Abstract
This prospective cohort study aimed to analyze the association between serum ferritin levels and the risk of abnormal glucose metabolism (AGM) in Southwestern Chinese population. The 383 subjects who are aged ≥20 years and free of AGM at baseline between in 2007 and in 2008 were included in Southwestern China, and their baseline serum ferritin levels were measured. Among these subjects, 140 subjects were developed into AGM during the follow-up (2008–2012). In logistic regression models, the relative risk in the top versus that in the lowest quartile of serum ferritin levels was 2.86 (p = 0.013) in females and 3.50 (p = 0.029) in males after adjusting the age, gender, family history of diabetes, current smoking, and alcohol; however, serum ferritin levels were not significantly associated with incident of AGM after controlling for metabolic factors (waist circumference, systolic pressure (SBP), triglyceride (TG), and homeostasis model assessment formula insulin resistance (HOMA-IR)). Elevated serum ferritin levels are associated with AGM but not an independent risk factor.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent years, the Western lifestyle has gradually pervaded the Chinese people’s daily life which characterizes with an increased consumption of red meat in the total amount of calories. The excess intake of red meat contributed to the rapid increase of the prevalence of type 2 diabetes mellitus (T2DM) in China [1]. It has been confirmed that the high content of iron in the red meat serves as a catalyst in the formation of reactive oxygen species (ROS) such as hydroxyl radical, which induced the oxidative stress of the liver and muscle, as well as the apoptosis of pancreatic β cells [2]. Serum ferritin is widely used as a clinical biomarker of body iron stores which is determined by the effect of genetic and dietary factors [3]. The pathologically increased ferritin level in genetic diseases, such as hereditary hemochromatosis, contributes to the occurrence of T2DM [4]. However, the association between moderately increased ferritin level caused by the over-intake of red meat and the risk of abnormal glucose metabolism (AGM) is still unclear.
The study investigates the association between serum ferritin levels with abnormal glucose metabolism risk, especially in the prospective cohort or nested case-control studies which have provided strong evidence about the interrelation of them, and most of these studies are done in western countries [5–8]; however, the relevant studies among the Asian countries are quite rare [9, 10] and no one reports them in Southwestern China to date. In this study, we designed a prospective cohort to explore the association between serum ferritin levels and AGM risk in Southwestern Chinese population.
Materials and Methods
Study Design and Subjects
The Chinese Diabetes Society conducted a cross-sectional study about diabetes and relevant metabolic risk factors by using multistage, stratified sampling method between 2007 and 2008 in China [11]. A total of 689 adults (age of 20 years old) who came from two urban communities and two rural villages in Southwestern China were involved in this study. Based on the previous cross-sectional study, these participants have been followed up for 4 years. The present study was reviewed and approved by The Ethics Committee of China-Japan Friendship Hospital (location: 2 Cherry Pank Street, Chaoyang District, Beijing 100029, China). Written informed consent was obtained from every subject before the study. Our study was registered in the Chinese clinical trial registry (#TR-CCH-ChiCTR-OCS-09000361).
Exclusion criteria included the following: the participants with AGM or who were taking medications for diabetes in the cross-sectional study at baseline, the subjects with chronic diseases such as hepatic or renal diseases, the subjects with thyroid or parathyroid diseases, the subjects taking osteoporosis medications or other drugs that might influence glucose metabolism such as glucocorticoid, pregnant women, and the subjects with incomplete data. According to the exclusion criteria, the final sample size for the present analysis was 383 participants (including 133 men and 250 women).
Subjects Classification
Our study got the blood samples of participants who did not have AGM at baseline between 2007 and 2008, and the serum ferritin levels were measured. The study was designed as a prospective cohort study, which used the baseline serum ferritin levels as the exposure. The subjects whether developed into AGM including type 2 diabetes (T2DM), impaired fasting glucose (IFG), and impaired glucose tolerance (IGT), according to the diagnosis criteria of World Health Organization (WHO) in 1999, during the follow-up, or maintained normal glucose tolerance (NGT) were considered as the result. We make use of the quartile methods in classifying the subjects into four groups according to the baseline serum ferritin levels in females and males, respectively, and compared the differences in developing AGM under different baseline serum ferritin levels and analyzed the association between baseline serum ferritin levels and AGM. During the follow-up, we got the blood samples of the 383 participants and measured their serum ferritin levels.
