation and function. Semin Cell Dev Biol 11 IssueID5 343–346 Occurrence Handle10.1006/scdb.2000.0188 Occurrence Handle1:CAS:528:DC%2BD3cXovVaqsrs%3D Occurrence Handle11105898
P Ducy G Karsenty (1999) ArticleTitleA Cbfa1-dependent genetic pathway controls bone formation beyond embryonic development. Genes Dev 13 IssueID8 1025–1036 Occurrence Handle1:CAS:528:DyaK1MXivFGkurc%3D Occurrence Handle10215629
P Ducy R Zhang V Geoffroy AL Ridall G Karsenty (1997) ArticleTitleOsf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell 89 IssueID5 747–754 Occurrence Handle1:CAS:528:DyaK2sXjs1ejsbg%3D Occurrence Handle9182762
C Banerjee LR McCabe JY Choi SW Hiebert JL Stein GS Stein JB Lian (1997) ArticleTitleRunt homology domain proteins in osteoblast differentiation: AML3/CBFA1 is a major component of a bone-specific complex. . 66 IssueID1 1–8 Occurrence Handle10.1006/adnd.1997.0740
B Lee K Thirunavukkarasu L Zhou L Pastore A Baldini J Hecht V Geoffroy P Ducy G Karsenty (1997) ArticleTitleMissense mutations abolishing DNA binding of the osteoblast-specific transcription factor OSF2/CBFA1 in cleidocranial dysplasia. Nat Genet 16 IssueID3 307–410 Occurrence Handle1:CAS:528:DyaK2sXktFKjsL0%3D Occurrence Handle9207800
S Mundlos F Otto C Mundlos JB Mulliken AS Aylsworth S Albright D Lindhout WG Cole W Henn JH Knoll MJ Owen R Mertelsmann BU Zabei BR Olsen (1997) ArticleTitleMutations involving the transcription factor CBFA1 cause cleidocranial dysplasia. Cell 89 IssueID5 773–779 Occurrence Handle1:CAS:528:DyaK2sXjs1ejt74%3D Occurrence Handle9182765
T Komori H Yagi S Nomura A Yamaguchi K Sasaki K Deguchi Y Shimizu RT Bronson YH Gao M Inada M Sato R Okamoto Y Kitamura S Yoshiki T Kishimoto (1997) ArticleTitleTargeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell 89 IssueID5 755–764 Occurrence Handle1:CAS:528:DyaK2sXjs1ejtr4%3D Occurrence Handle9182763
P Ducy M Starbuck M Priemel J Shen G Pinero V Geoffroy M Amling G Karsenty (1999) ArticleTitleA Cbfa1-dependent genetic pathway controls bone formation beyond embryonic development. Genes Dev 13 IssueID8 1025–1036 Occurrence Handle1:CAS:528:DyaK1MXivFGkurc%3D Occurrence Handle10215629
G Karsenty P Ducy M Starbuck M Priemel J Shen V Geoffroy M Amling (1999) ArticleTitleCbfa1 as a regulator of osteoblast differentiation and function. Bone 25 IssueID1 107–108 Occurrence Handle10.1016/S8756-3282(99)00111-8 Occurrence Handle1:STN:280:DyaK1MzkvFyqtw%3D%3D Occurrence Handle10423032
TC He S Zhou LT da Costa J Yu KW Kinzler B Vogelstein (1998) ArticleTitleA simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci USA 95 IssueID5 2509–2514 Occurrence Handle1:CAS:528:DyaK1cXhslehsLw%3D Occurrence Handle9482916
SL Cheng J Lou NM Wright CF Lai LV Avioli KD Riew (2001) ArticleTitle In vitro and in vivo induction of bone formation using a recombinant adenoviral vector carrying the human BMP-2 gene. Calcif Tissue Int 68 IssueID2 87–94 Occurrence Handle1:CAS:528:DC%2BD3MXkvFersr4%3D Occurrence Handle11310352
JY Lee D Musgrave D Pelinkovic K Fukushima J Cummins A Usas P Robbins FH Fu J Huard (2001) ArticleTitleEffect of bone morphogenetic protein-2-expressing muscle-derived cells on healing of critical-sized bone defects in mice. J Bone Joint Surg Am 83-A IssueID7 1032–1039 Occurrence Handle1:STN:280:DC%2BD3MvgtVGrtg%3D%3D Occurrence Handle11451972
JO Taboas RJ Ceremsak (1967) ArticleTitleA rapid hematoxylin and eosin stain. Tech Bull Regist Med Technol 37 IssueID4 119–120 Occurrence Handle1:STN:280:CCiB2MnptFE%3D Occurrence Handle4167192
I Reiter M Tzukerman G Maor (2002) ArticleTitleSpontaneous differentiating primary chondrocytic tissue culture: a model for endochondral oassification. Bone 31 IssueID2 333–339 Occurrence Handle10.1016/S8756-3282(02)00823-2 Occurrence Handle1:CAS:528:DC%2BD38XlvVWgtbg%3D Occurrence Handle12151087
