Abstract
Selenoprotein M (SelM) may function as thiol disulfide oxidoreductase that participates in the formation of disulfide bonds and can be implicated in calcium responses. SelM may have a functional role in catalyzing free radicals and has been associated with Alzheimer’s disease (AD). However, studies of SelM in chicken remain very limited. In this study, two groups of day-old broiler chicks (n = 40/group) were fed a corn-soy basal diet (BD, 13 μg Se/kg) and BD supplemented with Se (as sodium selenite) at 0.3 mg/kg. The brain was collected at 14, 21, 28, and 42 days of age. We performed a sequence analysis and predicted the structure and function of SelM. We also investigated the effects of Se deficiency on the expression of Selt, Selw, and Selm and the Se status in the chicken brain. The results show that Se deficiency induced the lower (P < 0.05) Se content, glutathione peroxidase (GPx), and catalase (CAT) activities; increased (P < 0.05) malondialdehyde (MDA) content; and reduced (P < 0.05) the expression of Selm messenger RNA (mRNA) and protein abundance of SelM in the brain. However, there were no significant brain Selt and Selw mRNA levels by dietary Se deficiency in chicks. The different regulations of these three redox (Rdx) protein expressions by Se deficiency represent a novel finding of the present study. Our results demonstrated that SelM may have an important role in protecting against oxidative damage in the brain of chicken, which might shed light on the role of SelM in human neurodegenerative disease. More studies are needed to confirm our conclusion.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Selenium (Se), an essential trace element for many living organisms, can assist cells to resist oxidative damage. Se in vivo is primary present as various selenoproteins to maintain the balance of the cellular redox state [1]. Selenoproteins are defined by incorporation of Se into amino acid selenocysteine. This human and avian family all consist 25 selenoproteins [2–4], which play important roles in redox regulation, detoxification, immune system protection, and viral suppression [5–7]. However, the biological functions of some selenoproteins identified recently remain largely unknown, including some redox (Rdx) proteins containing a diselenide bond in a common redox motif [8]. This motif that consist of CxxU (U designates selenocysteine) has been identified in a subset of selenoproteins, including selenoprotein M (SelM) [9], selenoprotein W (SelW) [10, 11], and selenoprotein T (SelT) [12]. It has been proposed that these Rdx proteins exhibit a thioredoxin-like fold and a conserved CxxU motif, suggesting a redox function. SelW, the first discovered selenoprotein, is the well-characterized protein. SelW is particularly abundant in muscle, and its deficiency has been implicated in white muscle disease [13, 14]. SelT is a protein involved in intracellular Ca2+ mobilization and neuroendocrine secretion, and SelT is largely regulated by pituitary adenylate cyclase-activating polypeptide (PACAP) gene [15]. Se and selenoproteins have been suggested to implicate in neurodegenerative disease, such as Alzheimer’s disease (AD) and Parkinson’s disease [16].
SelM was first reported as a new selenoprotein using bioinformatics methods [9, 17]. It is highly conserved from plants to humans and localizes to the endoplasmic reticulum. In pig, SelM is ubiquitously expressed in many tissues, including muscle, liver, kidney, cerebral cortex, pituitary, thyroid, and testis. In human, it is expressed in many tissues with the highest levels in the brain [9], supporting its possible function in AD. Although the distribution, function, and structure of SelM in mammals have been well studied, studies of SelM in chicken remain very limited. In this study, we aim to analyze the nucleotide and amino acid sequences of chicken SelM. We also compared the Se content; glutathione peroxidase (GPx) activity; catalase (CAT) activity; malondialdehyde (MDA) content; messenger RNA (mRNA) level of Selt, Selw, and Selm; and protein level of SelM in the brain between Se-deficient and Se groups.
Materials and Methods
Animal, Diet, and Experimental Design
All procedures in this experiment were approved by the Institutional Animal Care and Use Committee of China Agricultural University. A total of 80 day-old male broiler poults (Dafa Zhengda Poultry Co., Ltd., Beijing, China) were randomly divided into two groups. One group was treated with basal diet composed of corn and soybean that is obtained from Se-deficient area (Sichuan, China) (Se-deficient group) (n = 40), and the other group was treated with Se basal diet with sodium selenite at 0.3 mg/kg (Sigma-Aldrich, St. Louis, MO, USA) (Se group) (n = 40). The Se concentrations were 13 and 310 μg/kg in Se-deficient and Se groups, respectively. Food and water were provided ad libitum. All procedures, as well as the care, housing, and handling of the animals, were conducted according to accepted commercial management practices. On days 14, 21, 28, and 42, chickens (n = 10/group) were anesthetized with sodium pentobarbital and brain was collected and frozen in liquid nitrogen for analysis.
