Abstract
The aim of this study was to investigate microstructure and ultrastructure alterations in the pallium of immature mice exposed to cadmium. Forty immature mice were randomly divided into control, 1/100 LD50 (1.87 mg/kg, low), 1/50 LD50 (3.74 mg/kg, medium), and 1/25 LD50 (7.48 mg/kg, high) dose groups. After oral cadmium exposure for 40 days, the pallium of mice was obtained for microstructure and ultrastructure studies. The results showed that both microstructure and ultrastructure alterations of the pallium were observed in all treated mice and the most obvious alterations were in the high dose group. Microstructural analysis showed seriously congested capillary in the pia mater of the pallium in the high cadmium group. Meanwhile, vacuolar degenerate or karyopyknosis presented in some neurocytes, capillary quantity, and the number of apoptotic cells increased, some neurocytes became hypertrophy, the pia mater separated from the cortex, and local hemorrhage and accompanied inflammatory cell infiltration were also observed. Ultrastructural analysis showed that rough endoplasmic reticulum was expanded, heterochromatin marginalized, perinuclear space distinctly broadened, swelling and vacuolization mitochondria appeared, synapse was swelling, presynaptic and postsynaptic membranes presented fusion, and most of mitochondrial cristae were ambiguous. The results indicated that cadmium exposure for 40 days induced dose-dependent microstructure and ultrastructure alterations in pallium of immature mice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cadmium, a ubiquitous heavy metal, has been known as an industrial and environmental toxicant. Since cadmium is not degraded in the environment [1], natural and anthropogenic sources of cadmium may lead to the contamination of soils and water and to the increased uptake of this metal by crops and vegetables grown for human consumption [2]. Once absorbed, cadmium is rapidly transported by blood to different organs in the body, accumulated in various organs, and causes numerous undesirable effects on animals and people [3]. So it has been widely reported as a risk factor for animal and human health upon exposure. Today, besides toxicity and mutagenicity, teratogenicity, and carcinogenicity of many organs, cadmium is also considered as a neurotoxin. It is well known that pallium is not only the most important part of the brain but also the material basis of high nervous activity. It plays a key role in memory, attention, perception, awareness, thought, language, and consciousness. Pallium neural cells have been identified as targets of cadmium-mediated toxicity [4, 5]. In cortical neuron cell culture from fetal rats, cadmium modified the neuronal morphology after a 6-h treatment with 10 μM of cadmium, whereas at 24 h, it showed a great loss of neuronal integrity mainly evidenced by the almost complete disappearance of the axons [6]. Apoptotic morphological changes induced by cadmium in pallium neurons were assessed in some studies [5, 7]. However, histopathology alterations of pallium exposed to cadmium for long term were unclear.
In recent years, the neurotoxic mechanisms through which cadmium elicits its neurotoxic effects have been revealed in some papers [8–10]. However, it is still unresolved [8], especially in immature population. Normally, cadmium barely reaches the brain in adults due to the presence of blood–brain barrier. Nonetheless, this structure is not fully developed in young animals [11]. Due to differences in the blood–brain barrier integrity, cadmium is more toxic to young rats than to adult rats [8]. It has been reported that cadmium can induce its toxic effects on developing cortical cells and immature hippocampal slices in vitro [12, 13] and developing brain in vivo [14, 15]. It is well known that immature period is an important period characterized by rapid development of physical growth and the nervous system. However, very little attention has been paid to the susceptibility to cadmium during this period. The aim of the present study was to evaluate cadmium-induced neurotoxicity of immature mice brain by investigating microstructure and ultrastructure alterations in pallium.
