Abstract
Cadmium, a heavy metal, is a toxic environmental and industrial pollutant. Exposure to cadmium can lead to the toxic effects in a variety of tissues, also including the brain. The present study investigated the effect of cadmium exposure on the histopathology of cerebral cortex in juvenile mice. Juvenile mice were randomly divided into control, low (1.87 mg/kg), medium (3.74 mg/kg), and high (7.48 mg/kg) dose groups. After cadmium exposure by drinking water for 10 days, the cerebral cortex was obtained for histopathology studies. The medium and high dose of cadmium, rather than low dose, could induce the histopathology alterations of cerebral cortex in a dose-dependent manner. In the high-dose group, microstructure significantly showed pia mater encephali divorcing from cerebral cortex layer, serious hyperemia of blood capillary in pia mater encephali and cerebral cortex, broadening vessel peripheral clearance, a large number of eosinophil leukocyte infiltrating around blood vessel, vacuolar degeneration in part granule cells, and obviously increasing apoptotic cells. Ultrastructure obviously displayed marginalized heterochromatin, incomplete or fused nuclear membranes, broadened perinuclear space, ambiguous mitochondria cristae, decreased synaptic cleft, and fused presynaptic and postsynaptic membrane. Our results revealed that cadmium at the middle and high dose could induce obvious microstructure and ultrastructure alterations of cerebral cortex in juvenile mice, which may be one important mechanism of cadmium neurotoxicity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cadmium is known to be a toxic environmental and industrial pollutant and one of the most harmful heavy metals to the human health. Cadmium can be accumulated with biologic half-life as long as 15–20 years in humans [1]. Evidences have confirmed that occupational cadmium exposure can induce lung cancer and other cancers such as the prostate, renal, liver, hematopoietic system, urinary bladder, pancreatic, testis, and stomach cancers [2]. Thus, cadmium is classified as a human carcinogen. Due to its high blood brain barrier permeability [3], cadmium is also a potential neurotoxin in animals and humans; exposure to cadmium affects the function of the nervous system, which has been well studied in animals, while the neurotoxicity of cadmium in human has not been clearly demonstrated. Agency for Toxic Substances and Disease Registry (ATSDR) reported that oral exposure to cadmium resulted in significantly decreased motor activity, weakness and muscle atrophy, aggressive behavior, anxiety as manifested by increased passive avoidance behavior and ethanol consumption, alterations in brain biogenic amine content, and enzyme activities [4]. Cadmium-induced neurological symptoms of headache and vertigo, olfactory dysfunction, Parkinsonian-like symptoms, slowing of vasomotor functioning, peripheral neuropathy, decreased equilibrium, hyperactivity, and learning disabilities were observed in children [2, 5–7]. It also indicated that cadmium was an etiological factor of neurodegenerative diseases, such as Parkinsonism and Alzheimer [8, 9]. Although some mechanisms of cadmium neurotoxicity have been reviewed [2, 10, 11], it still remains incompletely understood.
In risk assessment of chemical hazards, it is widely recognized that juvenile individuals are one of the risk groups that need special attention. The neurotoxicity of cadmium in children was investigated in several studies in the 1970s and 1980s but has received little attention since then [2]. Wong et al. reported that the neurotoxic effects of cadmium were different in adult and young rats, and cadmium was more toxic to 4-day-old than adult rats [12]. Webster and Valois investigated the neurotoxic effects of cadmium on the neonatal mouse brain and revealed that cadmium exposure at 2 mg/kg dose on postnatal 1 day caused petechial hemorrhages, edema, and cellular pycnosis throughout much of the immature brain. However, the brain was apparently unaffected at 8 mg/kg cadmium on postnatal 22 days [13]. Juvenile period is characterized by rapid growth and development of the nervous and other systems, which may cause the juvenile to be more vulnerable to harmful substances than adults. However, very little attention has been paid to the susceptibility to the cadmium of the juvenile, and few studies have been performed to determine the histopathology and ultrastructural features of the juvenile mice in cerebral cortex. The present study aimed to investigate the histopathological changes of cerebral cortex in juvenile mice induced by cadmium and would be helpful to understanding the neurotoxicity of cadmium.