Demographic Characteristics
The clinical characteristics and lifestyle of participants were recorded by trained staff using a standard questionnaire [12]. Cigarette smoking was defined as having smoked at least 100 cigarettes in one’s lifetime. Alcohol consumption was defined as consumption of ≥30 g of alcohol per week for 1 year or more. Leisure-time physical activity was defined as participation in ≥30 min of moderate or vigorous activity per day at least 3 days per week. A physical examination was performed on all the participants and included an assessment of body height, weight, blood pressure, pulses, and waist circumference. Body mass index (BMI) was calculated as weight in kilograms divided by the square of height in meters.
After an overnight fast ≥8 h, the Oral Glucose Tolerance Test (OGTT) using a 75 g glucose load was performed to measure fasting plasma glucose (FPG), 2 h-plasma glucose (2 h-PG), fasting serum insulin (FINS) and 2 h-serum insulin (2 h- INS).
Biochemical Parameter Measured
Plasma glucose levels were measured by using a hexokinase enzymatic method. Insulin was measured by a radioimmunoassay with human insulin as a standard (Linco, St. Charles, MO). Triglyceride (TG), total cholesterol (TC), high-density lipoprotein-cholesterol (HDL-C), low-density lipoprotein-cholesterol (LDL-C), aspartate aminotransferase (AST), alanine transaminase (ALT), and blood creatinine (Cr) values were measured for all the patients by using the automated standardized equipment from the Clinical Laboratory of West China Hospital, Sichuan University. Serum ferritin level was measured with a radioimmunoassay kit (Beijing North Institute of Biological Technology). Both at baseline and during the follow-up, the questionnaire, physical examination, OGTT, biochemical indexes, and serum ferritin level were recorded and measured. At baseline, homeostasis model assessment formula insulin resistance (HOMA-IR) was used to measure the insulin resistance as the equation,\( \mathrm{HOMA}-IR\kern0.50em = \left[\mathrm{FINS}\ \left(\frac{\mathrm{mIU}}{\mathrm{L}}\right)\times FPG\left(\frac{\mathrm{mmol}}{\mathrm{L}}\right)\right]/22.5 \), and homeostasis model assessment formula beta-cell function (HOMA-β) was used to measure the function of pancreatic β-cells as the equation \( \mathrm{HOMA}-\upbeta \kern0.5em = \left[20\times \mathrm{FINS}\ \left(\frac{\mathrm{mIU}}{\mathrm{L}}\right)\ \right]/\ \left[FPG\ \left(\frac{\mathrm{mmol}}{\mathrm{L}}\right)-3.5\right] \).
Statistical Analysis
Normally distributed data are expressed as means ± standard deviation (SD) for continuous variables and percentage for categorical variables. Skewed variables were logarithmically transformed before analysis and were expressed as median (interquartile range). The quantitative variables were compared using Student’s t test, while the qualitative variables were compared using the chi-square (χ 2) test. The association of serum ferritin levels at baseline with AGM risk was analyzed using a logistic regression model to calculate the relative risk (RR) and 95 % confidence interval (CI). The variables included in the logistic regression model were those that were statistically significant in univariate analyses or were biologically relevant. Values with p ≤ 0.05 were considered statistically significant. All the statistical analysis was performed using SPSS 16.0 (SPSS, Inc., Chicago, IL).
Results
Baseline and Follow-Up Characteristics
During 4-years follow-up, 243 non-AGM participants did not develop into AGM, and 140 non-AGM participants (91 women and 49 men) developed into AGM. The 140 subjects developed into AGM included 28 subjects with T2DM,15 subjects with IFG, and 107 subjects with IGT; there were 10 subjects developed into IFG and IGT at the same time.
