Abstract
Whether advanced biological waste treatment technologies, such as hydrothermal pretreatment (HTP) integrated anaerobic digestion (AD), could enhance the removal of different antibiotics remains unclear. This study investigated the outcome of antibiotics and methane productivity during pig manure treatment via HTP, AD, and HTP + AD. Results showed improved removal efficiency of sulfadiazine (SDZ), oxytetracycline (OTC), and enrofloxacin (ENR) with increased HTP temperatures (70, 90, 120, 150, and 170 °C). OTC achieved the highest removal efficiency of 86.8% at 170 °C because of its high sensitivity to heat treatment. For AD, SDZ exhibited resistance with a removal efficiency of 52.8%. However, OTC and ENR could be removed completely within 30 days. When HTP was used prior to AD, OTC and ENR could achieve complete removal. However, residual SDZ levels reduced to 20% and 16% at 150 and 170 °C, respectively. The methanogenic potential showed an overall upward trend as the HTP temperature increased. Microbial analysis revealed the antibiotics-induced enrichment of specific microorganisms during AD. Firmicutes were the dominant bacterial phylum, with their abundance positively correlated with the addition of antibiotics. Methanobacterium and Methanosarcina emerged as the dominant archaea that drove methane production during AD. Thus, HTP can be a potential pretreatment before AD to reduce antibiotic-related risks in manure waste handling.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The conventional practices of livestock husbandry have evolved into intensive livestock production systems as the demand for animal protein increases worldwide, particularly in developing countries [1]. Using antibiotics as feed additives has been proved to be an effective means to boost livestock production by stimulating growth and preventing disease infections [2]. The most commonly detected antibiotics in pig manure include sulfadiazine (SDZ), oxytetracycline (OTC), and enrofloxacin (ENR) [3, 4]. For example, in 2013, the use of ENR was more than 3000 tons in Chinese pig farms [5]. Studies have shown that a large proportion of the fed antibiotics, ranging from 30 to 90%, are excreted via the urine and feces of the livestock because of animals’ limited digestive capability [4, 6, 7]. Their detected antibiotics in pig manure can reach as high as 235.1 mg/kg SDZ, 59.06 mg/kg OTC, and 33.26 mg/kg ENR, respectively [8, 9]. The presence of residual antibiotics in livestock manures can exacerbate the spread of antibiotic resistance genes across environmental media, posing unforeseeable risks to human health [10]. At the same time, antibiotics can affect the microbial activity and disrupt the normal operation during the pig manure treatment process. Previous report that OTC reduced the role of some microbial bacteria (such as Clostridium sp. and Corynebacterium) in nitrogen conversion during aerobic treatment of pig manure [11]. Thus, seeking effective means for mitigating the spread of residual antibiotics during the disposal and handling of animal manure generated from the livestock farming industry is necessary.
As a sustainable alternative for biogas production, anaerobic digestion (AD) can reduce certain antibiotic residuals in animal manure. Under anaerobic conditions, antibiotics can be eliminated from the system through degradation, adsorption, volatilization, and hydrolysis [12]. The effects of AD on antibiotic removal are not always consistent. According to a study, the removal efficiency of tetracycline in fecal liquid is approximately 29% [13]. By contrast, erythromycin exhibits highly effective degradation with a removal efficiency of 99% within 40 days of pig manure AD under thermophilic conditions [14]. The inconsistent effects of AD on antibiotics may be related to antibiotics in raw materials and digestion conditions, such as temperature, inoculum, and residence [15]. Nevertheless, most antibiotics remaining in raw livestock and poultry manure can inhibit microbial activities during AD, compromising the efficiency of the entire AD system [16]. For instance, Hu et al. [17] reported that at a concentration of 0.24 mg/L, sulfamethazine reduces methane production by 48% during sludge anaerobic fermentation. Thus, a comprehensive approach along the process is necessary because AD is inefficient in removing antibiotics completely.
Hydrothermal pretreatment (HTP) involves the partial degradation of raw biomass materials in saturated steam under high temperature and pressure; thus, the hydrolysis of dissolved macromolecular organic matter is ultimately enhanced [18]. HTP not only reduces the hydrolysis half-life of antibiotics by increasing the pretreatment temperature but also demonstrates effective removal capabilities for certain antibiotics, such as tetracyclines, penicillins, erythromycins, and sulfonamides [19]. When used as a pretreatment process for AD feedstock, HTP can intensify methane production and shorten digestion time [20]. Previous study has shown that HTP can effectively remove high concentrations of oxytetracycline (OTC) and its intermediates in fermentation residue [21]. All these works emphasize the beneficial effects of HTP on antibiotic degradation and the improvement of the methanogenic performance during subsequent AD.