Materials and Methods
Animals
Forty Kunming juvenile mice (20 female and 20 male mice), postnatal (20 ± 1) days, were obtained from the Centre for Laboratory Animal of Xinxiang Medical University, China. All animal experiments were reviewed and approved by the Institutional Animal Care and Use Committee of Henan Province, China. The mice were kept in plastic cages with free access to standard commercial rodent diet and water, in a room with controlled temperature (22 ± 2 °C) and on a 12-h light/12-h dark cycle.
Animal Groups and Treatments
The juvenile mice were allowed to acclimatize for 5 days and then randomly divided into four groups (Five male and five female juvenile mice per group): control (deionized water, control), low dose (1/100 LD50, 1.87 mg/kg of body weight, low), medium dose (1/50 LD50, 3.74 mg/kg of body weight, medium) and high dose (1/25 LD50, 7.48 mg/kg of body weight, high) group. Cadmium chloride was orally administered in drinking water to the mice for 10 days. LD50, the median lethal dose of cadmium chloride via oral administration to mice, was 187 mg/kg of body weight obtained from our previous study [14].
Normally, oral intake via water or food is the dominant route of cadmium exposure in the non-smoking and non-occupationally exposed juvenile. Hence, three different levels of cadmium chloride solution, dissolved in deionized water, were provided ad libitum in drinking water for 10 days. Deionized water was given the juvenile mice of the control group in the same manner as the treatment groups.
After cadmium exposure for 10 days, ten juvenile mice were perfused transcardially with cold normal saline solution for sampling. Craniotomy was performed and the intact cerebrum was immediately removed, weighed, and fixed for histopathology studies.
Processing for Microstructure Study by Light Microscope
Frontal lobe cortex of left cerebral cortex from three mice per group were isolated, fixed in 4 % paraformaldehyde in 0.1 M phosphate-buffered saline (PBS), pH 7.2, for 24 h, and then routinely processed for histological studies using the hematoxylin and eosin (HE) staining method. Briefly, after fixation, the samples were dehydrated in a graded series of ethanol, cleared with xylene, impregnated in molten paraffin, embedded in fresh molten paraffin, and sectioned into 5-μm thickness sections using a microtome (Leica, RM 2235, Germany). Subsequently, the sections were stained with hematoxylin and eosin (HE) to be examined by a light microscope (Axiostar plus, Carl Zeiss, Germany).
Processing for Ultrastructure Study by Transmission Electron Microscope
Frontal lobe cortex of left cerebral cortex from the other three mice per group were isolated, chopped to get pieces of approximately l mm × 1 mm ×l mm, and immediately fixed in 2.5 % glutaraldehyde solution for 4 h. Then, the samples were washed three times with PBS and post-fixed in 1 % osmium tetroxide solution for 1.5 h, dehydrated with acetone, immersed in propylene oxide, and embedded in EPON 812 at 60 °C. Ultrathin sections (40–60 nm) were obtained by Leica EM UC6rt ultramicrotome, and were doubly stained with 0.1 % uranyl acetate and 3 % lead citrate solution. Afterwards, the sections were examined and photographed under transmission electron microscope (Hitachi H-7500, Japan) at different magnifications.
Statistical Analysis
The data of body weight and cerebrum weight were expressed as mean ± SD and freely compared for significance using the Student’s t test in SPSS 10.0 (SPSS Inc., Chicago, IL, USA). Significance was accepted as P < 0.05.
Results
Behavioral Changes and Weight Parameters in Cadmium-exposed Juvenile Mice
With the extension of exposure time, cadmium-exposed juvenile mice exhibited decreased activities, lethargy, and insensitivity to outside stimulation compared to control. None of the mice died during the exposure period. The weight parameters in cadmium-exposed juvenile mice were shown in Table 1. The final body weight of cadmium-exposed juvenile mice declined, cerebrum weight and cerebrum to body weight ratio increased with increasing of cadmium dose, but they did not differ significantly from control (P > 0.05).