Material and Methods
Ethics Statement, Animals and Housing
All animal experiments were conducted in accordance with Measures for Administration of Laboratory Animals of Henan Province, China (Approval No.1992-24). Forty Kunming immature mice (20 female and 20 male mice), weaned on postnatal (20 ± 1) days, were obtained from Centre for Laboratory Animal of Xinxiang Medical University, China. The mice were housed in plastic cages and maintained under conventional conditions with 12:12 h light/dark cycle and a controlled temperature of 22 ± 2 °C. During the experiment period, free access to standard commercial rodent diet and water was allowed.
Treatment
After 5-day acclimation, immature mice were weighed and randomly assigned to 4 equal groups of 10 animals each. For the control group, deionized water was given to mice ad libitum in the same manner as the following cadmium exposure groups. For the low-dose, medium-dose, and high-dose cadmium group, cadmium chloride dissolved in deionized water at dose of 1/100 LD50 (1.87 mg/kg body weight, low), 1/50 LD50 (3.74 mg/kg, medium), and 1/25 LD50 (7.48 mg/kg, high) were orally administered in drinking water to the corresponding animals for 40 days, respectively. LD50, lethal dose of cadmium chloride by oral gavage administration to mice, was 187 mg/kg body weight based on our previous study [16].
The oral route is the principal route of cadmium exposure in humans. After oral cadmium exposure for 40 days, 10 immature mice grew into adult mice and they were perfused through the heart using cold normal saline solution for sampling. The intact cerebrum was immediately extracted from the skull, weighed, and preserved in the fixative for the following studies.
Processing for Microstructure Study by Light Microscope
Frontal lobe cortices of the left pallium from three mice per group were excised, fixed with 4 % paraformaldehyde in 0.1 M PBS (pH 7.2) for 24 h, and then routinely processed and stained using hematoxylin-eosin staining method. Briefly, after fixation, the samples were dehydrated in a graded series of ethanol, cleared with xylene, impregnated in molten paraffin, embedded in fresh molten paraffin, and sectioned to 5-μm-thick sections using a microtome (Leica, RM 2235, Germany). Sections were then stained with hematoxylin-eosin and examined with a light microscope (Axiostar plus, Carl Zeiss, Germany).
Processing for Ultrastructure Study by Transmission Electron Microscope
Frontal lobe cortices of the left pallium from other three mice per group were excised, chopped to get pieces of approximately l mm × 1 mm × l mm, and immediately kept in 2.5 % glutaraldehyde solution for 4 h at room temperature. Then samples were washed three times with PBS and then post-fixed in 1 % osmium tetroxide solution for 1.5 h, dehydrated with graded acetone, immersed in propylene oxide, and included in Epon812 at 60 °C. Ultrathin sections (40–60 nm) were made by Leica EM UC6rt ultramicrotome, placed on grids, and doubly stained with 0.1 % uranyl acetate and 3 % lead citrate solution. Subsequently, the grids with thin sections were examined and photographed in a transmission electron microscope (Hitachi H-7500, Japan).
Statistical Analysis
The data of weight parameters were expressed as mean ± standard deviation. Comparison test using Student’s t test in SPSS 10.0 (SPSS Inc., Chicago, IL, USA) was carried out to assess any significant differences. P < 0.05 was considered as being significant statistically.
Results
The Changes of Weight Parameters
Compared with the control mice, the cadmium-exposed mice showed decreased daily activities, sleepy, and slower response to outside stimulation with increasing of exposed duration. No incidence of death was recorded during the experiment period. The weight parameters were shown in Table 1. Final body weight of treated mice declined with increasing of cadmium-exposed dose, and a statistically significant difference was found between the high-dose treated group and control group (P < 0.05). The cerebrum weight and cerebrum to body weight ratio increased with increasing of cadmium dose. However, there was no significant difference in the cerebrum weight when all groups were freely compared to each other (P > 0.05). The difference of cerebrum to body weight ratio was significant between the medium-dose treated group and control group (P < 0.05) and highly significant between the high-dose treated group and control group (P < 0.01).