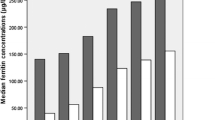
The characteristics of participants at baseline and after the follow-up were shown in Tables 1 and 2, separately. At baseline, compared with the participants who did not develop into AGM, participants who developed into AGM were older and the waist, BMI, systolic blood pressure (SBP), TG, FPG, 2 h-PG, FINS, 2 h-INS, and HOMA-IR were higher significantly both in females and males. The baseline serum ferritin levels were higher in men than the ferritin concentration in women. Moreover, the concentration of serum baseline ferritins were markedly higher in subjects who developed into AGT compared to those who remained NGT, 110.9 (124.8) vs. 63.9 (108.6) in women and 199.5 (169.2) vs. 150.8 (159.8) in men, separately. During the follow-up, the age, waist, BMI, SBP, and DBP were significantly higher and the levels of TG, FPG, and 2 h-PG increased significantly both in women and men who developed AGM compared with those who did not.
The Relationship Between Baseline Serum Ferritin Levels and AGM Risk in Females and Males
Table 3 showed the logistic regression analysis of the relationship between baseline serum ferritin levels and AGM risk in females and males, respectively. After adjusting the confounders of age, the family history of diabetes, current smoking, and alcohol, compared with the lower quartile of baseline serum ferritin levels, the females in the upper quartile of baseline serum ferritin levels had an increased trend of developing AGM [RR 2.86 (1.25–6.56), p = 0.013]. After adjusting the confounders of age, the family history of diabetes, current smoking, alcohol, and components of metabolic factors (waist circumference, SBP, TG, and HOMA-IR), compared with females in the lower quartile of baseline serum ferritin levels, the females in the upper quartile of baseline serum ferritin levels had no significant difference in the risk of developing AGM [RR 2.23 (0.94–5.30), p = 0.068].
For the males, the study results were consistent with those in females. In multivariable-adjusted models, the RRs for developing AGM compared subjects in the highest with those in the lowest quintile serum ferritin levels were 3.50 (p = 0.029) after adjusting age, the family history of diabetes, current smoking, and alcohol and 2.74 (p = 0.103) after adjusting age, the family history of diabetes, current smoking, alcohol, and components of metabolic factors (waist circumference, SBP, TG, and HOMA-IR).
In order to find the most effective factor of AGT, confounding factors, including ferritin, age, the family history of diabetes, current smoking, alcohol, waist circumference, SBP, TG, and HOMA-IR, were analyzed using logistic regression model (forward, conditional). Waist and SBP were included in the final equation of logistic regression in females. Unlike the result of logistic regression in females, serum ferritin, age, and waist appeared in the final equation of logistic regression in males.
Discussion
In this prospective cohort study, we found that incident AGM patients had higher baseline and follow-up serum ferritin levels than those in non-AGM subjects. Subjects with the upper quartile of baseline serum ferritin levels had the highest risk of developing AGM. These results indicated that elevated serum ferritin levels might be associated with the increased risk of AGM in Southwestern Chinese. However, after adjusting the age and components of metabolic factors (waist circumference, SBP, TG, and HOMA-IR), the subjects with the highest quartile of baseline serum ferritin levels did not have higher risk of developing AGM, which suggested that high serum ferritin levels might not be an independent factor for the occurrence of AGM.
It is widely recognized that the pathologically high level of serum ferritin is the major cause of AGM in hereditary hemochromatosis [13]. The excess iron in patients with hereditary hemochromatosis could reduce the insulin secretion by destroying pancreatic β cells and induce insulin resistance based on which deposited in liver and muscle tissues [13]. However, it is still unclear whether moderately increased ferritin level directly affects the AGM. From the aspect of cause and effect, although the extremely high level of ferritin contributes directly to the pathogenesis of AGM, it is highly possible that moderately elevated levels of serum ferritin only play a role in metabolic intermediate or a biomarker of metabolic abnormalities.
