Abstract
Cadmium exposure may result in a variety of pulmonary diseases. No studies have evaluated tracheal damage or even whether cadmium intake can damage the respiratory tract. We evaluated the possible injuries caused by cadmium poisoning via intake into the respiratory tract and the possible effects of water pH in their genesis. Ninety male Wistar rats were divided into six groups (n = 15): GC5—received CdCl2 (400 mg/l) in the drinking water at an acidic pH of 5; GC7 received CdCl2 (400 mg/l) in the drinking water at an acidic pH of 7.0; GC8—received CdCl2 (400 mg/l) in the drinking water at an acidic pH of 8.0; GW5—received water at an acidic pH of 5.0; GW7—received water at an acidic pH of 7.0; GW8—received water at an acidic pH of 8.0. Animals were euthanized after 6 months. Samples of the trachea and lung were removed for histopathologic analysis. The animals exposed to cadmium presented with goblet cells in the trachea at an average rate of 65.83 cells/6 high-power fields (HPF), whereas unexposed animals showed 85.16 cells/6HPF (p = 0.012). Further, pulmonary emphysema was demonstrated in 71.43 to 100% of exposed cases, whereas the unexposed animals presented emphysema in 6.66 to 15.38% of cases (p < 0.001). The respiratory tract is a target for cadmium-related injuries after intake; however, the pH of the water did not influence the development of these lesions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cadmium has been included as a group 1 carcinogen since 1993, as a cause of lung cancer among workers who handle it, and there is a strong suspicion of it being related to the time and intensity of exposure (Terra Filho and Kitamura, 2006) High levels of cadmium have been found in tissue from patients with lung cancer and in smokers (Demir et al. 2014).
Exposure to cadmium can cause a variety of lung diseases, including emphysema, interstitial fibrosis, pneumonitis, and cancer (WHO 1992; Fortoul et al. 2005; Zhao et al. 2010).
The airway epithelium serves as a biological barrier that protects against the ingress of exogenous substances (Forti et al. 2010). Studies assessing the bronchial epithelium have shown a decrease in the number of non-ciliated bronchial cells and an increase in the number of dividing cells clones after 8 weeks of inhaled cadmium (Fortoul et al. 2005). Furthermore, exposure to cadmium chloride results in disruption of the respiratory epithelium barrier function at sub-cytotoxic concentrations (Forti et al. 2010). However, studies in the literature are restricted to evaluating the bronchial epithelium, and none have assessed the tracheal epithelium.
Cadmium is a metal that contaminates many foods, such as vegetables, cereals, and crustaceans (Olsson et al. 2002); thus, it can also infect humans without occupational exposure. Hence, there is a need for alternative and simple ways to remedy the toxicity caused by this element. No studies have evaluated whether ingested cadmium can damage the respiratory tract or whether this respiratory damage is largely influenced by the resultant change in the pH of drinking water by cadmium.
The objective of this study was to evaluate the injuries caused by cadmium poisoning via intake to the respiratory tract and the possible effects of the pH of the water in the genesis of these lesions.
Materials and methods
Animal experiment
We evaluated 90 male adult Wistar rats (Rattus norvegicus Albinus), weighing 200–250 g. The rats were divided into groups of four and placed in large rectangular boxes (measuring 49 × 34 × 16 cm) that could accommodate up to five adult rats.
The animals were maintained under a controlled temperature of 25 ± 2 °C, relative humidity of 50 ± 15% and a normal photoperiod (12–12 h light-dark cycles).
The cadmium source was cadmium chloride (CdCl2—Sigma Chemical Company, St. Louis, MO, USA) with a hydration level of at least 98% and water content of approximately 2.5 mol/mol. For 6 months, the animals were treated with CdCl2 in their drinking water on a daily basis at a concentration of 400 mg/L (adapted from Motta et al. 2004). The pH of the water was adjusted using hydrochloric acid or sodium hydroxide. The drinking water was changed three times a week to maintain the pH. Any wastewater containing cadmium was sent to the central reservoir of the UNOESTE and neutralized for disposal. The amount of water remaining in the rat troughs was measured every time the solution was changed to estimate the average intake for each animal. Additionally, the pH of the remaining water was measured to ensure that the pH remained the same.