Microstructure Alterations
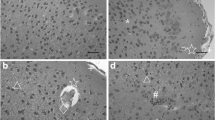
Light microscope examination showed no lesions in the pallium of control mice (Fig. 1a). However, in the low dose group, the pia mater slightly separated from pallium layer, capillary quantity increased, neurocyte displayed slight vacuolar degeneration, and a few granule cells displayed karyopyknosis (Fig. 1b). Microstructure lesions of the pallium in the medium dose group were more serious compared with those in the control group, as a part of the pia mater distinctly separated from pallium, capillary quantity increased, some neurocyte were slightly swelled, more granule cells displayed karyopyknosis, the number of apoptotic cells increased, and local cortex tissue hemorrhage and neurocyte displayed vacuolar degeneration (Fig. 1c, d). The microstructure lesions in the pallium of mice exposed to high-dose cadmium were the most serious. The pallium showed seriously congestive capillary under pia mater, vacuolar degeneration, increasing capillary quantity, karyopyknosis, increasing number of hypertrophy cells and apoptotic cells, pia mater separating from the cortex, and local hemorrhage accompanied with inflammatory cell infiltration (Fig. 1e–g).
Light micrographs of the pallium from each group mice. a Photomicrograph of the pallium of normal mice. The microstructure of pallium layer is intact. b Photomicrograph of the pallium of mice treated with low-dose cadmium. ←: pia mater slightly separated from pallium layer, ↑: capillary quantity increased, →: cells of the slight vacuolar degeneration, ↖: a few granule cells of karyopyknosis. c, d Photomicrograph of pallium of mice treated with medium-dose cadmium. c ←: pia mater distinctly separated from pallium, ↑: capillary quantity increased, ↘: slightly swelling cells, ↖: granule cells of karyopyknosis, ↙: increasing apoptotic cells. d ↗: local hemorrhage, →: vacuolar degeneration cells, ↑: capillary quantity increased, ↘: slightly swelling cells, ↙: increasing apoptotic cells. e, f, g Photomicrograph of the pallium of mice treated with high-dose cadmium. e ↓: seriously congestive capillary under pia mater, →: vacuolar degeneration cells, ↑: capillary quantity increased, ↖: granule cells of karyopyknosis. f ▲: some hypertrophy cells, ↑: capillary quantity increased, ↙: increasing apoptotic cells. g ←: pia mater separated from pallium, ↗: local hemorrhage, △: inflammatory cells infiltration. Magnification, ×400 (a–g). Scale bars (a–g), 200 nm. There was HE staining
Ultrastructure Alterations
The ultrastructure of cerebral cortical neurons and synapse of normal mice were separately shown in Fig. 2a, b. Electron microscope examination showed that ultrastructure alterations of the pallium were all present in each treated group. The ultrastructure alterations of cerebral cortical neurons from mice treated with low-dose cadmium were characterized by swelling and broadened rough endoplasmic reticulum, ambiguous mitochondrial cristae and vacuolization mitochondria (Fig. 2c). However, alterations of synapse structure were not found (not shown). The ultrastructure lesions in the pallium of mice exposed to medium-dose cadmium were more serious compared with control. The rough endoplasmic reticulum was swelling, syncretic nuclear membrane of neuron appeared, some mitochondrial cristae were ambiguous, and some mitochondria were swelling and vacuolar (Fig. 2d). Furthermore, synapse vesicles were fracted and syncretic and reduced in vesicle numbers (Fig. 2e). In high-dose cadmium group, ultrastructure lesions of the pallium were the most serious compared with control. The ultrastructure revealed expanded rough endoplasmic reticulum, marginalized heterochromatin, distinctly broadened perinuclear space, swelling and vacuolization mitochondria, swelling synapse, membrane fusion between presynaptic and postsynaptic membrane, and most of ambiguous mitochondrial cristae (Fig. 2f, g). However, no ultrastructure lesions of blood–brain barrier were found in all treated groups (not shown).