However, very few studies were conducted on antibiotic degradation in pig manure through the integration of HTP with AD. Therefore, the present study aims to investigate the removal efficiencies of common antibiotics, including sulfadiazine (SDZ), OTC, and enrofloxacin (ENR), during HTP, AD, and HTP + AD of pig manure. Furthermore, the response of methane production and microbial community to different antibiotics and HTP temperatures were evaluated.
Materials and Methods
Raw Materials
Samples of pig manure, sourced from breeding practices that did not involve the use of antibiotics, were collected from the Changping Base of Animal Husbandry Research Institute, Chinese Academy of Agricultural Sciences. The total solid (TS) content of the raw pig manure sample was approximately 35%. The sample was diluted and stirred using a blender for 5 min to create a slurry. The initial feeding stock of AD had a TS of 21.6 ± 0.1%. The prepared pig manure sample was stored at 4 °C and used for subsequent HTP and AD. The inoculum sludge, sampled from a full-scale AD reactor at the Beijing Drainage Group, was fed into the lab-scale AD reactor along with pig manure at a dose of 0.75 gVS/L. The mixture was incubated under 37 °C for 10 days. The characteristics of the pig manure and the inoculum are described in Table 1.
Preparation of Pig Manure Containing Antibiotics
Different concentrations of OTC, SDZ, and ENR were manually dosed into the antibiotic-free pig manure samples to prepare of pig manure samples containing antibiotics. The doses of these three antibiotics were estimated based on the doses administered for disease treatments in pigs. The daily drug concentration (C) in fresh manure of each pig was calculated based on Eq. (1), with the assumptions that 1 in 10 pigs received antibiotics every day, each pig weighs 70 kg, and the volatile solids (VS) of the fresh manure was 16 ± 0.3%. The typical doses administered included 15 mg/kgbody weight twice a day for OTC, 25 mg/kgbody weight once a day for SDZ, and 2.5 mg/kgbody weight twice a day for ENR. Therefore, M was 2100 mg OTC, 1750 mg SDZ, and 350 mg ENR for each pig. The proportion of drugs excreted was assumed to be 60%, and the daily excretion coefficient per pig was 3.95 kg/head·day.
where C represents the drug concentration in fresh manure of each pig (mg/L), M represents the total daily dose per pig (mg/head·day), P implies the proportion of drug excreted (%), E means the excretion coefficient (kg/head·day), k represents the VS of the fresh manure, R represents the pig manure added quality (gVS), and V represents the working volume (L).
Experimental Design and Operation
HTP Setup and Operation
The HTP of the prepared pig manure was conducted in a reaction kettle with a working volume of 1 L. Heat transfer oil was used as the medium to conduct heat to the reaction kettle. In each reaction kettle, 0.5 L of pig manure (Table 1) containing a mix of antibiotics, including 2.39 mg/L SDZ, 2.88 mg/L OTC, and 0.47 mg/L ENR, was added. Subsequently, the pig manure with mixed antibiotics (MIX) was hydrothermally pretreated for 30 min at 70, 90, 120, 150, and 170 °C in triplicate. The HTP-pretreated pig manure samples were placed in a cold tank for forced cooling before being analyzed for TS, VS, and the residual concentrations of OTC, SDZ, and ENR. The remaining pretreated samples were used as the feedstock for the subsequent AD experiments.
AD Setup and Operation
AD experiments were performed in 100-mL bottles, with each bottle having a working volume of 80 mL. Inoculum sludge and pig manure were mixed and loaded into the bottles with a sludge-to-pig manure ratio of 0.5 based on VS. Then, each bottle was sealed by a rubber stopper with an outlet for biogas collection. Five experimental groups were established, with each group having a distinct addition of antibiotics: control group (CK; without antibiotics), SDZ group (2.39 mg/L), OTC group (2.88 mg/L), ENR group (0.47 mg/L), and MIX group (2.39 mg/L SDZ, 2.88 mg/L OTC, and 0.47 mg/L ENR). In addition, a blank group only with inoculum was prepared to provide a baseline. All six groups were incubated at 37 ± 1 °C for 30 days in triplicate. The changes in antibiotic concentrations during AD were monitored every 5 days, and the collected biogas were analyzed for methane content and yield determination.
HTP-Enhanced AD Setup and Operation
For the HTP and AD integrated study, the pig manure containing MIX from the “HTP Setup and Operation” section was used as raw material. Moreover, six experimental groups were created, with each group having a distinct temperature for HTP, i.e., CK without HTP and the experimental groups with HTP operated according to aforementioned methods at 70, 90, 120, 150, and 170 °C, respectively. The effluent from all six groups was incubated at 37 ± 1 °C for 30 days in the AD bottles described above in triplicate. In addition, a blank group only with inoculum was prepared to serve as the baseline.