The mechanism of excess iron in hereditary hemochromatosis leading to AGM is summarized as above (elevated serum ferritin levels → AGM). However, many literatures have demonstrated that the abnormalities in metabolic factors, such as hyperlipidemia, obesity, and hypertension, may increase the level of serum ferritin (metabolic factors → elevated serum ferritin levels) [14, 15, 16]. The hyperinsulinemia in metabolic syndromes could interfere with iron metabolism by stimulating iron uptake and ferritin synthesis [14]. Oxidative stress also exists in metabolic syndromes, which induced insulin resistance of peripheral tissues and increases the ferritin mRNA expression [15]. Qin Tang found that serum ferritin level is correlated with higher waist circumference, SBP, HOMA-IR, and concentration of TG in Chinese males [16]. Based on these, the moderately elevated serum ferritin might be the result of metabolic syndromes and AGM or the metabolic intermediate for the occurrence of AGM.
Salonen et al. [17], who designed a case-control study, reported that men with the lowest quarter of the ratio of transferrin receptors to ferritin had higher risk of developing AGM [17]. Some case-control or cross-sectional studies found that AGM subjects had higher ferritin levels than those in non-AGM subjects [9, 18]. Jiang et al. [6] published the first follow-up study (a nested case-control study), explored the association between serum ferritin levels and T2DM risk [6], and confirmed the higher serum levels increased the T2DM risk [RR 2.68 (95 % CI, 1.75–4.11)].
A systematic review and meta-analysis summarized the published literatures about the association between body iron stores and T2DM risk [19]. Most of the literatures supported that the elevated body iron stores were significantly associated with increased risk of AGM. Some studies showed that gender difference might significantly influence on these results. Dekker et al. [20] and Shi et al. [21] found that only females who had higher ferritin levels had increased risk of developing T2DM while Le et al. [8] came out with the opposite conclusion. Jehn et al. [22] demonstrated when the components of metabolic factors and inflammatory indexes were not adjusted, a higher plasma ferritin level was significantly associated with increased T2DM risk [RR 1.74 (95 % CI, 1.14–2.65)]; however, the result was not significant when these factors were adjusted, which implicated that metabolic factors play an important role in association of serum ferritins levels and T2DM risk. They concluded that elevated ferritin might not be a causal factor for T2DM, but a biomarker of metabolic abnormalities related to the pathogenesis of T2DM [22], which is consistent with our findings.
Previously, many studies among the Chinese population were designed as cross-sectional or case-control studies, and their results indicated that the high ferritin levels could merely reflect the systemic inflammation rather than the cause of AGM [9, 22–28] and no one studies them in the community of Southwestern China. Our study investigated the association between serum ferritin and AGM in Southwestern Chinese population. In addition, the limitation of the present study, during the follow-up, many participants only received anthropometric measurements, but did not want to provide blood sample; thus, the development of AGM between visits was unknown. Due to the small study sample, the power to study the relationship between AGM and serum ferritin in males was low; prospective cohort studies with larger samples were needed to confirm the relationship between serum ferritin levels and AGM.
Conclusion
In a word, elevated serum ferritin levels are associated with AGM, but not an independent risk factor in Southwestern Chinese population. The higher levels of serum ferritin might play a metabolic intermediate part in the occurrence of AGM.