Electron micrographs of the pallium from each group mice. a Electron micrographs of cerebral cortical neurons of normal mice. b Electron micrographs of synapse of normal mice. c Electron micrographs of cerebral cortical neurons of mice treated with low-dose cadmium. +: swelling and broadened rough endoplasmic reticulum, ↗: ambiguous mitochondrial cristae, *: vacuolization mitochondria. d, e Electron micrographs of cerebral cortical neurons of mice treated with medium-dose cadmium. d +: swelling rough endoplasmic reticulum, ↓: syncretic nuclear membrane of neuron, ↗: ambiguous mitochondrial cristae, ↑: swelling mitochondria, *: vacuolization mitochondria. e ◇: fracted and syncretic synapse vesicle and reduction in vesicle numbers. f, g Electron micrographs of cerebral cortical neurons of mice treated with high-dose cadmium. f +: expanded rough endoplasmic reticulum, ←: marginalized heterochromatin, →: distinctly broadened perinuclear space, ↑: swelling mitochondria, *: vacuolization mitochondria. g ↔: swelling synapse, —: membrane fusion between presynaptic and postsynaptic membrane, ↑: swelling mitochondria, *: increasing vacuolization mitochondria, ↗: most of ambiguous mitochondrial cristae. Magnification, ×12,000 (a, c, d, f); ×25,000 (b, e, g). Scale bars (a, c, d, f), 833 nm; b, e, g, 400 nm. There were uranyl acetate and lead citrate staining
Discussion
Most of body tissues are susceptible to cadmium-induced toxicity, including the brain [17]. Various studies reported that cadmium can enter into the brain and neurons across the blood–brain barrier [14, 18], produce neurological changes in humans [19] and adult mice [20], and lead to lower attention, olfactory dysfunction, and memory deficits [21]. In the present study, oral exposure to cadmium caused some behavioral aberrations such as decreased activities, sleepy, and slower response compared with the control, especially in high-dose cadmium group. The cerebrum weight and cerebrum to body weight ratio increased with increasing of cadmium-exposed dose. Moreover, respectively compared with the control, the difference of cerebrum to body weight ratio was significant in medium-dose cadmium group (P < 0.05) and highly significant in high-dose cadmium group (P < 0.01). The data revealed that cadmium exposure can influence weight parameters of the brain.
It has been reported that cadmium exposure in critical developmental periods, at doses much lower than those which affect adults, can cause damage to the brain [21]. The cadmium distribution in the brain was found to decrease with age [22]. Although the entrance of cadmium in the adult central nervous system is limited, developmental neurotoxicity has been evidenced as a result of the blood–brain barrier (BBB) immaturity [23]. The neurotoxic effects of cadmium have been indicated to be different in adult and immature rats, and cadmium was more toxic to 4-day-old than adult rats [24]. In addition, cadmium is a specific metal because it produces toxicity even at low dose and has a long biological half-life and low excretion rate in human (15–30 years) [2, 25]. Therefore, cadmium exposure even at low doses can also cause lesions to the brain as well as other tissues in immature period. In the present study, 40-day exposure duration is almost equivalent to a whole growth duration of immature mice and is long enough for the metal accumulation in a variety of tissues including the brain. The microscope examination indicated that cadmium could induce microstructure and ultrastructure alterations in the pallium of immature mice exposed to cadmium for 40 days in a dose-dependent manner. The microstructures and ultrastructures affected by cadmium included pia mater, blood capillary, neurocytes, mitochondria, rough endoplasmic reticulum, nuclear membrane, and synapse of ultrastructure. However, in our previous study, histopathology changes of the pallium were found at medium and high doses of cadmium exposed to juvenile mice for 10 days [26]. These alterations not only illustrated that cadmium could enter the brain but also indicated that long-term cadmium exposure could lead to microstructure and ultrastructure alterations in the pallium of immature mice even at low doses. The microstructure and ultrastructure alterations in the pallium of immature mice may be an important neurotoxic mechanism induced by cadmium and might impair physiological functions of the brain.