Selm Sequence Analysis, Selenocysteine Insertion Sequence Element Prediction, 3D Molecular Modeling, and Phylogenetic Analysis
With selenocysteine insertion sequence (SECIS), UGA codon can be translated into Sec, which has been regarded as a marker of selenoprotein mRNA. In order to confirm the novel RNA sequence and to identify the SECIS element, we used the SECISearch engine (http://seblastian.crg.es/, SECISearch3) [18] to analyze the primary sequence and secondary structure of chicken Selm mRNA. The complementary DNA (cDNA) sequence and deduced amino acid sequence were analyzed using the BLAST algorithm (http://www.ncbi.nlm.nih.gov/blast) and the Expert Protein Analysis System (http://www.expasy.org/). The 3D structure and biological function of chicken SelM were predicted using the I-TASSER server (http://zhanglab.ccmb.med.umich.edu/I-TASSER/) [19, 20]. The evolutionary and tree homology analysis was constructed using the DNAMAN 6 (Lynnon BioSoft, Canada) and MEGA 5 [21].
Biochemical Assays
Se concentrations in brains obtained from chickens were measured using the hydride generation-atomic fluorescence spectrometer (AFS-3200; Yongtuo Instruments) against the standard reference of Se (GBW(E)080441, National Research Center for Certified Reference Materials) [22]. Briefly, brain tissues were homogenized on ice in physiological saline and centrifuged at 1000×g and supernatants were collected for GPx, CAT, and MDA activity analysis using kits provided by Jiancheng Biotechnology Research Institute (Nanjing, China). GPx activity was measured spectrophotometrically at 37 °C at 412 nm as described previously [23]. The CAT assay was measured spectrophotometrically at 520 nm. The formation of MDA was determined as an indicator of lipid peroxidation measuring at 532 nm [24]. Protein concentration was determined using the method by Bradford [25].
Real-Time Quantitative PCR Analysis of Selm, Selw, and Selt
Literature search [26] allowed us to target on Selm, Selw, and Selt genes in chicks. To determine effects of dietary Se on the mRNA expression of these genes, we isolated total mRNA from the brain (50 to 100 mg tissue) of five most representative chicks from each group. The RNA sample preparation, quantitative PCR (Q-PCR) procedure, and the relative mRNA abundance qualification were the same as previously described by our group [4]. Primers (Table 1) for the three selenoprotein genes and two reference genes, β-actin gene (Actb) and glyceraldehyde 3-phosphate dehydrogenase gene (Gapdh), were designed using Primer Express 3.0 (Applied Biosystems, Foster City, CA, USA).
Western blot Analysis
Tissues were homogenized with cell lysis buffer for western and IP (catalog no. P0013; Beyotime Institute of Biotechnology) and centrifuged at 12,000×g for 10 min at 4 °C. The supernatants (10∼40 μg protein/lane) were loaded onto a SDS-PAGE gel (12.5 %), followed by transfer of proteins to polyvinylidene difluoride membranes (Millipore, Temecula, CA, USA). The membrane was incubated with primary anti-SelM antibody (1:500; Santa Cruz Biotech, Santa Cruz, CA, USA) and anti-β-actin (1:1000; Biosynthesis Biotechnology, Beijing, China), followed by incubation with horseradish peroxidase-conjugated secondary antibody (1:10,000; ZSGB Biotech, Beijing, China). The washed blot was developed using enhanced chemiluminescence reagent (Millipore) and exposed to X-ray films.
Statistical Analysis
Statistical analysis was performed using SPSS, version 13 (Chicago, IL, USA). Data were presented as mean ± SD. Difference between groups was determined by one-way ANOVA using a significance level of P < 0.05. The data were assessed using the Tukey-Kramer method for multiple comparisons.