Microstructure Changes of Cerebral Cortex in Cadmium-exposed Juvenile Mice
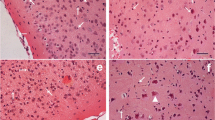
Photomicrograph of HE-stained sections showed intact microstructure of cerebral cortex of juvenile mice from the control group (Fig. 1a). In the low-dose group, microstructure of each layer of cerebral cortex was similar to that of control mice, and remarkable pathological changes were not found. However, in medium-dose group, pia mater encephali slightly divorced from cerebral cortex layer, vacuolar degeneration appeared in part granule cells, hyperemia was observed in blood capillary, and vessel peripheral clearance broadened (Fig. 1b). Microstructural damage of cerebral cortex in juvenile mice exposed to high-dose cadmium was more serious. Pia mater encephali remarkably divorced from cerebral cortex layer, serious hyperemia of blood capillary was observed in pia mater encephali, vessel peripheral clearance broadened, and the numbers of apoptotic cells obviously increased (Fig. 1c). Additionally, a large number of eosinophil leukocyte infiltrated around the blood vessel and vacuolar degeneration also appeared in part granule cells (Fig. 1d).
Effect of cadmium exposure on the microstructure of cerebral cortex of juvenile mice. a Photomicrograph of normal cerebral cortex of juvenile mice. Intact microstructure of cerebral cortex layer. b Photomicrograph of cerebral cortex of juvenile mice exposed medium dose cadmium. Pia mater encephali slightly divorcing from cerebral cortex layer (cross), vacuolar degeneration in part granule cells (triangle), hyperemia of blood capillary (diamond), and broadening vessel peripheral clearance (star). c and d Photomicrograph of cerebral cortex of juvenile mice exposed to high-dose cadmium. c Pia mater encephali of juvenile mice seriously divorcing from cerebral cortex layer (cross), serious hyperemia of blood capillary in pia mater encephali (arrow), broadening vessel peripheral clearance (star) and obviously increasing apoptotic cells (asterisk). d Hyperemia of blood capillary (diamond), a large number of eosinophil leukocyte infiltrating around blood vessel (pound sign), vacuolar degeneration in part granule cells (triangle) and obviously increasing apoptotic cells (asterisk). Magnification: ×400 (a–d). Scale bars a–d 200 nm. There was HE staining
Ultrastructural Changes of Cerebral Cortex Neurons in Cadmium-exposed Juvenile Mice
Electron micrographs of normal cerebral cortex neurons were shown in Fig. 2a, f. The ultrastructure of cerebral cortical neurons in juvenile mice treated with low-dose cadmium was not significantly affected. However, the ultrastructure of cerebral cortical neurons in the medium-dose group was characterized by the fusion of nuclear membranes, ambiguous ultrastructure, vacuolization, and vague cristae of some mitochondria (Fig. 2g). The ultrastructure of cerebral cortical neurons of juvenile mice treaded with high-dose cadmium was more seriously damaged. Heterochromatin was observed marginalization, nuclear membrane was not intact, and perinuclear space broadened (Fig. 2h). Nuclear membrane of neuron fused. The ultrastructure of mitochondria was ambiguous and mitochondria cristaes were vague (Fig. 2i). Additionally, synaptic ultrastructure was abnormal, some of synaptic cleft decreased, some of presynaptic and postsynaptic membrane fused (Fig. 2j). However, no ultrastructural changes of blood brain barrier were observed after 10 days of cadmium treatment in all groups (no shown).