References
Himsworth HP (1935). Diet and the incidence of diabetes mellitus. Clin Sci 2: 117–48. doi:
Opara EC (2004) Role of oxidative stress in the etiology of type 2 diabetes and the effect of antioxidant supplementation on glycemic control. J Investig Med 52:19–23
Finch C (1994) Regulators of iron balance in humans. Blood 84:1697–1702
Merryweather-Clarke AT, Pointon JJ, Shearman JD, Robson KJ (1997) Global prevalence of putative haemochromatosis mutations. J Med Genet 34:275–278
Salomaa V, Havulinna A, Saarela O, Zeller T, Jousilahti P, Jula A, Muenzel T, Aromaa A, Evans A, Kuulasmaa K, Blankenberg S (2010) Thirty one novel biomarkers as predictors for clinically incident diabetes. PLoS One 5(4):e10100. doi:10.1371/journal.pone.0010100
Jiang R, Manson JE, Meigs JB, Ma J, Rifai N, Hu FB (2004) Body iron stores in relation to risk of type 2 diabetes in apparently healthy women. JAMA 91(6):711–717. doi:10.1001/jama.291.6.711
Rajpathak SN, Wylie-Rosett J, Gunter MJ, Negassa A, Kabat GC, Rohan TE, Crandall J, Diabetes Prevention Program (DPP) Research Group (2009) Biomarkers of body iron stores and risk of developing type 2 diabetes. Diabetes Obes Metab 11(5):472–479. doi:10.1111/j.1463-1326.2008.00985.x Epub 2009 Jan 21
Le TD, Bae S, Ed Hsu C, Singh KP, Blair SN, Shang N (2008) Effects of cardiorespiratory fitness on serum ferritin concentration and incidence of type 2 diabetes: evidence from the Aerobics Center Longitudinal Study (ACLS). Rev Diabet Stud 5(4):245–252. doi:10.1900/RDS.2008.5.245 Epub 2009 Feb 10
Wu H, Yu Z, Qi Q, Li H, Sun Q, Lin X (2011) Joint analysis of multiple biomarkers for identifying type 2 diabetes in middle-aged and older Chinese a cross-sectional study. BMJ Open 1(1):e000191. doi:10.1136/bmjopen-2011-000191
Sun L, Zong G, Pan A, Ye X, Li H, Yu Z, Zhao Y, Zou S, Yu D, Jin Q, Hu FB, Lin X (2013) Elevated plasma ferritin is associated with increased incidence of type 2 diabetes in middle-aged and elderly Chinese adults. J Nutr 143(9):1459–1465. doi:10.3945/jn.113.177808 Epub 2013 Jul 31
Yang WY, Lu JM, Weng JP, et al (2010) Prevalence of diabetes among men and women in China. N Engl J Med 362:1090–1101. doi:10.1056/NEJMoa0908292
Luepker R, Evans A, McKeigue P, Reddy K (2004) Cardiovascular survey methods. World Health Organization, Geneva
McClain DA, Abraham D, Rogers J, Brady R, Gault P, Ajioka R, Kushner JP (2006) High prevalence of abnormal glucose homeostasis secondary to decreased insulin secretion in individuals with hereditary haemochromatosis. Diabetologia 49:1661–1669. doi:10.1007/s00125-006-0200-0
Davis RJ, Corvera S, Czech MP (1986) Insulin stimulates cellular iron uptake and causes the redistribution of intracellular transferrin receptors to the plasma membrane. J Biol Chem 261:8708–8711
Bertelsen M, Anggard EE, Carrier MJ (2001) Oxidative stress impairs insulin internalization in endothelial cells in vitro. Diabetologia 44:605–613
Tang Q, Liu Z, Tang Y, Tan A, Gao Y, Lu Z, Wang Q, Chen Y, Wu C, Zhang H, Yang X, Mo Z (2015) High serum ferritin level is an independent risk factor for metabolic syndrome in a Chinese male cohort population. Diabetol Metab Syndr 7:11. doi:10.1186/s13098-015-0004-9. eCollection 2015
Salonen JT, Tuomainen TP, Nyyssönen K, Lakka HM, Punnonen K (1998) Relation between iron stores and non-insulin dependent diabetes in men case-control study. BMJ 317(7160):727. doi:10.1136/bmj.317.7160.727
Kim C, Cheng YJ, Beckles GL (2008) Inflammation among women with a history of gestational diabetes mellitus and diagnosed diabetes in the Third National Health and Nutrition Examination Survey. Diabetes Care 31(7):1386–1388. doi:10.2337/dc07-2362 Epub 2008 Mar 28