The blood–brain barrier is a protective barrier which prevents many harmful substances from entering the brain, protecting the brain from an assortment of potential risks. High cadmium levels are known to impair the function of blood–brain barrier [27]. In another study, when rats of 21 days of age were exposed to cadmium in drinking water for 30 days, cadmium did not affect the blood–brain barrier permeability, whereas 90-day exposure increased its permeability [28]. In this study, ultrastructure lesions in blood–brain barrier of 40-day cadmium exposure mice were not found in all groups, which indicated that cadmium at the used doses did not disrupt the ultrastructure of blood–brain barrier. It is regrettable that the blood–brain barrier permeability was not investigated.
Increasing evidences indicated that multifactorial mechanisms might be involved in cadmium neurotoxicity. It was suggested that the effect of cadmium neurotransmitter, oxidative damage, interaction with other metals such as cobalt and zinc, estrogen-like effect, and epigenetic modification may be the underlying mechanisms [8]. Cadmium was reported to penetrate and accumulate in the brain of developing and adult rats, which led to intracellular accumulation, cellular dysfunction, and cerebral edema [29]. Cadmium-induced brain dysfunction may be related to the disruption of metal ion homeostasis, reduction of the total brain antioxidant status, inhibition of oxidative DNA repair systems, alteration in signal transduction, and stimulation in the production of ROS, which may act as signaling molecules in the induction of gene expression and apoptosis [9]. Biochemical mechanisms were reported by disturbances of the cellular antioxidant system, generation of reactive oxygen and nitrogen species, changes in energy production in the metabolic pathways, changes in the metabolism of biogenic amines, neurotransmitter amino acids and calcium ions, and inhibition of enzymatic proteins [10]. In the present study, the obtained results suggested that cadmium induced microstructure and ultrastructure alterations in the pallium of immature mice, and the alterations incremented in severity as the exposure dose increased. There is no doubt that alterations induced by cadmium in the pallium will influence physiological functions of the nervous system. In addition, the obtained results also implied cadmium-induced neurotoxicity may be associated with microstructure and ultrastructure alterations of the pallium in immature mice.
Conclusions
In summary, the present study manifested that cadmium could induce dose-dependent microstructure and ultrastructure alterations in the pallium of immature mice exposed for 40 days. For this reason, measures should be taken to reduce cadmium exposure in order to minimize the risk of adverse health effects on immature population.
References
Järup L, Berglund M, Elinder CG, Nordberg G, Vahter M (1998) Health effects of cadmium exposure—a review of the literature and a risk estimate. Scand J Work Environ Health 24:1–51
Abdalla FH, Cardoso AM, Pereira LB, Schmatz R, Gonc alves JF, Stefanello N, Fiorenza AM, Gutierres JM, da Silva Serres JD, Zanini D, Pimentel VC, Vieira JM, Schetinger MRC, Morsch VM, Mazzanti CM (2013) Neuroprotective effect of quercetin in ectoenzymes and acetylcholinesterase activities in cerebral cortex synaptosomes of cadmium-exposed rats. Mol Cell Biochem 381:1–8. doi:10.1007/s11010-013-1659-x