Analytical Methods and Statistical Analysis
Physicochemical Analysis
The analyses of TS and VS were conducted in accordance with the standard method of the American Public Health Association [22]. The pH measurements were performed using a pH meter (FE28, Mettler-Toledo, Switzerland). The biogas production throughout AD was continuously monitored using glass syringes. The methane content was determined using an SP-2100 gas chromatograph equipped with a Φ10-m × 2-mm stainless steel column. The parameters used for methane detection were as follows: sample volume injection, 0.5 mL; nitrogen partial pressure, 0.6 MPa; flow rate, 60 mL/min; inlet temperature, 150 °C; column temperature, 230 °C; detector temperature, 150 °C.
Quantification of Antibiotics
The antibiotics were quantified using the online solid phase extraction combined with high-performance liquid chromatography (HPLC) and UV detection. The HPLC system (Dionex Ultimate U3000, Sunnyvale, USA) was equipped with a UV detector and an Acclaim C18 (5 μm, 4.6 mm × 250 mm) column. It used acetonitrile and 0.1% oxalate solution as the mobile phase operated at a flow rate of 0.8 mL/min. The liquid samples were filtered through 0.45-μm water filter membranes. Phosphoric acid was added to adjust the pH level within the range of 2 to 3, followed by filtering adjusted samples through 0.22-μm filter membranes. The solid samples were freeze-dried at − 80 °C for 10 h with acetonitrile + Na2EDTA-phosphate buffer solution (pH 3) with a volume ratio of 1. The supernatant was extracted and filtered through 0.45-μm organic filter membranes. Phosphoric acid was added to adjust the pH level within the range of 2 to 3. The antibiotics were quantified by the external standard method.
Microbial Community Structure Analysis
The samples for DNA extraction were collected on day 30 of the AD batch tests. The DNA extraction was performed using the E.Z.N.A. Soil DNA Kit (Omega Bio-Tek, USA). The DNA purity and concentration were analyzed using NanoDrop2000 and TBS-380. The DNA integrity was measured using 1% agarose gel electrophoresis operated at 5 V/cm for 20 min. An M220 ultrasonic crusher (Covaris, Woburn, MA, USA) was employed to crush the DNA into fragments with approximately 400 bp for PCR amplification and sequencing, which were completed by Shanghai Majorbio Bio-pharm Technology Co., Ltd. The PCR amplification was performed using an ABI Gene Amp 9700 PCR thermocycler (ABI, CA, USA). The 16S rRNA gene regions of archaea and bacteria were used for PCR-DGGE analysis. The bacterial 16S rRNA gene was amplified with specific primers 338F (5′-ACTCCTACGGGAGGCAGCAG-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′), whereas 524F10extF (5′-TGYCAGCCGCCGCGGTAA-3′) and Arch958RmodR (5′-YCCGGCGTTGAVTCCAATT-3′) were used for the archaea 16S rRNA gene. The PCR reactions were performed using 4 μL of 5 × FastPfu Buffer, 2 μL of 2.5 mM dNTPs, 5 μM of forward and reverse primers, approximately 10 ng of template DNA, and ddH2O to achieve a total volume of 20 μL. The thermal cycle of the PCR process consisted of the following steps: initial denaturation at 95 °C for 3 min, denaturation at 95 °C for 30 s, annealing at 55 °C for 30 s, extension at 72 °C for 45 s repeated for 30 cycles, and a final holding at 72 °C for 10 min. The PCR products were detected using 2% agarose gel electrophoresis and quantified using the QuantiFluor-ST Blue fluorescence quantification system (Promega, USA). Then, sequencing was performed on the Illumina MiSeq platform (Illumina, San Diego, USA). The results were analyzed using the Majorbio Cloud platform (https://www.majorbio.com).
Calculation of the Contribution Rate
The calculation of the contribution rate of AD and HTP to the antibiotic removal is as follows:
where I represents the initial antibiotic concentration (mg/L), AHT represents the antibiotic concentration after HTP (mg/L), AAD represents the antibiotic concentration after AD (mg/L), HR implies the contribution rate of HTP to the antibiotic removal (%), AR means the contribution rate of AD to the antibiotic removal (%), and PR is the percentage of antibiotic residue (%).
The Modified Gompertz Model
As demonstrated in a previous study by Yin et al. [4], the modified Gompertz model has been proved to be a well-established kinetic model for methane production during AD, which is shown as follows:
where MP represents the cumulative methane yield (mL/gVS), t represents the digestion time (days), P0 implies the ultimate maximum methane yield (mL/gVS), Rmax means the maximum methane production rate (mL/gVS·day), and λ is the lag phase of gas production (days).