The animals were divided into the following six groups: GC5—received CdCl2 (400 mg/l) in the drinking water at an acidic pH of 5; GC7 received CdCl2 (400 mg/l) in the drinking water at an acidic pH of 7.0; GC8—received CdCl2 (400 mg/l) in the drinking water at an acidic pH of 8.0; GW5—received water at an acidic pH of 5.0; GW7—received water at an acidic pH of 7.0; and GW8—received water at an acidic pH of 8.0. Animals from all groups received water and food ad libitum.
The animals in all groups were euthanized 6 months after the beginning of the experiment. Euthanasia was performed by intraperitoneal injection of thiopental (Syntec, USA) at a dose of 100 mg/kg (Paiva et al. 2005). Necropsy was performed, and samples of the trachea (proximal, medium, and distal) and each lobe of lung from each rat were removed for histopathologic analysis.
Histopathologic analysis
The tissue samples were fixed in 10% formalin (Chemical Kinetics, Brazil) for 24 h, processed with standard histological procedures, and paraffin embedded (Dynamic Analytical Reagents, Brazil). Sections with a 5 μm thickness were obtained and stained with hematoxylin-eosin (Dolles, Brazil) and PAS-Alcian blue staining (Merck, Germany) to evaluate the type of mucus (acid or basic) and quantification of goblet cells.
Histopathologic analysis was blinded and performed by an experienced observer using an optical microscope (NIKON Labophot, Japan). The following parameters were evaluated with the following respective scoring scheme: (1) trachea: interstitial inflammatory infiltrate (0 = absent, 1 = mild, 2 = moderate, and 3 = severe) and inflammatory cell-type present (polymorphonuclear and/or monuclear); tissue congestion (0 = absent, 1 = mild, 2 = moderate, and 3 = severe); hypertrophy of the muscles (0 = absent, 1 = present); non-neoplastic changes in the mucosa (atrophy and hyperplasia); dysplastic lesions (0 = absent, 1 = mild dysplasia, 2 = moderate dysplasia, and 3 = severe dysplasia) and benign and malignant neoplastic lesions (0 = absent and 1 = present); type of mucus (acid or basic); and the number of goblet cells counted in six high-power fields (HPF), 2HPF in each tracheal segment sampled; (2) lung: interstitial inflammatory infiltrate (0 = absent, 1 = mild, 2 = moderate, 3 = severe), type of inflammatory cells (polymorphonuclear cells and/or monuclears) and location (alveolar, interstitial, and peribronchial); tissue congestion (0 = absent, 1 = mild, 2 = moderate, 3 = severe); interstitial fibrosis (0 = absent, 1 = focal, 2 = diffuse); emphysema (0 = absent, 1 = present); dysplastic lesions (0 = absent, 1 = mild dysplasia, 2 = moderate dysplasia, 3 = severe dysplasia) and presence of benign and malignant neoplastic lesions (0 = absent, 1 = benign, 2 = malignant).
Data analysis
The data showed normality using the Kolmogorov-Smirnov test (p = 0.849), and the Levene test showed no homogeneity of variances (p = 0.024). To compare the groups, we used one-way ANOVA and Tukey’s test. All statistical tests were performed at a significance level of <0.05.
Results
Mortality
Five animals died during the course of our study (one rat each from groups GC7, GC8, and GW5 and two rats from group GW8). The cause of death for the animals in groups GC7 and GC8 was acute pulmonary edema, a complication that is associated with cadmium exposure (Järup and Akesson 2009). It was not possible to establish the cause of death for the rats from groups GW5 and GW8.
Water intake
The average water intake per animal per day was 57 ml for group GC5 (approximately 22.8 mg of cadmium), 55 ml for group GC7 (approximately 22 mg of cadmium), 52 ml for group GC8 (approximately 20.8 mg of cadmium), 60 ml for group GW5, 73 ml for group GW7, and 70 ml for group GW8. There was no significant difference between the groups with respect to the cadmium or water intake (p > 0.05).
Histopathologic analysis
Trachea
There were no signs of tissue congestion, non-neoplastic changes in the mucosa, dysplastic lesions, or benign or malignant neoplastic lesions in tracheal segments assessed in any of the groups.
There was no statistically significant difference among groups with respect to the presence of inflammation (p = 0.654) and epithelial metaplasia (p = 0.105) (Table 1).
The average number of goblet cells in each group is shown in Table 1. There were significant differences between groups GC7 and GW7 (p = 0.018), GC8 and GW7 (p = 0.026), and GW5 and GW7 (p = 0.040). When we evaluated the average number of goblet cells in groups exposed to cadmium (GC groups), it was 65 cells/6HPF, but in the non-exposed groups (GW groups), it was 85 cells/6HPF (p = 0.012). All goblet cells of the animals of all groups contained neutral mucus (Fig. 1).