Du J, Cheng SY, Hou WX, Shi BM, Shan AS (2013) Effectiveness of maifanite in reducing the detrimental effects of cadmium on growth performance, cadmium residue, hematological parameters, serum biochemistry, and the activities of antioxidant enzymes in pigs. Biol Trace Elem Res 155:49–55. doi:10.1007/s12011-013-9769-6
Xu B, Chen S, Luo Y, Chen Z, Liu L, Zhou H, ChenW ST, Han X, Chen L, Huang S (2011) Calcium signaling is involved in cadmium-induced neuronal apoptosis via induction of reactive oxygen species and activation of MAPK/mTOR network. PLoS One 6:e19052. doi:10.1371/journal.pone.0019052
Yuan Y, Jiang CY, Xu H, Sun Y, Hu FF, Bian JC, Liu XZ, Gu JH, Liu ZP (2013) Cadmium-induced apoptosis in primary rat cerebral cortical neurons culture is mediated by a calcium signaling pathway. PLoS One 8:e64330. doi:10.1371/journal.pone.0064330
López E, Figueroa S, Oset-Gasque MJ, González MP (2003) Apoptosis and necrosis: two distinct events induced by cadmium in cortical neurons in culture. Br J Pharmacol 138:901–911. doi:10.1038/sj.bjp.0705111
Chen L, Liu L, Huang S (2008) Cadmium activates the mitogen activated protein kinase (MAPK) pathway via induction of reactive oxygen species and inhibition of protein phosphatases 2A and 5. Free Radic Biol Med 45:1035–1044. doi:10.1016/j.freeradbiomed.2008.07.011
Wang B, Du YL (2013) Cadmium and its neurotoxic effects. Oxidative Med Cell Longev 2013:898034. doi:10.1155/2013/898034
Carageorgiou H, Katramadou M (2012) Aspects of cadmium neurotoxicity. In: Li YV, Zhang JH (eds) Metal ion in stroke, 1st edn. Springer, New York, pp. 703–749
Labudda M (2011) Biochemical mechanisms of neurotoxicity caused by cadmium. Rocz Panstw Zakl Hig 62:357–363
Pal R, Nath R, Gill KD (1993) Influence of ethanol on cadmium accumulation and its impact on lipid peroxidation and membrane bound functional enzymes (Na+, K (+)-ATPase and acetylcholinesterase) in various regions of adult rat brain. Neurochem Int 23:451–458. doi:10.1016/0197-0186(93) 90129-s
Ohtani-Kaneko R, Tazawa H, Yokosuka M, Yoshida M, Satoh M, Watanabe C (2008) Suppressive effects of cadmium on neurons and affected proteins in cultured developing cortical cells. Toxicology 253:110–116. doi:10.1016/j.tox.2008.08.021
Rigon AP, Cordova FM, Oliveira CS, Posser T, Costa AP, Silva IG, Santos DA, Rossi FM, Rocha JB, Leal RB (2008) Neurotoxicity of cadmium on immature hippocampus and a neuroprotective role for p38 MAPK. Neurotoxicology 29:727–734. doi:10.1016/j.neuro.2008.04.017
Rai A, Maurya SK, Khare P, Srivastava A, Bandyopadhyay S (2010) Characterization of developmental neurotoxicity of As, Cd and Pb mixture: synergistic action of metal mixture in glial and neuronal functions. Toxicol Sci 118:586–601. doi:10.1093/toxsci/kfq266
Amara S, Douki T, Garrel C, Favier A, Ben Rhouma K, Sakly M, Abdelmelek H (2011) Effects of static magnetic field and cadmium on oxidative stress and DNA damage in rat cortex brain and hippocampus. Toxicol Ind Health 27:99–106. doi:10.1177/0748233710381887
Yang XF, Ge YM, Jiang JQ, Xu ZY, Cui YH, Wang ZL (2012) Acute toxic effect of cadmium chloride in mice. Chin J Vet Sci 32:467–471
Govil N, Chaudhary S, Waseem M, Parvez S (2012) Postnuclear supernatant: an in vitro model for assessing cadmium-induced neurotoxicity. Biol Trace Elem Res 146:402–409. doi:10.1007/s12011-011-9263-y
Nishimura Y, Yamaguchi JY, Kanada A, Horimoto K, Kanemaru K, Satoh M, Oyama Y (2006) Increase in intracellular Cd2+ concentration of rat cerebellar granule neurons incubated with cadmium chloride: cadmium cytotoxicity under external Ca2+-free condition. Toxicol in Vitro 20:211–216. doi:10.1016/j.tiv.2005.06.006