Statistical Analysis
All experiments in this study were conducted in triplicate. Microsoft Office Excel 2019 was used for data organization, analysis, and graphical representation. The significant difference of different treatments was compared using SPSS 18.0, and the p-value was considered statistically significant at p < 0.05.
Results and Discussions
Antibiotic Removal During HTP and AD
Effect of HTP on Antibiotic Removal
As depicted in Fig. 1, SDZ, OTC, and ENR in pig manure exhibited notable reduction after HTP, and the removal efficiency of SDZ, OTC, and ENR showed a positive correlation with HTP temperature. In particular, the removal efficiency of OTC (86.82%) was higher than that of ENR (64.65%) and SDZ (67.23%) under an HTP temperature of 170 °C. This finding indicates that the resistance of SDZ and ENR to high HTP temperatures was greater than that of OTC. However, the removal efficiency of SDZ and ENR remained mostly unchanged within the 70–120 °C range. Moreover, their removal efficiencies increased dramatically beyond 150 °C. These findings are consistent with previous reports indicating that OTC can be effectively removed as the HTP temperature increases. For instance, the OTC concentration decreases from 3.9 mg/g to below the detection limit when the HTP temperature is 130 °C for 5 min [23]. Most antibiotics are sensitive to heat and even degrade at temperatures below 100 °C; however, certain antibiotic classes, such as aminoglycosides, chloramphenicol, and tetracycline, can tolerate temperatures exceeding 100 °C [24]. A previous study showed that with an initial concentration of 50 ng/g, ENR can remain stable for 3 h when subjected to heating at 100 °C [25]. Thus, OTC can probably be effectively removed by HTP. However, SDZ and ENR exhibited a certain degree of resistance to degradation at low HTP temperatures.
Effect of AD on Antibiotic Removal
The removal of single antibiotics in pig manure during AD is shown in Table 2. The OTC and ENR concentrations sharply decreased on day 15 and day 5, respectively. However, the SDZ concentration reduced slowly, and the removal efficiency of SDZ remained unchanged at 52.72% starting from day 30 of AD. These findings are aligned with previous studies showing that OTC and ENR are more effectively removed than SDZ during AD [26]. In addition, an SDZ concentration of 1 mg/L manure essentially remained unchanged during AD for 40 days [14]. Cheng et al. [27] also emphasized that SDZ is slightly susceptible to degradation during the AD of pig manure, despite achieving sufficient degradation. Therefore, the resistance of SDZ to degradation during AD tends to be greater than that of OTC and ENR.
The liquid and solid fractions of digestate during AD were assessed to understand the reduction performance of antibiotics. The degradation dynamics of MIX, including SDZ, OTC, and ENR, during AD is illustrated in Fig. 2. Among these antibiotics, SDZ exhibited the lowest removal efficiency of 41.65% after 30 days of AD (Fig. 2a). SDZ was predominately distributed in the liquid fraction rather than in the solid fraction, and it was detected only in the solid fraction on day 5. The SDZ concentration in the liquid fraction was reduced by 41.31% during the first 20 days. This reduction was followed by a much slower decline, with the removal efficiency stabilizing at 41.65% until the end. OTC was detected only in the solid fraction on day 5, and the removal efficiency of OTC in the liquid fraction reached 100% by day 20 (Fig. 2b). ENR was detected only in the liquid fraction and was immediately removed with the concentration dropping below the detection limit on day 5 (Fig. 2c). Compared with the degradation of single antibiotics, that of the mixed antibiotics did not substantially impact the ENR removal (Table 2). By contrast, the complete removal of OTC in the presence of mixed antibiotics occurred 5 days later than that in the OTC-only environment. Similarly, the removal of SDZ in the presence of mixed antibiotics was slower than that in the presence of a single SDZ. The above results suggest that the presence of MIX had an inhibition effect on the removal of individual antibiotics. The reason is that the antibacterial properties of antibiotics affected the anaerobic microorganisms in the digestive system, possibly inhibiting the bio-removal and fermentation efficiency of organic matter [28]; this phenomenon may have ultimately affected the removal of antibiotics.