Lung
There were no signs of non-neoplastic changes in the mucosa, dysplastic lesions, or benign or malignant neoplastic lesions in the lungs in all groups.
All animals in all groups showed mild congestion in the lungs. Further, all animals in all groups showed foci of mild or moderate peribronchial inflammation, with no significant difference regarding the degree (p = 0.135). Just a few animals (n = 9) showed foci of mild interstitial chronic inflammation and three animals showed bronchopneumonia.
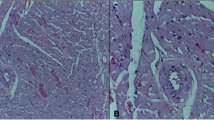
There was a statistically significant difference when comparing the groups exposed to cadmium vs. not exposed, for the presence of pulmonary emphysema (p < 0.001). There was a predominance of cases of pulmonary emphysema in animals exposed to cadmium (Fig. 2), but the animals in group GW8, which included animals that were exposed to water with a basic pH (pH 8.0), did not differ from the animals in GC groups (p > 0.05).
Light microscopy of the lung. a Normal lung parenchyma with mild congestion and normal bronchial area (single asterisk) (animal from group GW8). b Pulmonary emphysema. Note the dilation of the alveoli and the retraction of the septa forming small balls on the end of these (arrows) (animal from group GC7). Hematoxylin-eosin staining, ×200 magnification (23 μm bar for scale)
Discussion
Cadmium exposure by ingestion is the main source of contamination for the non-smoking population. Industrialization and increasing agricultural productivity in recent decades have led to an increase in cadmium in the environment. The soil can contain cadmium, and the use of phosphate fertilizers can increase these concentrations. Plants easily absorb cadmium, and the acidification of soil makes it more prone to absorbing cadmium (Olsson et al. 2002). In humans, the acidic or basic pH in which cadmium is carried may interfere with its absorption due to the fact that cadmium intestinal transporters also are cotransporters of H− and HCO3− ions (Waisberg et al. 2004). So the pH of ingested water could influence in the blood concentration of cadmium.
Others studies showed that pH of water can influence the cadmium injuries in some organs, such as stomach (Nai et al. 2015a), prostate (Nai et al. 2015b), and aorta (Nai et al. 2015c), so we wanted to assess whether a difference in pH would influence effects of cadmium in the respiratory tract. Nevertheless, there are lesser studies evaluating the toxicity of cadmium via intake than via inhalation.
The maximum daily intake of cadmium should be 1 μg/kg of body weight (WHO 1992). In the current study, animals were exposed to 400 mg of cadmium/l of water ingested, a value well above the permissible daily intake, therefore, simulating cases of environmental contamination on a large scale.
The lung is a target organ for various metals when inhaled. Due to the low solubility of metals in the pulmonary alveoli, these compounds remain in the lung parenchyma for a long time, thus allowing for the appearance of lesions (Demir et al. 2014). Cadmium may accumulate in the hair, nails, skin, and lungs by inhalation (Demir et al. 2014). At inhalation, the respiratory tract is the first to have contact with cadmium, but no study has evaluated whether the respiratory tract is the target for cadmium toxicity, independent of the route of exposure, or whether it is a target only when cadmium is inhaled. In the present study, we observed that the respiratory tract was the target for cadmium toxicity even when it was ingested, which shows that the respiratory tract is a target independent of the cadmium exposure route.
Previous studies have shown changes in the bronchial epithelium via inhalation exposure to cadmium (Fortoul et al. 2005; Chen et al. 2014), but this was the only area of the airways evaluated in the literature. Injuries of the tracheal epithelium may favor tracheobronchitis or even a pneumonic disease, so the importance of evaluating the tracheal epithelium is high. This study showed a decrease in the number of goblet cells in groups that were exposed to cadmium via water intake, although this effect was not demonstrated to be dependent on changes in the pH of the water. A previous study showed that cadmium has a cytotoxic effect on bronchial epithelial cells (Chen et al. 2014); this same effect may have occurred in the tracheal epithelium and may underlie the reduction in goblet cells. This decrease in the number of goblet cells could lead to a decrease in mucus production, and it is an important factor in protecting the respiratory epithelium against injury (Forti et al. 2010).