Rose CS, Heywood PG, Costanzo RM (1992) Olfactory impairment after chronic occupational cadmium exposure. J Occup Med 34:600–605
Lukawski K, Nieradko B, Sieklucka-Dziuba M (2005) Effects of cadmium on memory processes in mice exposed to transient cerebral oligemia. Neurotoxicol Teratol 27:575–584. doi:10.1016/j.ntt.2005.05.009
Leal RB, Rieger DK, Peres TV, Lopes MW, Gonçalves CAS (2012) Cadmium neurotoxicity and its role in brain disorders. In: Li YV, Zhang JH (eds) Metal ion in stroke, 1st edn. Springer, New York, pp. 751–766
Choudhuria S, Liu WL, Berman NEJ, Klaassen CD (1996) Cadmium accumulation and metallothionein expression in brain of mice at different stages of development. Toxicol Lett 84:127–133. doi:10.1016/0378-4274(95)03444-7
Abib RT, Peres KC, Barbosa AM, Peres TV, Bernardes A, Zimmermann LM, Quincozes-Santos A, Fiedler HD, Leal RB, Farina M, Gottfried C (2011) Epigallocatechin-3-gallate protects rat brain mitochondria against cadmium-induced damage. Food Chem Toxicol 49:2618–2623. doi:10.1016/j.fct.2011.07.006
Wong KL, Klaassen CD (1982) Neurotoxic effects of cadmium in young rats. Toxicol Appl Pharmacol 63:330–337. doi:10.1016/0041-008X(82)90261-7
Haider S, Anis L, Batool Z, Sajid I, Naqvi F, Khaliq S, Ahmed S (2015) Short term cadmium administration dose dependently elicits immediate biochemical, neurochemical and neurobehavioral dysfunction in male rats. Metab Brain Dis 30:83–92. doi:10.1007/s11011-014-9578-4
Yang XF, Fan GY, Liu DY, Zhang HT, Xu ZY, Ge YM, Wang ZL (2015) Effect of cadmium exposure on the histopathology of cerebral cortex in juvenile mice. Biol Trace Elem Res 165:167–172. doi:10.1007/s12011-015-0246-2
Bar-Sela S, Reingold S, Richter ED (2001) Amyotrophic lateral sclerosis in a battery-factory worker exposed to cadmium. Int J Occup Environ Health 7:109–112. doi:10.1179/107735201800339470
Shukla A, Shukla GS, Srimal RC (1996) Cadmium-induced alterations in blood-brain barrier permeability and its possible correlation with decreased microvessel antioxidant potential in rat. Hum Exp Toxicol 15:400–405. doi:10.1177/096032719601500507
Tobwala S, Wang HJ, Carey JW, Banks WA, Ercal N (2014) Effects of lead and cadmium on brain endothelial cell survival, monolayer permeability, and crucial oxidative stress markers in an in vitro model of the blood-brain barrier. Toxics 2:258–275. doi:10.3390/toxics2020258
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All procedures performed in studies involving animals were in accordance with the ethical standards of the institution at which the studies were conducted.
Funding
This study was supported by the Grant of Aid Project for Leading Young Teachers in Henan Provincial Institutions of Higher Education of China (Grant No.2010GGJS-136) and Science and Technology Research Important Project of Education Department Henan Province (Grant No.13A230142).
Conflict of Interest
The authors declare that they no conflict of interest.
Additional information
X. F. Yang and Q. G. Han contributed equally to this work.
Rights and permissions
About this article
Cite this article
Yang, X.F., Han, Q.G., Liu, D.Y. et al. Microstructure and Ultrastructure Alterations in the Pallium of Immature Mice Exposed to Cadmium. Biol Trace Elem Res 174, 105–111 (2016). https://doi.org/10.1007/s12011-016-0657-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-016-0657-8