Effect of cadmium exposure on the ultrastructure of cerebral cortex of juvenile mice. a and f Electron microscopic image of normal cerebral cortex neurons. g Electron microscopic image of cerebral cortex neurons of juvenile mice treated with medium dose cadmium. Fused nuclear membranes (leftwards arrow), vacuolate mitochondria (asterisk), and vague mitochondria cristae (cross). h–j Electron microscopic image of cerebral cortex neurons of juvenile mice treated with high-dose cadmium. h. Marginalized heterochromatin (star), incomplete nuclear membranes (rightwards arrow) and broadened perinuclear space (downwards arrow). i Fused nuclear membrane (leftwards arrow), ambiguous mitochondria cristae (cross). j Decreased synaptic cleft (bold line), fused presynaptic and postsynaptic membrane (upwards arrow). Magnification: ×12,000 (e, g, h, and i); ×25,000 (f and j). Scale bars e, g, h, and i 833 nm; f and j 400 nm. There were uranyl acetate and lead citrate staining
Discussion
Human exposure to cadmium is inevitable due to its excessive use in various products and disposal of these products along with household waste [15], and intake via contaminated drinking water is a common and main source of cadmium exposure for the non-smoking and non-occupational exposed population. So, cadmium in drinking water was orally administered to the experimental animals in the present study. Although neurotoxic effects induced by cadmium on the adults have been intensely investigated, few studies were conducted to investigate the effects of cadmium to the juvenile. The postnatal (20 ± 1) days mice, acclimatized for 5 days, were used for risk assessment of cadmium neurotoxicity in the present study as postnatal 21–27 days are considered as the juvenile period in rodents [16].
This study assessed the effects of cadmium exposure on microstructure and ultrastructure of cerebral cortex in juvenile mice brain. The cadmium-exposed juvenile mice displayed decreased activities, lethargy, and insensitivity. Mice treated with cadmium weighed less and the cerebrum weight and cerebrum to body weight ratio tended to be increased; however, the differences did not reach significance (P > 0.05). Compared to the control, microstructure and ultrastructure alterations of cerebral cortex in juvenile mice treaded with low-dose cadmium were not obvious. However, the effects of cadmium on the juvenile mice treaded with medium- and high-dose cadmium were definite and in a dose-dependent manner. The high dose of cadmium could disrupt the microstructure of cerebral cortex in juvenile mice and significantly showed pia mater encephali divorcing from cerebral cortex layer, serious hyperemia of blood capillary in pia mater encephali and cerebral cortex, broadening vessel peripheral clearance, a large number of eosinophil leukocyte infiltrating around blood vessel, vacuolar degeneration in part granule cells, and obviously increasing apoptotic cells. High-dose cadmium exposure could also destroy the ultrastructure of cerebral cortex which displayed marginalized heterochromatin, incomplete or fused nuclear membranes, broadened perinuclear space, ambiguous mitochondria cristae, decreased synaptic cleft, and fused presynaptic and postsynaptic membrane. Our results indicated that both medium and high doses of cadmium could gain access to the brain. Cadmium can enter the brain through blood brain barrier, and then bioaccumulated and leaded to alter the microstructure and ultrastructure of cerebral cortex. The microstructure and ultrastructure alterations could affect the function of cerebral cortex, which may be one of the important mechanisms of the neurotoxic effects induced by cadmium exposure.
Cerebral cortical neurons have been identified as targets of cadmium-mediated toxicity and cadmium-induced cell apoptosis [17, 18]. Cadmium has been reported to penetrate and accumulate in the brain of developing and adult rats leading to intracellular accumulation, cellular dysfunction, cerebral edema, lower attention, olfactory dysfunction, and memory deficits [19, 20]. In normal conditions, cadmium barely reaches the brain in adults due to the presence of blood brain barrier. However, this structure is not fully developed in young animals [2], leading to the severer toxicity of cadmium in newborn and young rats than in adult rats [21–23]. Prenatal cadmium exposure-induced endothelial cell alteration in the fetal brain has been reported [24]. In another study, cadmium exposure for 90 days rather than 30 days changed the permeability of blood brain barrier [25]. In our study, no significant ultrastructural changes of blood brain barrier were observed after 10 days of cadmium treatment in all groups, which may be due to the dose and the treatment time we used. Regretfully, the permeability change of blood brain barrier was not determined.
The neurotoxic mechanisms through which cadmium elicits its neurotoxic effects are complicated and have been carefully reviewed in recent years. Cadmium-induced brain dysfunction was indicated by the disruption of metal ion homeostasis, reduction of the total brain antioxidant status, inhibition of oxidative DNA repair systems, alteration in signal transduction pathway, and stimulation in the production of ROS [10]. Biochemical mechanism was reflected by disturbances of the cellular antioxidant system, generation of reactive oxygen and nitrogen species, changes in energy production in the metabolic pathways, changes in the metabolism of biogenic amines, neurotransmitter amino acids and calcium ions, and inhibition of enzymatic proteins [11]. Oxidative damage interacting with other metals such as cobalt and zinc, estrogen-like effect, and epigenetic modification, was also considered as the neurotoxic mechanisms [2]. Our research indicated that the neurotoxic effects of cadmium in juvenile mice may be associated with microstructure and ultrastructure alterations of the cerebral cortex.