Cadmium inhalation can result in emphysema (Zhao et al. 2010). Cadmium exposure in rat lung fibroblasts increases the levels of metal scavenging thiol, e.g., the metallothionein (MT) and glutathione, and heavy chain γ-glutamylcysteine synthetase, which is a key enzyme for the biosynthesis of glutathione, with concomitant downregulation of lysyl oxidase, a copper-dependent enzyme for the cross-linking of collagen and elastin in the extracellular matrix. Cd downregulation of lysyl oxidase in treated cells was closely accompanied by suppression of synthesis of collagen, a major structure component of the lung extracellular matrix. Four to 6 weeks of instillation of cadmium chloride in an animal model resulted in a reduction of lysyl oxidase and collagen expression and increased MT and glutathione. After 6 weeks, there was emphysema formation (Zhao et al. 2010).
In another study, inhalation exposure to cadmium continued for 20 weeks and showed a significant increase in the secretion of metalloproteinases (MMPs) -2 (×3.5 more than in normal tissue) and increased expression of MT, especially that of isoforms, MT-1A and 2A, which are characteristics of cancer cells (Person et al. 2013). However, exposure via ingestion may also contribute to the formation of emphysema.
In this study, we observed diffuse emphysema formation in the groups that were exposed to cadmium via water intake, although it was not demonstrated to be dependent on changes in the water pH. This finding suggests that the enzyme and protein expression changes that occur in exposure to inhaled cadmium can also occur in exposure via ingestion, ultimately resulting in the formation of pulmonary emphysema. Probably, the exposure via intake has its action by a cadmium accumulation in the blood circulation. Lung is a highly vascularized organ, so the ingested cadmium can have a higher concentration in this organ.
Since 1950, there have been descriptions of lung, bladder, kidney, liver, gallbladder, and breast cancer due to chronic exposure to cadmium, and non-neoplastic changes can occur in these organs (Waalkes et al. 2003). Recently, a study showed that the blood levels of cadmium are associated with the stage of lung cancer, that is, the higher the stage of cancer is, the higher the level of blood cadmium is (Demir et al. 2014). In this study, there was no development or dysplastic lesions related to lung cancer, although these have mainly been demonstrated during chronic exposure to this heavy metal in higher concentrations, and previous studies have shown the development of dysplastic lesions in the stomach (Nai et al. 2015a) and prostate (Nai et al. 2015b) in chronic dietary exposure to cadmium. This lack might have occurred because only approximately 5% of the cadmium dose is absorbed from the gastrointestinal tract, but the pulmonary absorption is approximately 90% of the inhaled dose (Godt et al. 2006); maybe for the development of lung neoplasia, a greater burden of this heavy metal is required.
More studies with exposure for longer periods and further analysis focusing oxidative stress are needed to better assess the damage caused by cadmium exposure on the tracheal epithelium. Also, more studies are needed to evaluate the possible influence of the pH of the water on the toxicity of cadmium in the respiratory tract.
Conclusion
Our findings indicate that the respiratory tract is a cadmium target in chronic exposure via ingestion, but the pH of the water did not influence the development of these lesions.
Abbreviations
- CdCl2 :
-
cadmium chloride
- H− ions:
-
hydrogen ions
- H:
-
hour
- HCO3−ions:
-
bicarbonate ions
- HPF:
-
high-power field
- MMPs:
-
metalloproteinases
- MT:
-
metallothionein
- PAS:
-
periodic acid Schiff
- pH:
-
hydrogen potential
References
Chen DJ, Xu YM, Du JY, Huang DY, Lau AT (2014) Cadmium induces cytotoxicity in human bronchial epithelial cells through upregulation of eIF5A1 and NF-kappaB. Biochem Biophys Res Commun 445(1):95–99. doi:10.1016/j.bbrc.2014.01.146
Demir N, Enon S, Turksoy VA, Kayaalti Z, Kaya S, Cangir AK, Soylemezoglu T, Savas I (2014) Association of cadmium but not arsenic levels in lung cancer tumor tissue with smoking, histopathological type and stage. Asian Pac J Cancer Prev 15(7):2965–2970
Forti E, Bulgheroni A, Cetin Y, Hartung T, Jennings P, Pfaller W, Prieto P (2010) Characterisation of cadmium chloride induced molecular and functional alterations in airway epithelial cells. Cell Physiol Biochem 25(1):159–168. doi:10.1159/000272060
Fortoul TI, Saldivar LO, Espejel-Maya G, Bazarro PN, Mussaligalante P, Avila-Casado MC, Colin-Barenque L, Avila-Costa MR (2005) Inhalation of cadmium, lead or its mixture. Effects on the bronchiolar structure and its relation with metal tissue concentrations. Environ Toxicol Pharmacol 19:329–334
Godt J, Scheidig F, Grosse-Siestrup C, Esche V, Brandenburg P, Reich A, Groneberg DA (2006) The toxicity of cadmium and resulting hazards for human health. J Occup Med Toxicol 1:22. doi:10.1186/1745-6673-1-22
Järup L, Akesson A (2009) Current status of cadmium as an environmental health problem. Toxicol Appl Pharmacol 238:201–208. doi:10.1016/j.taap.2009.04.020
Motta ACF, Migliari DA, Gioso MA, Komesu MC, Sala MA, Lopes RA (2004) The carcinogenic potential of cadmium in the palatal and gingival epithelium of rats: a morphologic and morphometric analysis. Braz J Vet Res Anim Sci 41:183-188.