Results
Sequence and Structure of Chicken Selm
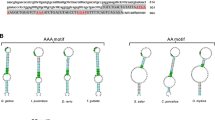
The characteristic of chicken Selm cDNA and its SECIS element is illustrated in Fig. 1. The Selm coding sequence (CDS) had 459 bases with an in-frame TGA triplet. The sequence theoretically encoded 152 amino acid residues, and the Sec was the 37th residue (Fig. 1a). The chicken Selm had 78 % CDS homology to human Selm. According to the generally used classification method [27] and SECISearch program analysis, a SECIS element, with the conserved denosine (–AAA–) rather than the cytidine (–CC–) motif in the apical loop was found in the 3′-untranslated region of the cDNA (mRNA), which is similar to the majority of avian species. Figure 1b compares the Selm SECIS elements in chick with other species, finding that the SECIS element had the –AAA– motif in Gallus gallus (chick), Meleagris gallopavo (turkey), Anas platyrhynchos (duck), Danio rerio (zebrafish), and Xenopus tropicalis (frog). In contrast, the SECIS element had the –CC– motif in Homo sapiens (human), Sus scrofa (pig), Mus musculus (mouse), Equus caballus (horse), and Bos taurus (cattle).
Chicken Selm cDNA and its selenocysteine insertion sequence (SECIS). a The cDNA sequence of chicken Selm. The sequence in uppercase is the coding sequence from ATG to TAA (in bold), and the framed TGA at 37th triplet from the initiation codon encodes selenocysteine (Sec). The underlined letters in bold in the 3′-untranslated region indicate that the highly conserved nucleotides are in the SECIS. b The SECIS structures were predicted using the SECISearch program with appropriate patterns. The highly conserved nucleotides are in bold. Accession numbers of the sequences mentioned above are NM_001277859.1 (G. gallus), XM_003211094.1 (M. gallopavo), XM_005016259.1 (A. platyrhynchos), NM_178286.3 (D. rerio), NM_001092248.1 (X. laevis), NM_080430.2 (H. sapiens), NM_001161648.1 (S. scrofa), NM_053267.2 (M. musculus), NM_001168402.1 (E. caballus), and NM_001163171.1 (B. taurus)
Homology and Phylogeny Analyses
The chicken SelM protein was aligned with 16 species (Fig. 2a), including Anolis carolinensis (green anole), X. tropicalis (frog), Stegastes partitus (bicolor damselfish), D. rerio (zebrafish), H. sapiens (human), M. musculus (mouse), S. scrofa (pig), E. caballus (horse), Physeter catodon (sperm whale), B. taurus (cattle), Eptesicus fuscus (bat), Canis lupus (dog), Leptonychotes weddellii (Weddell seal), Oryctolagus cuniculus (rabbit), Macaca mulatta (monkey), and Rattus norvegicus (rat). It shows an overall 58 % identity with a highly conserved thioredoxin-like domain containing a CXXU motif across all the species.
Alignment and phylogeny analyses of SelM based on the amino acid sequences. a The conserved CXXU motif (CGGU in the chicken) is indicated by the asterisks. The gray-colored letters indicate conserved amino acid residue across the 17 species. b The phylogenetic analyses were conducted using MEGA5 and encompassed 20 SelM proteins. The bootstrap consensus tree generated using the neighbor-joining method with 500 replicates and pairwise deletion options was taken to represent the evolutionary history of the 20 proteins. The statistics and frequency are presented at each of the nodes, and the length of the distance scale bar at the bottom of the panel defines 0.05 of the genetic distance
The amino acid sequence of chicken SelM shares a 79, 84, 97, and 58 % identity with SelM, in humans, duck, turkey, and zebrafish, respectively (Table 2). The nucleotide identity range of CDS of Selm is 72 to 94 % between chicken and other animals, including mammals and aquatic invertebrates (Table 2). In phylogeny (Fig. 2b), when SelM from the 20 species was genetically clustered, the Phasianinae, chicken, and turkey had the shortest distance.
The Predicted 3D Structure of SelM
Figure 3 shows that chicken SelM consists of a two stranded α-helices with four extended β-sheets. The molecule is characterized by a β1 (25–31)-α1(38–54)-β2(58–63)-β3(69–73)-β4(79–85)-α2(115–127) secondary structure pattern, wherein β1 and β2 are parallel strands forming one classical β-α-β motif. The predicted active Sec (U) site is located in the loop (residues 37) between β1 and α1.