S Spinella-Jaegle S Roman-Roman C Faucheu FW Dunan S Kawai S Gallea V Stiot AM Blanchet B Courtois R Baron G Rawadi (2001) ArticleTitleOpposite effects of bone morphogenetic protein-2 and transforming growth factor-beta1 on osteoblast differentiation. Bone 29 IssueID4 323–330 Occurrence Handle10.1016/S8756-3282(01)00580-4 Occurrence Handle1:CAS:528:DC%2BD3MXnsVSlsLg%3D Occurrence Handle11595614
G Karsenty (1999) ArticleTitleThe genetic transformation of bone biology. Genes Dev 13 IssueID23 3037–3051 Occurrence Handle10.1101/gad.13.23.3037 Occurrence Handle1:CAS:528:DC%2BD3cXhtFOitQ%3D%3D Occurrence Handle10601030
A Vortkamp S Pathi GM Peretti EM Caruso DJ Zaleske CJ Tabin (1998) ArticleTitleRecapitulation of signals regulating embryonic bone formation during postnatal growth and in fracture repair. Mech Dev 71 IssueID1–2 65–76 Occurrence Handle10.1016/S0925-4773(97)00203-7 Occurrence Handle1:CAS:528:DyaK1cXhs1Ggtro%3D Occurrence Handle9507067
C Ferguson E Alpem T Miclau JA Helms (1999) ArticleTitleDoes adult fracture repair recapitulate embryonic skeletal formation?. Mech Dev 87 IssueID1–2 57–66 Occurrence Handle10.1016/S0925-4773(99)00142-2 Occurrence Handle1:CAS:528:DyaK1MXmtVyjtrc%3D Occurrence Handle10495271
DA Oakes JR Lieberman (2000) ArticleTitleOsteoinductive applications of regional gene therapy: ex vivo gene transfer. Clin Orthop 379 IssueIDsupp S101–112 Occurrence Handle11039758
SP Bruder N Jaiswal NS Ricalton JD Mosca KM Kraus S Kadiyala (1998) ArticleTitleMesenchymai stem cells in osteobiology and applied bone regeneration. Clin Orthop 355 IssueIDsupp S247–256 Occurrence Handle9917644
MJ Jimenez M Balbin JM Lopez J Alvarez T Komori C Lopez-Otin (1999) ArticleTitleCollagenase 3 is a target of Cbfa1, a transcription factor of the runt gene family involved in bone formation. Mol Cell Biol 19 IssueID6 4431–4442 Occurrence Handle1:CAS:528:DyaK1MXjt1yis7w%3D Occurrence Handle10330183
H Harada S Tagashira M Fujiwara S Ogawa T Katsumata A Yamaguchi T Komori M Nakatsuka (1999) ArticleTitleCbfa1 isoforms exert functional differences in osteoblast differentiation. J Biol Chem 274 IssueID11 6972–6978 Occurrence Handle1:CAS:528:DyaK1MXhvFWqtL4%3D Occurrence Handle10066751
C Banerjee LR McCabe JY Choi SW Hiebert JL Stein GS Stein JB Lian (1997) ArticleTitleRunt homology domain proteins in osteoblast differentiation: AML3/CBFA1 is a major component of a bone-specific complex. J Cell Biochem 66 IssueID1 1–8 Occurrence Handle10.1002/(SICI)1097-4644(19970701)66:1<1::AID-JCB1>3.0.CO;2-V Occurrence Handle1:CAS:528:DyaK2sXktl2isL0%3D Occurrence Handle9215522
A Banfi A Muraglia B Dozin M Mastrogiacomo R Cancedda R Quarto (2000) ArticleTitleProliferation kinetics and differentiation potential of ex vivo expanded human bone marrow stromal cells: implications for their use in cell therapy. Exp Hernatol 28 IssueID6 707–715 Occurrence Handle10.1016/S0301-472X(00)00160-0 Occurrence Handle1:STN:280:DC%2BD3czpsFeisQ%3D%3D
S Tamura H Kataoka Y Matsui Y Shionoya K Ohno KI Michi K Takahashi A Yamaguchi (2001) ArticleTitleThe effects of transplantation of osteoblastic cells with bone morphogenetic protein (BMP)/carrier complex on bone repair. Bone 29 IssueID2 169–175 Occurrence Handle10.1016/S8756-3282(01)00498-7 Occurrence Handle1:CAS:528:DC%2BD3MXlvFWkurs%3D Occurrence Handle11502479
Acknowledgements
We thank Prof. Karsenty G (Department of Molecular and Human Genetics, Baylor College of Medicine, Huston, TX) for providing cDNA of mouse cbfa1/osf2 and Dr. He TC (Howard Hughes Medical Institute, Baltimore, MD) for providing the AdEasy System.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zheng, H., Guo, Z., Ma, Q. et al. Cbfa1/osf2 Transduced Bone Marrow Stromal Cells Facilitate Bone Formation In Vitro and In Vivo . Calcif Tissue Int 74, 194–203 (2004). https://doi.org/10.1007/s00223-003-0004-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-003-0004-x