Nai GA, Gonçalves Filho MA, Estrella MPS, Teixeira LDS (2015a) Study of the influence of the pH of water in the initiation of digestive tract injury in cadmium poisoning in rats. Toxicol Rep 2:1033–1038. doi:10.1016/j.toxrep.2015.07.012
Nai GA, Golghetto GM, Estrella MPS, Teixeira LDS, Moura FC, Bremer Neto H, Parizi JLS (2015b) Influence of pH of water in the genesis of cancer in cadmium poisoning: an experimental study in rats. Histol Histopathol 30:61–67
Nai GA, Golghetto JJ, Estrella MP, Alves JA, Garcia LA (2015c) pH dependence of cadmium-contaminated drinking water on the development of cardiovascular injury in Wistar rats. Biol Trace Elem Res 165(1):81–85. doi:10.1007/s12011-014-0216-0
Olsson IM, Bensryd I, Lundh T, Ottosson H, Skerfving S, Oskarsso NA (2002) Cadmium in blood and urine—impact of sex, age, dietary intake, iron status, and former smoking—association of renal effects. Environ Health Perspect 110:1185–1190
Paiva FP, Mafilli VV, Santos ACS (2005) Course handling of laboratory animals. Fundação Osvaldo Cruz. Centro de Pesquisas Gonçalo Muniz. 2005. Available from: http://www.bioteriocentral.ufc.br/arquivos/apostilha_manipulacao.pdf. Accessed 10 Nov 2014
Person RJ, Tokar EJ, Xu Y, Orihuela R, Ngalame NN, Waalkes MP (2013) Chronic cadmium exposure in vitro induces cancer cell characteristics in human lung cells. Toxicol Appl Pharmacol 273(2):281–288
Terra Filho M, Kitamura S (2006) Occupational lung cancer. J Bras Pneumol 32 (Supl 1): S60-S68.
Waalkes MP (2003) Cadmium carcinogenesis. Mutat Res 533:107–120
Waisberg M, Black WD, Waisberg CM, Hale B (2004) The effect of pH, time and dietary source of cadmium on the bioaccessibility and adsorption of cadmium to/from lettuce (Lactuca sativa L. cv. Ostinata). Food Chem Toxicol 42:835–842
WHO. World Health Organization (1992) Environmental Health Criteria, Cadmium. 1st ed. World Health Organization, Geneva, Switzerland, vol. 134.
Zhao Y, Chen L, Gao S, Toselli P, Stone P, Li W (2010) The critical role of the cellular thiol homeostasis in cadmium perturbation of the lung extracellular matrix. Toxicology 267(1–3):60
Acknowledgments
The authors thank Edvaldo Mamede dos Santos for solution preparation. L. M. M. Queiroz is a fellow of the Institutional Program for Scientific Initiation Scholarships of the National Council for Scientific and Technological Development (PIBIC/CNPq).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflicts of interest.
Ethical approval
The Ethics Committee on Animal Use at the Universidade do Oeste Paulista (UNOESTE) approved this study (Protocol 2459). All procedures performed in this study involving animals were in accordance with the ethical standards of the institution or practice at which the study was conducted.
Rights and permissions
About this article
Cite this article
Nai, G.A., Marin, F.F., Queiroz, L.M.M. et al. Respiratory tract cadmium-induced injuries—poisoning via intake and water pH could influence their genesis? An experimental study in rats. Comp Clin Pathol 26, 997–1002 (2017). https://doi.org/10.1007/s00580-017-2474-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00580-017-2474-7