Se Content, GPx, and CAT Activities and MDA Content in Brain Tissue
In brains collected at each time point, Se level was significantly lower in Se-deficient group than in Se group (P < 0.05) (Fig. 4(a)). In the Se group, compared with Se level in brains collected at 14 and 21 days, Se level at 28 and 42 days was decreased (P < 0.05). In Se-deficient group, GPx activity in the brain was significantly lower than that in Se group collected at different time points (P < 0.05) (Fig. 4(b)). In the Se-deficient group, GPx activity was decreased in the sampling time point from 14 to 42 days (P < 0.05). However, there was no significant difference in GPx activity at different time points in the Se group. In the Se-deficient group, CAT activity in the brain was significantly lower than that in the Se group brain collected at different time points (P < 0.05) (Fig. 4(c)). In the Se-deficient group, CAT activity was decreased in the sampling time point from 14 to 42 days (P < 0.05). However, there was no significant difference in CAT activity at different time points in the Se group. In the Se-deficient group, MDA content in the brain was significantly greater than that in the Se group brain collected at different time points (P < 0.05) (Fig. 4(d)). In the Se-deficient group, MDA content was increased in the sampling time point from 14 to 42 days (P < 0.05). However, there was no significant difference in MDA content at different time points in the Se group.
Effects of dietary Se concentration on Se content (a), GPx activity (b), CAT activity (c), and MDA content (d) in the brain of chickens fed a basal selenium-deficient diet (BD) plus 0.0 and 3.0 mg Se/kg in four sampling points (d = days). Data are presented as means ± standard error of the mean (SEM), n = 4 (a) or n = 5 (b–d). Within the same sampling points, asterisk (P < 0.05) shows differences under different dietary Se concentrations. At the same concentrations of dietary Se, bars with different letters (capital letter in 0.0 mg Se/kg group and small letter in 0.3 mg Se/kg group) are statistically significantly different at each time point by one-way analysis of variance followed by Tukey-Kramer multiple comparison test (P < 0.05)
The mRNA Level of Selt, Selw, and Selm and Protein Expression of SelM in Brain
Figure 5(a) shows that mRNA levels of Selt in the brain collected at all time points are non-comparable between the two groups. The mRNA level of Selw in the brain of Se-deficient group collected at 42 days was significantly lower than in the brain collected from the Se group (P < 0.05). No significant difference in Selw level was detected among the other time points (Fig. 5(b)). Figure 6(a) shows that mRNA levels of Selm in the brain collected from the Se-deficient group were significantly lower than those in the brain from the Se group collected at all time points (P < 0.05). We also examined the protein expression of SelM in brains, finding that the SelM protein in the Se-deficient group was significantly lower than that in Se group collected at 14 days (Fig. 6(b)), 21 days (Fig. 6(c)), 28 days (Fig. 6(d)), and 42 days (Fig. 6(e)) (P < 0.05).
Effects of dietary Se concentration on Selt mRNA abundance (a) and Selw mRNA abundance (b) in the brain of chickens fed a basal selenium-deficient diet (BD) plus 0.0 and 3.0 mg Se/kg in four sampling points (d = days). Data are presented as means ± standard error of the mean (SEM), n = 5. Within the same sampling points, asterisk (P < 0.05) shows differences under different dietary Se concentrations
Effects of dietary Se concentration on the a brain tissue Selm mRNA abundance and brain tissue protein level of SelM in 14 days (b), 21 days (c), 28 days (d), and 42 days (e) of chickens fed a basal selenium-deficient diet (BD) plus 0.0 and 3.0 mg Se/kg. Data are presented as means ± standard error of the mean (SEM), n = 5 (a) or n = 4 (b–e). Within the same sampling points, asterisk (P < 0.05) shows differences under different dietary Se concentrations
Discussion
In our study, we found that chicken SelM contains one well-conserved Sec. The chicken SelM protein is characterized by a β-α-β-β-β-α secondary structure pattern that forms a classical motif observed in thioredoxin-like fold proteins. In the Se-deficient group, GPx and CAT activities were decreased in the sampling time point. In contrast, the MDA content was increased by Se deficiency at all time points. Meanwhile, the mRNA and protein levels of Selm in the brain of Se-deficient group were significantly lower than those in the brain of Se group collected at all time points [28]. To the best of our knowledge, this is the first work that reports the structural and functional predictions of SelM in chicken.