Effects of HTP Integration with AD on Antibiotic Removal
As discussed in the “Effect of HTP on Antibiotic Removal” and “Effect of AD on Antibiotic Removal” sections, the previous results demonstrated that OTC, SDZ, and ENR exhibited varying degrees of degradation during standalone HTP and AD. SDZ could not achieve the ideal treatment efficiency during HTP at 70–120 °C, with the contribution rate of HTP ranging only from 22.36 to 24.87% (Table 3). During the subsequent AD, the residual SDZ was further degraded, with the removal efficiency reaching 50.67% to 51.73% with 70–120 °C HTP. However, the contribution rate of HTP for SDZ removal was up to 55.34% when the HTP temperature increased to 150 °C. The degradation efficiency of SDZ during AD also increased to 80.07% because of HTP enhancement. The removal efficiency increased to 83.75% (Fig. 3) when the HTP temperature was further increased to 170 °C. Ultimately, the residual SDZ was reduced to 0.48 and 0.39 mg/L at 150 and 170 °C HTP, respectively (Table 3). Given the energy consumption and economic cost, HTP at 150 °C appears to be appropriate for removing SDZ from the subsequent AD. On the contrary, HTP demonstrated a pronounced degradation effect on OTC (2.88 mg/L), with the contribution rate of HTP ranging from 69.99 to 86.82%. After 30 days of AD, OTC was finally almost complete degraded during AD, followed with the HTP being operated at 70, 90, 120, 150, and 170 °C, respectively. Under the condition of MIX, ENR could reach levels below the detection limit in AD (CK). Moreover, the treatment efficiency of AD was superior to that of HTP. These findings are aligned with those in the “Effect of HTP on antibiotic removal” and “Effect of AD on Antibiotic Removal” sections. In conclusion, the effect of HTP integration with AD on antibiotic removal surpassed that of either a standalone process for SDZ or MIX.
Methane Production
Biochemical Methane Potential (BMP) of Pig Manure Containing Antibiotics
The pig manure samples, whether containing individual or mixed antibiotics, were subjected to BMP tests. The methane production results are presented in Table 4. The R2 values fitted from the modified Gompertz model were all greater than 0.95, indicating a decent fit of the model to the AD experiment. In line with the modeled results, the cumulative methane production of the OTC group (380.23 mL/gVS) and the ENR group (390.03 mL/gVS) was 1.1% to 3.7% higher than that of CK (376.11 mL/gVS). The ENR group exhibited a superior methanogenic potential, which could be attributed to the low ENR presence during AD. Zhi et al. [29] reported that the cumulative methane production under minimal antibiotic conditions is significantly higher than that under conditions without antibiotic presence. Their report indicated that sulfadimethoxine can stimulate the growth of Methanosarcina, and this finding may be the reason for the strong stimulating effect of sulfadimethoxine on methane yield. Yin et al. [4] found a strong positive correlation between antibiotic reduction and methane production for ENR. However, opposite results in methane production were observed in the SDZ and MIX groups, with cumulative methane production decreasing by 2.44–5.92% (Table 4). This observation may be attributed to the inhibition effect because of the high levels of antibiotic residues present in the SDZ and MIX groups during AD. This finding is aligned with the study by Wu et al. [30]. They reported that antibiotics can reduce biogas production during AD primarily by inhibiting the activity of methanogenic bacteria. The study demonstrated that mixed antibiotics (OTC + tetracycline + tylosin) exhibited the highest inhibition effect of up to 76% on specific methanogenic activity, resulting in the most significant decrease in methane production (− 56%) [31]. Therefore, OTC and ENR may promote the production of methane during AD, whereas SDZ and MIX may exhibit inhibitory effects on methanogenesis.
According to previous report, antibiotics have various interference effects on anaerobic digestion, such as the accumulation of organic acids, inhibition of biogas production, and imbalance of microbial communities [32]. Most antibiotics can inhibit methane production and methanogenesis rates at low concentrations. Sulfamethazine at 0.24 mg/L promoted the accumulation of VFAs by 73.2% mainly by increasing the content of acetic acid, while reducing methane production by 48% [33]. Moreover, the combined effect of multiple antibiotics also inhibited methane production from anaerobic digestion. However, a few antibiotics (e.g., oxytetracycline) can have a positive effect on AD by increasing methane production. The 100 mg/L OTC antibiotic largely improved the CH4 yield during the high solid anaerobic digestion [34]. The effects of antibiotics on CH4 production might be due to the following. On the one hand, some antibiotics, as organic substrates, are easily decomposed and metabolized by active microorganisms relating to AD during biological processes. This phenomenon positively affects methane production. On the other hand, given their broad-spectrum activity and proven ability to destroy and inhibit a wide range of bacteria, some antibiotics can adversely affect AD microbial activity. Consequently, some antibiotics adversely affect the AD methane production to some extent [4]. This study shows that the methane yield can be enhanced with the high removal efficiency antibiotics in the AD process for OTC and ENR. The opposite result was obtained for SDZ and MIX. The antibiotic reduction was strongly correlated with the effect of antibiotic on AD methane production. It is possible that high antibiotic degradation efficiency reduces the inhibitory effect of antibiotics on microorganisms and increases methane production. Thus, the high removal efficiency of antibiotics may be increasing the methane production. However, the effect of antibiotics on the methanogenic potential of AD also depends on the type and concentration of antibiotics.