Conclusions
To the best of our knowledge, this preliminary study firstly demonstrated the effects of cadmium exposure on the histopathology of cerebral cortex in juvenile mice. It is concluded that both medium and high dose of cadmium, rather than low dose, can induce the histopathological alterations of cerebral cortex in juvenile mice. The microstructure and ultrastructure alterations observed in the present study may be one important mechanism of cadmium neurotoxicity.
References
Son YO, Wang X, Hitron JA, Zhang Z, Cheng S, Budhraja A, Ding S, Lee JC, Shi X (2011) Cadmium induces autophagy through ROS-dependent activation of the LKB1-AMPK signaling in skin epidermal cells. Toxicol Appl Pharmacol 255:287–296. doi:10.1016/j.taap.2011.06.024
Wang B, Du YL (2013) Cadmium and its neurotoxic effects. Oxid Med Cell Longev 2013:898034. doi:10.1155/2013/898034
López E, Figueroa S, Oset-Gasque MJ, González MP (2003) Apoptosis and necrosis: two distinct events induced by cadmium in cortical neurons in culture. Br J Pharmacol 138:901–911. doi:10.1038/sj.bjp. 0705111
Agency for Toxic Substances and Disease Registry (1999) Toxicological profile for cadmium. Atlanta, Georgia
Kim SD, Moon CK, Eun SY, Ryu PD, Jo SA (2005) Identification of ASK1, MKK4, JNK, c-Jun, and caspase-3 as a signaling cascade involved in cadmium-induced neuronal cell apoptosis. Biochem Biophys Res Commun 328:326–334. doi:10.1016/j.bbrc.2004.11.173
Monroe RK, Halvorsen SW (2006) Cadmium blocks receptor-mediated Jak/STAT signaling in neurons by oxidative stress. Free Radic Biol Med 41:493–502. doi:10.1016/j.freeradbiomed.2006.04.023
Pihl RO, Parkes M (1977) Hair element content in learning disabled children. Science 198:204–206. doi:10.1126/science.905825
Okuda B, Iwamoto Y, Tachibana H, Sugita M (1997) Parkinsonism after acute cadmium poisoning. Clin Neurol Neurosurg 99:263–265. doi:10.1016/S0303-8467(97)00090-5
Jiang LF, Yao TM, Zhu ZL, Wang C, Ji LN (2007) Impacts of Cd (II) on the conformation and self-aggregation of Alzheimer’s tau fragment corresponding to the third repeat of microtubule-binding domain. Biochim Biophys Acta 1774:1414–1421. doi:10.1016/j.bbapap.2007.08.014
Carageorgiou H, Katramadou M (2012) Aspects of cadmium neurotoxicity. In: Li YV, Zhang JH (eds) Metal ion in stroke, 1st edn. Springer, New York, pp 703–749
Labudda M (2011) Biochemical mechanisms of neurotoxicity caused by cadmium. Rocz Panstw Zakl Hig 62:357–363
Wong KL, Klaassen CD (1982) Neurotoxic effects of cadmium in young rats. Toxicol Appl Pharmacol 63:330–337. doi:10.1016/0041-008X(82)90261-7
Webster WS, Valois AA (1981) The toxic effects of cadmium on the neonatal mouse CNS. J Neuropathol Exp Neurol 40:247–257. doi:10.1097/00005072-198105000-00003
Yang XF, Ge YM, Jiang JQ, Xu ZY, Cui YH, Wang ZL (2012) Acute toxic effect of cadmium chloride in mice. Chin J Vet Sci 32:467–471
Haider S, Anis L, Batool Z, Sajid I, Naqvi F, Khaliq S, Ahmed S (2014) Short term cadmium administration dose dependently elicits immediate biochemical, neurochemical and neurobehavioral dysfunction in male rats. Metab Brain Dis. doi:10.1007/s11011-014-9578-4