Consistent with the Selm SECIS elements in other species (i.e., turkey, duck, zebrafish, and frog), the chicken Selm SECIS elements also contains –AAA– sequence but not –CC– in the apical loop. However, the substitution of adenosines (A) for cytidines (C) shows no observable effect on Sec insertion into SelM. Pervious study has demonstrated that the SECIS element with –CC– motif appears to be universal in the Selm genes of mammalian animals [9]. In order to understand the evolutionary meaning of forms and motifs in the Selm SECIS element, we did a phylogenetic analysis encompassing a greater number of species. Our finding indicates that similar to its mammalian homolog, chicken SelM may play a role in redox regulation [17]. The observed highly homology of SelM indicates that chicken SelM was derived from a common ancestor. Our 3D structural analysis demonstrated that chicken SelM was similar to that of pig SelM [9], chicken SelW [10, 11], and chicken SelT [12], suggesting that they might exhibit similar functions.
In this study, we examined mRNA level of Selm, Selw, and Selt to assess the response of SelM to Se. In the Se-deficient group, we found decreased level of Se content, GPx and CAT activities, and Selm mRNA level in the brain compared with those in Se groups. However, no significant difference in Selt and Selw mRNA levels between Se-deficient and Se groups was observed. In contrast, the Se-deficient chicks had higher MDA content in the brain than the Se chicks at each time point. In general, antioxidant defense systems eliminate reactive oxygen species (ROS) or other radical species to prevent oxidative stress, which is defined as an alteration in the steady-state balance between oxidant and antioxidant agents. It has been shown that under Se-deficient status, the activity of some antioxidative enzymes, such as GPx and CAT, the first line of cellular defense against potential oxidative damage [29], might be inhibited in various tissues, such as liver and muscle in poults [4], plasma in mice [30], and liver and brain of rat [31]. Consistent with previous studies, we observed decreased GPx, CAT, and Se in the Se-deficient group. Se levels in the brain are not high compared with those in most other organs and remain remarkably stable even during Se deficiencies [32, 33]. The results indicated that Se may be critical for the maintenance of brain function and may first meet the Se demand of the brain at the expense of other organs. However, in chicken feed with low-Se diet for a long time, the brain is also influenced by Se deficiency.
Compared with the Se group, we observed similar mRNA level of Selt and Selw, decreased mRNA level of Selm, and decreased protein level of SelM in the Se-deficient group. In fact, SelM, SelT, and SelW are the three important Rdx proteins in selenoprotein family and are proposed to possess a thioredoxin-like fold and a conserved CxxC or CxxU motif with redox function [34]. Previous study has showed that SelT was expressed in neural progenitors in various regions, such as the cortex and cerebellum, but was undetectable in most adult nervous cells [35]. SelT expression was maintained at stable level in several adult endocrine tissues, such as pituitary, thyroid, and testis. SelW was widespread in neurons and neuropil of mouse brain and appeared in soma and processes of neurons in culture [36]. Pyramidal neurons of the cortex and hippocampus express high levels of SelW. In our study, we did not observe significant difference in Selt and Selw mRNA level (14, 21, and 28 days), indicating the stable Se dietary-independent expression pattern. Se deficiency may cause irreversible changes in the neuronal cells and brain injury [37]. Clinical studies have revealed that Se deficiency is associated with various neuro diseases, such as cognitive impairment, seizures, Parkinson’s disease, and Alzheimer disease [38]. Taken together with the observed decreased Selm mRNA level in our study, we speculated that SelM is one of the major selenoperoxidases contributing to the prevention of brain injury possibly through the peroxide scavenging and antioxidant functions. Compared with other avian selenoproteins [4, 28, 39], in the present study, SelM was more sensitive to Se deficiency in the brain. This might indicate that SelM could be a molecular biomarker that predicts Se status in the brain.
In conclusion, the sequence of SelM in chicken is similar to that in other species. In the Se-deficient group, GPx and CAT activities and mRNA level of Selm were decreased significantly than those in the Se group. Our results demonstrated that SelM may have an important role in protecting against oxidative damage in the brain, which might shed light on the role of SelM in neurodegenerative disease in human. More studies in other species are needed to confirm our conclusion.