BMP of Pig Manure Containing Antibiotic via HTP
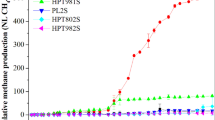
In this section, the effect of coupling HTP with AD on methane production was assessed. Figure 4 shows the methanogenic potential predicted by the modified Gompertz model and the methanogenic rate curves under different HTP temperatures (R2 > 0.95). As shown in Table 5, the methanogenic potentials of pig manure containing mixed antibiotics in CK and groups with different HTP temperatures (70, 90, 120, 150, and 170 °C) was 348.76, 348.79, 362.95, 376.63, 380.83, and 379.91 mL/gVS, respectively. There was no significant difference between the control and the 70 °C HTP group (p > 0.05). The same was found in 150 °C and 170 °C HTP groups. However, the significant differences were found in the groups with 90–150 °C HTP temperatures (p < 0.05). The results are in consensus with the findings regarding the methanogenic potentials in the “Biochemical Methane Potential (BMP) of Pig Manure Containing Antibiotics” section. As the HTP temperature increased, the methanogenic potential of each group showed an overall upward trend. These results confirmed the substantial enhancing effect of HTP on the AD of pig manure under specific conditions [35]. Huang et al. [36] also reported that even a relatively lower temperature (110 °C) in HTP can improve the AD of pig manure by 34% more CH4 production. Additionally, Awad et al. [23] showed that the BMP cumulative methane productions over 23 days of AD was 73.7, 215.9, 656.8, and 439.0 mL CH4/gVS when the raw feedstock was pretreated at 110, 130, 150, and 170 °C for 5 min, respectively. For the groups with HTP temperatures less than or equal to 150 °C, the time required to reach the maximum methanogenesis rate was approximately 6 days. For the group with an HTP temperature of 170 °C, the time required was extended to approximately 10 days. The group with an HTP temperature of 150 °C exhibited the highest methane production rate (34.53 mL/gVS·day) among all comparative groups (p < 0.05). This observation aligns with the above findings of the high degradation efficiency of SDZ in HTP (150 °C) coupled with AD (“Effects of HTP Integration with AD on Antibiotic Removal” section). HTP at high temperatures (> 170 °C) may lead to the creation of chemical bonds and result in particle agglomeration [37]. One of the most known phenomena is the Maillard reaction. The Maillard reaction is a complex chemical reaction that carbonyl compounds (e.g., reducing sugar) reacts with the amino group of proteins, peptides, or amino acids to produce brown melanoidins, which usually occurs at temperatures in the range of 140 °C to 170 °C. This reaction is the nonenzymatic browning reaction widely found in the food industry [38]. There has been previous study pretreatment of pig manure at temperatures higher than 110 °C. They observed hardening and darkening of manure, which resulted in a low biogas yield [39]. Hardening and the dark brownish color development of the substrate indicated the occurrence of Maillard reaction [39, 40]. The decrease and lag phase of methane production may be due to the Maillard reaction. Biomethane production decreased by 11.7% during fruit and vegetable residue AD with 175 °C thermal pretreatment [41]. Thus, our study suggests that HTP at 150 °C offers the most distinct advantages over HTP at other temperatures in terms of antibiotic removal and AD performance, making it a promising option for future studies.
Effects of Antibiotics on AD Microbial Structure
Bacterial Community Structure
Table 6 provides an overview of the α-diversity indices for bacterial and archaea communities during AD. The Chao and Ace values were used to estimate the number of operational taxonomic units (OTUs) in the community and reflect the overall flora abundance. The Chao and Ace values observed in the SDZ, OTC, ENR, and MIX groups were generally lower than those in CK, suggesting that the presence of antibiotics contributed to a decrease in bacterial diversity after AD. The Shannon and Simpson indices serve as metrics for assessing relative germline abundance and microbial diversity. The values in Table 6 suggest that antibiotics can reduce bacterial diversity. This observation may be attributed to the selective enrichment of certain microbial species caused by the presence of different classes of antibiotics [42]. As shown in Fig. 5a and b, the total number of bacterial OTUs in the five groups at the phylum and genus levels was 15 and 109, respectively. Compared with CK, the groups exposed to antibiotics exhibited several specific bacterial OTUs. The MIX sample had a relatively high number of unique OTUs. This finding indicates that the presence of multiple antibiotics exerted certain stimulating effects on bacterial diversity.