Mitchell NC, Gould GG, Smolik CM, Koek W, Daws LC (2013) Antidepressant-like drug effects in juvenile and adolescent mice in the tail suspension test: relationship with hippocampal serotonin and norepinephrine transporter expression and function. Front Pharmacol 4:131. doi:10.3389/fphar.2013. 00131
Xu B, Chen S, Luo Y, Chen Z, Liu L, Zhou H, Chen W, Shen T, Han X, Chen L, Huang S (2011) Calcium signaling is involved in cadmium-induced neuronal apoptosis via induction of reactive oxygen species and activation of MAPK/mTOR network. PLoS One 6:e19052. doi:10.1371/journal.pone.0019052
Yuan Y, Jiang CY, Xu H, Sun Y, Hu FF, Bian JC, Liu XZ, Gu JH, Liu ZP (2013) Cadmium-induced apoptosis in primary rat cerebral cortical neurons culture is mediated by a calcium signaling pathway. PLoS One 8:e64330. doi:10.1371/journal.pone. 0064330
Gonçalves JF, Fiorenza AM, Spanevello RM, Mazzanti CM, Bochi GV, Antes FG, Stefanello N, Rubin MA, Dressler VL, Morsch VM, Schetinger MR (2010) N-acetylcysteine prevents memory deficits, the decrease in acetylcholinesterase activity and oxidative stress in rats exposed to cadmium. Chem Biol Interact 186:53–60. doi:10.1016/j.cbi.2010.04.011
Méndez-Armenta M, Ríos C (2007) Cadmium neurotoxicity. Environ Toxicol Pharmacol 23:350–358. doi:10.1016/j.etap.2006.11.009
Leret ML, Millán JA, Antonio MT (2003) Perinatal exposure to lead and cadmium affects anxiety-like behaviour. Toxicology 186:125–130. doi:10.1016/S0300-483X(02)00728-X
Yargiçoğlu P, Ağar A, Oğuz Y, Izgüt-Uysal VN, Sentürk UK, Oner G (1997) The effect of developmental exposure to cadmium (Cd) on visual evoked potentials (VEPs) and lipid peroxidation. Neurotoxicol Teratol 19:213–219. doi:10.1016 /S0892-0362(97)00016-0
Antonio MT, Corredor L, Leret ML (2003) Study of the activity of several brain enzymes like markers of the neurotoxicity induced by perinatal exposure to lead and/or cadmium. Toxicol Lett 143:331–340. doi:10.1016/S0378-4274(03)00194-2
Rohrer SR, Shaw SM, Lamar CH (1978) Cadmium induced endothelial cell alterations in the fetal brain from prenatal exposure. Acta Neuropathol 44:147–149. doi:10.1007/BF00691482
Shukla A, Shukla GS, Srimal RC (1996) Cadmium-induced alterations in blood-brain barrier permeability and its possible correlation with decreased microvessel antioxidant potential in rat. Hum Exp Toxicol 15:400–405. doi:10.1177/096032719601500507
Compliance with Ethical Standards
ᅟ
Funding
This study was funded by the grant of Aid Project for Leading Young Teachers in Henan Provincial Institutions of Higher Education of China (Grant No.2010GGJS-136) and Science and Technology Research Important Project of Education Department Henan Province (Grant No.13A230142 and 13A230289).
Conflict of Interest
The authors declare no conflict of interest.
Ethical Approval
All procedures performed in studies involving animals were in accordance with the ethical standards of the institution at which the studies were conducted.
Author information
Authors and Affiliations
Corresponding author
Additional information
XF Yang and GY Fan contributed equally to this work.
Rights and permissions
About this article
Cite this article
Yang, X., Fan, G., Liu, D. et al. Effect of Cadmium Exposure on the Histopathology of Cerebral Cortex in Juvenile Mice. Biol Trace Elem Res 165, 167–172 (2015). https://doi.org/10.1007/s12011-015-0246-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-015-0246-2