Zhao Z, Li S, Liu G, Yan F, Ma X, Huang Z, Tian H (2012) Body iron stores and heme-iron intake in relation to risk of type 2 diabetes: a systematic review and meta-analysis. PLoS One 7(7):e41641. doi:10.1371/journal.pone.0041641 Epub 2012 Jul 26
Dekker LH, Nicolaou M, van der A DL, Busschers WB, Brewster LM, Snijder MB, Stronks K, van Valkengoed IG (2013) Sex differences in the association between serum ferritin and fasting glucose in type 2 diabetes among south Asian Surinamese, African Surinamese, and ethnic Dutch: the population-based SUNSET study. Diabetes Care 36(4):965–971. doi:10.2337/dc12-1243 Epub 2012 Nov 19
Shi Z, Hu X, Yuan B, Pan X, Meyer HE, Holmboe-Ottesen G (2006) Association between serum ferritin, hemoglobin, iron intake, and diabetes in adults in Jiangsu, China. Diabetes Care 29(8):1878–1883. doi:10.2337/dc06-0327
Jehn ML, Guallar E, Clark JM, Couper D, Duncan BB, Ballantyne CM, Hoogeveen RC, Harris ZL, Pankow JS (2007) A prospective study of plasma ferritin level and incident diabetes the Atherosclerosis Risk in Communities (ARIC) Study. Am J Epidemiol 165(9):1047–1054. doi:10.1093/aje/kwk093
Luan de C, Li H, Li SJ, Zhao Z, Li X, Liu ZM (2008) Body iron stores and dietary iron intake in relation to diabetes in adults in North China. Diabetes Care 31(2):285–286. doi:10.2337/dc07-0921
Sun L, Franco OH, Hu FB, Cai L, Yu Z, Li H, Ye X, Qi Q, Wang J, Pan A, Liu Y, Lin X (2008) Ferritin concentrations, metabolic syndrome, and type 2 diabetes in middle-aged and elderly Chinese. J Clin Endocrinol Metab 93(12):4690–4696. doi:10.1210/jc.2008-1159 Epub 2008 Sep 16
Jiang F, Sun ZZ, Tang YT, Xu C, Jiao XY (2011) Hepcidin expression and iron parameters change in type 2 diabetic patients. Diabetes Res Clin Pract 93(1):43–48. doi:10.1016/j.diabres.2011.03.028 Epub 2011 Apr 21
Ren Y, Tian HM, Li XJ, Liang JZ, Zhao GZ (2004) Elevated serum ferritin concentrations in a glucose-impaired population and in normal glucose tolerant first-degree relatives in familial type 2 diabetic pedigrees. Diabetes Care 27(2):622–623. doi:10.2337/diacare.27.2.622
Chen T, Ren Y, Liu Y, Long Y, Zhang X, Yu H, Xu J, Yu T, Tian H (2010) Serum gamma-glutamyl transferase, ferritin and the risk of type 2 diabetes in women from a Chinese minority. Diabetes Res Clin Pract 90(3):352–357. doi:10.1016/j.diabres.2010.09.017
Chen T, Zhang X, Yu H, Ran X, Gao Y, Lu H, Xie X, Chen X, Ren Y, Shi J, Tian H (2012) The association of plasma free amino acids with liver enzymes in type 2 diabetic patients. J Endocrinol Investig 35(8):772–775
Acknowledgments
We give our best thank to the residents at Yulin and Longquan Community for their work on collecting demographic data and blood samples in this study. This study was supported by grants from the Chinese Medical Association Foundation and Chinese Diabetes Society (no. 07020470055).
Compliance with Ethical Standards
The authors declare that there is no conflict of interests regarding the publication of this paper. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Written informed consents were obtained from every subject before the study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Fangli Zhou and Zhuoxian Zhao contributed equally to this work
Rights and permissions
About this article
Cite this article
Zhou, F., Zhao, Z., Tian, L. et al. Association of Serum Ferritin Level with Risk of Incident Abnormal Glucose Metabolism in Southwestern China: a Prospective Cohort Study. Biol Trace Elem Res 169, 27–33 (2016). https://doi.org/10.1007/s12011-015-0393-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-015-0393-5