The bacterial community distribution is shown in Fig. 5. The predominant bacterial phyla consisted of Firmicutes (63.6–86.2%), Caldatribacteriota (3.5–11.7%), Bacteroidota (4.3–7.3%), Synergistota (2.5–11.4%), and Patescibacteria (2.3–4.9%) at the end of AD (Fig. 5c). Firmicutes were the dominant bacteria in all groups, with their relative abundance showing an increase in groups exposed to antibiotics. As a type of organic acid producer, Firmicutes play a pivotal role in maintaining system stability because of their unique physiological structure and robust adaptability [43]. Furthermore, Firmicutes demonstrate efficiency in degrading complex organic compounds [44]. A slightly increased relative abundance (86.2%) of Firmicutes was observed in ENR. This increase might be one of the reasons why the CH4 yield in the ENR group was higher than that in other groups. By contrast, the relative abundance of Caldatribacteriota and Synergistota decreased, indicating an inhibitory effect associated with the presence of antibiotics during AD (Fig. 5d).
At the genus level, norank_f__norank_o_norank_c__D8A-2 and Caldicoprobacter, which belong to the phylum Firmicute, were predominant at 15.6–37.48% and 5.5–9.2% relative abundances, respectively, as shown in Fig. 5d. The genera D8A-2 belongs to the phylum Firmicutes and is classified as syntrophic acetate-oxidizing bacteria [45]. The relative abundance of norank_f__norank_o_norank_c__D8A-2 was extremely high in ENR (37.48%), corresponding to the enhanced methanogenic potential observed previously in the ENR group (Table 5). As a hydrolytic bacterium, Caldicoprobacter was affected by the presence of different antibiotics during AD. The relative abundance of HN-HF0106 was 4.7–8.9% in different groups. HN-HF0106 can utilize cellulose for growth and ferment saccharides to acetate and H2 [46]. The relative abundance of norank_f_Dethiobacteraceae in MIX (3.8%) was lower than that in SDZ, OTC, ENR (6.5–9.0%), and CK (4.9%). Li et al. [47] predicted that Dethiobacteraceae is involved in the syntrophic acetate oxidation followed by hydrogenotrophic methanogenesis. Thus, MIX can inhibit Dethiobacteraceae and reduce CH4 production. Candidatus caldatribacterium and Acetomicrobium exhibited a decrease in relative abundance because of their high sensitivity to antibiotics. In conclusion, Firmicutes played an important role in methane production during AD, and the addition of antibiotics can further promote the abundance of Firmicutes. The presence of multiple antibiotics appeared to have a certain combined inhibitory effect on microorganisms and CH4 production.
Archaea community structure
In contrast to bacteria, the archaeal richness remained relatively unchanged, as indicated by the Chao and Ace indices. The total number of OTUs observed at the archaea level and genus level was 4 and 9, respectively, with no distinct difference among the five groups (Fig. 6a and b). These findings were in consensus with the results outlined in Table 6, reaffirming that the presence of antibiotics exhibited a limited effect on the archaeal community structure.
Euryarchaeota and Halobacterota emerged as the predominant phyla of archaea, collectively accounting for more than 99% of the relative abundance (Fig. 6c). Given that methanogens belong to Euryarchaeota at the phylum level [48], the abundance of Euryarchaeota and Halobacterota may be associated with the methane production during AD. The results showed that Euryarchaeota exhibited the highest relative abundance in the ENR group, which may correlate to the highest cumulative methane production, as mentioned in Table 5. Halobacterota, a novel methanogenic archaeon found in recent studies, represents one of the most primitive archaeal branches on the phylogenetic tree. It can convert H2/CO2, methyl compounds (formate, methanol, and methylamide), and acetate into CH4 [49].
As shown in Fig. 6d, Methanobacterium, Methanosarcina, and Methanobrevibacter were the predominant genera within the archaeal community. As a typical genus of hydrotrophic methanogens, Methanobacterium plays a key role by interacting with other genera, such as Syntrophomonas and Clostridium [50]. This finding can be observed from Fig. 6d. The relative abundance of Methanobacterium in the ENR group was the highest (70.36%) among the five experimental groups. This finding corresponds with the highest cumulative methane production achieved by the ENR group in this study (Table 5). No significant differences were observed in the archaeal community distribution in other groups. Methanosarcina is a hybrid methanogenic bacterium that can utilize acetic acid or hydrogen to reduce CO2 and produce methane [51]. The genus Methanosarcina was resistant to harsh or even extreme conditions [52]. Therefore, all these findings underscore the close correlation between the percentage of Methanobacterium and methane yield during AD.