Abbreviations
- AD:
-
Alzheimer’s disease
- Se:
-
Selenium
- SECIS:
-
Selenocysteine insertion sequence
- SelT:
-
Selenoprotein T
- SelM:
-
Selenoprotein M
- SelW:
-
Selenoprotein W
References
McKenzie RC, Arthur JR, Beckett GJ (2002) Selenium and the regulation of cell signaling, growth, and survival: molecular and mechanistic aspects. Antioxid Redox Signal 4:339–351
Kryukov GV, Castellano S, Novoselov SV, Lobanov AV, Zehtab O, Guigó R, Gladyshev VN (2003) Characterization of mammalian selenoproteomes. Science 300:1439–1443
Mariotti M, Ridge PG, Zhang Y, Lobanov AV, Pringle TH, Guigo R, Hatfield DL, Gladyshev VN (2012) Composition and evolution of the vertebrate and mammalian selenoproteomes. PLoS One 7:e33066
Huang JQ, Li DL, Zhao H, Sun LH, Xia XJ, Wang KN, Luo X, Lei XG (2011) The selenium deficiency disease exudative diathesis in chicks is associated with downregulation of seven common selenoprotein genes in liver and muscle. J Nutr 141:1605–1610
Sun LH, Li JG, Zhao H, Shi J, Huang JQ, Wang KN, Xia XJ, Li L, Lei XG (2013) Porcine serum can be biofortified with selenium to inhibit proliferation of three types of human cancer cells. J Nutr 143:1115–1122
Papp LV, Lu J, Holmgren A, Khanna KK (2007) From selenium to selenoproteins: synthesis, identity, and their role in human health. Antioxid Redox Signal 9:775–806
Yao HD, Wu Q, Zhang ZW, Zhang JL, Li S, Huang JQ, Ren FZ, Xu SW, Wang XL, Lei XG (2013) Gene expression of endoplasmic reticulum resident selenoproteins correlates with apoptosis in various muscles of Se-deficient chicks. J Nutr 143:613–619
Fomenko DE, Gladyshev VN (2003) Identity and functions of CxxC-derived motifs. Biochem 42:11214–11225
Zhou JC, Zhao H, Tang J-Y, Li JG, Liu XL, Zhu YM (2011) Molecular cloning, chromosomal localization and expression profiling of porcine selenoprotein M gene. Genes Genom 33:529–534
Li JL, Ruan HF, Li HX, Li S, Xu SW, Tang ZX (2011) Molecular cloning, characterization and mRNA expression analysis of a novel selenoprotein: avian selenoprotein W from chicken. Mol Biol Rep 38:4015–4022
Ou BR, Jiang MJ, Lin CH, Liang Y-C, Lee KJ, Yeh JY (2011) Characterization and expression of chicken selenoprotein W. Biometals 24:323–333
You L, Liu C, Yang Z-j, Li M, Li S (2014) Prediction of selenoprotein T structure and its response to selenium deficiency in chicken immune organs. Biol Trace Elem Res 160:222–231
Yao H, Liu W, Zhao W, Fan R, Zhao X, Khoso PA, Zhang Z, Xu S (2014) Different responses of selenoproteins to the altered expression of selenoprotein W in chicken myoblasts. RSC Adv 4:64032–64042
Yao HD, Wu Q, Zhang ZW, Li S, Wang XL, Lei XG, Xu SW (2013) Selenoprotein W serves as an antioxidant in chicken myoblasts. BBA-Gen Subjects 1830:3112–3120
Grumolato L, Ghzili H, Montero-Hadjadje M, Gasman S, Lesage J, Tanguy Y, Galas L, Ait-Ali D, Leprince J, Guérineau NC (2008) Selenoprotein T is a PACAP-regulated gene involved in intracellular Ca2+ mobilization and neuroendocrine secretion. FASEB J 22:1756–1768
Chen J, Berry MJ (2003) Selenium and selenoproteins in the brain and brain diseases. J Neurochem 86(1):1–12
Korotkov KV, Novoselov SV, Hatfield DL, Gladyshev VN (2002) Mammalian selenoprotein in which selenocysteine (Sec) incorporation is supported by a new form of Sec insertion sequence element. Mol Cell Biol 22:1402–1411
Mariotti M, Lobanov AV, Guigo R, Gladyshev VN (2013) SECISearch3 and Seblastian: new tools for prediction of SECIS elements and selenoproteins. Nucleic Acids Res 41:e149
Roy A, Kucukural A, Zhang Y (2010) I-TASSER: a unified platform for automated protein structure and function prediction. Nat Protoc 5:725–738
Zhang Y (2008) I-TASSER server for protein 3D structure prediction. BMC Bioinform 9:40
Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol 24:1596–1599
Moreno ME, Pérez-Conde C, Cámara C (2000) Speciation of inorganic selenium in environmental matrices by flow injection analysis-hydride generation-atomic fluorescence spectrometry. Comparison of off-line, pseudo on-line and on-line extraction and reduction methods. J Anal Atom Spectrom 15:681–686
Rotruck J, Pope A, Ganther H, Swanson A, Hafeman DG, Hoekstra W (1973) Selenium: biochemical role as a component of glutathione peroxidase. Science 179:588–590
Sun LH, Zhang NY, Zhai QH, Gao X, Li C, Zheng Q, Christopher SK, Qi DS (2014) Performance, hematology, serum biochemistry, antioxidant status, and tin retention in broilers. Biol Trace Elem Res 162:302–308
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Jongeneel CV (2000) Searching the expressed sequence tag (EST) databases: panning for genes. Brief Bioinform 1:76–92
Grundner-Culemann E, Martin GW, Harney JW, Berry MJ (1999) Two distinct SECIS structures capable of directing selenocysteine incorporation in eukaryotes. RNA 5:625–635
Huang JQ, Ren FZ, Jiang YY, Xiao C, Lei XG (2015) Selenoproteins protect against avian nutritional muscular dystrophy by metabolizing peroxides and regulating redox/apoptotic signaling. Free Radic Biol Med 83:129–138
Lei XG, Cheng W-H, McClung JP (2007) Metabolic regulation and function of glutathione peroxidase-1. Annu Rev Nutr 27:41–61
Cheng WH, Ho YS, Ross DA, Valentine BA, Combs GF, Lei XG (1997) Cellular glutathione peroxidase knockout mice express normal levels of selenium-dependent plasma and phospholipid hydroperoxide glutathione peroxidases in various tissues. J Nutr 127:1445–1450
Turan B, Acan NL, Ulusu NN, Tezcan EF (2001) A comparative study on effect of dietary selenium and vitamin E on some antioxidant enzyme activities of liver and brain tissues. Biol Trace Elem Res 81:141–152
Nakayama A, Hill KE, Austin LM, Motley AK, Burk RF (2007) All regions of mouse brain are dependent on selenoprotein P for maintenance of selenium. J Nutr 137:690–693
Schweizer U, Schomburg L, Savaskan NE (2004) The neurobiology of selenium: lessons from transgenic mice. J Nutr 134:707–710
Dikiy A, Novoselov SV, Fomenko DE, Sengupta A, Carlson BA, Cerny RL, Ginalski K, Grishin NV, Hatfield DL, Gladyshev VN (2007) SelT, SelW, SelH, and Rdx12: genomics and molecular insights into the functions of selenoproteins of a novel thioredoxin-like family. Biochem 46:6871–6882
Tanguy Y, Falluel-Morel A, Arthaud S, Boukhzar L, Manecka DL, Chagraoui A, Prevost G, Elias S, Dorval-Coiffec I, Lesage J (2011) The PACAP-regulated gene selenoprotein T is highly induced in nervous, endocrine, and metabolic tissues during ontogenetic and regenerative processes. Endocrinology 152:4322–4335
Raman AV, Pitts MW, Seyedali A, Hashimoto AC, Bellinger FP, Berry MJ (2013) Selenoprotein W expression and regulation in mouse brain and neurons. Brain Behav 3:562–574
Schweizer U, Bräuer AU, Köhrle J, Nitsch R, Savaskan NE (2004) Selenium and brain function: a poorly recognized liaison. Brain Res Rev 45:164–178
Zhang S, Rocourt C, Cheng WH (2010) Selenoproteins and the aging brain. Mech Ageing Dev 131:253–260
Jiang YY, Huang JQ, Lin GC, Guo HY, Ren FZ, Zhang H (2015) Characterization and expression of chicken selenoprotein U. Biol Trace Elem Res 166:216–224
Acknowledgments
This study was funded in part by Chinese Natural Science Foundation: the Major International (Regional) Joint Research Program of China (No. 31320103920) and the Project of Creating Excellent of the Capital (Beijing) Food Safety Technology (No. Z141100002614011).
Conflict of Interest
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Huang, JQ., Ren, FZ., Jiang, YY. et al. Characterization of Selenoprotein M and Its Response to Selenium Deficiency in Chicken Brain. Biol Trace Elem Res 170, 449–458 (2016). https://doi.org/10.1007/s12011-015-0486-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-015-0486-1