Conclusions
This study explored the roles of different treatment processes (HTP, AD, and HTP + AD) in antibiotic removal and methane production. The removal efficiencies of SDZ, OTC, and ENR improved with the increase in HTP temperature. OTC was highly temperature sensitive with the highest removal efficiency. SDZ exhibited high resistance, and OTC and ENR could be removed completely during AD. For HTP + AD process, there was a great improvement in SDZ removal, with residual SDZ levels reduced to 20% at 150 °C. Compared with the standalone AD, HTP augmented the methanogenic potential of AD in all groups. A significant (p < 0.05) increase in methanogenic potential was observed in the 150 °C HTP groups. Microbial analysis revealed that ENR and OTC demonstrated a catalytic effect on methane production, whereas the presence of SDZ and mixed antibiotics was associated with an inhibitory effect. These findings revealed HTP’s potential application values as a pretreatment process for AD in controlling antibiotic residuals in pig manure.
Data Availability
Data will be made available on request.
References
Tian, M., He, X., Feng, Y., Wang, W., Chen, H., Gong, M., Liu, D., Clarke, J. L., & van Eerde, A. (2021). Pollution by antibiotics and antimicrobial resistance in liveStock and poultry manure in China, and countermeasures. Antiboitics, 10, 539.
Peng, S., Zhang, H., Song, D., Chen, H., Lin, X., Wang, Y., & Ji, L. (2022). Distribution of antibiotic, heavy metals and antibiotic resistance genes in livestock and poultry feces from different scale of farms in Ningxia China. Journal of Hazardous Materials, 440, 129719.
Wang, H., Chu, Y., & Fang, C. (2017). Occurrence of veterinary antibiotics in swine manure from large-scale feedlots in Zhejiang Province China. Bulletin of environmental contamination and toxicology, 98, 472–477.
Yin, F., Dong, H., Zhang, W., Wang, S., Shang, B., & Zhu, Z. (2021). Ability of anaerobic digestion to remove antibiotics contained in swine manure. Biosystems Engineering, 212, 175–184.
Chen, T., Xie, G., Mi, J., Wen, X., Cao, Z., Ma, B., Zou, Y., Zhang, N., Wang, Y., Liao, X., & Wu, Y. (2022). Recovery of the structure and function of the pig manure bacterial community after enrofloxacin exposure. Microbiology Spectrum, 10(3), e02004–21.
Massé, D., Saady, N., & Gilbert, Y. (2014). Potential of biological processes to eliminate antibiotics in livestock manure: An overview. Animals, 4(2), 146–163.
Cheng, D., Ngo, H. H., Guo, W., Chang, S. W., Nguyen, D. D., Liu, Y., Zhang, X., Shan, X., & Liu, Y. (2020). Contribution of antibiotics to the fate of antibiotic resistance genes in anaerobic treatment processes of swine wastewater: A review. Bioresource Technology, 299, 122654.
Zhao, L., Dong, Y. H., & Wang, H. (2010). Residues of veterinary antibiotics in manures from feedlot livestock in eight provinces of China. Science of the Total Environment, 408, 1069–1075.
Wohde, M., Berkner, S., Junker, T., Konradi, S., Schwarz, L., & Düring, R. A. (2016). Occurrence and transformation of veterinary pharmaceuticals and biocides in manure: A literature review. Environmental Sciences Europe, 28, 23.
Wu, X., Tian, Z., Lv, Z., Chen, Z., Liu, Y., Yong, X., Zhou, J., Xie, X., Jia, H., & Wei, P. (2020). Effects of copper salts on performance, antibiotic resistance genes, and microbial community during thermophilic anaerobic digestion of swine manure. Bioresource Technology, 300, 122728.
Guo, H., Gu, J., Wang, X., Song, Z., Qian, X., & Sun, W. (2020). Negative effects of oxytetracycline and copper on nitrogen metabolism in an aerobic fermentation system: Characteristics and mechanisms. Journal of Hazardous Materials, 403, 123890.
Ben, W., Qiang, Z., Yin, X., Qu, J., & Pan, X. (2014). Adsorption behavior of sulfamethazine in an activated sludge process treating swine wastewater. Journal of Environmental Sciences, 26, 1623–1629.
Wallace, J. S., Garner, E., Pruden, A., & Aga, D. S. (2018). Occurrence and transformation of veterinary antibiotics and antibiotic resistance genes in dairy manure treated by advanced anaerobic digestion and conventional treatment methods. Environmental Pollution, 236, 764–772.
Feng, L., Casas, M. E., Ottosen, L. D. M., Møller, H. B., & Bester, K. (2017). Removal of antibiotics during the anaerobic digestion of pig manure. Science of the Total Environment, 603, 219–225.
Aziz, A., Sengar, A., Basheer, F., Farooqi, I. H., & Isa, M. H. (2021). Anaerobic digestion in the elimination of antibiotics and antibiotic-resistant genes from the environment – A comprehensive review. Journal of Environmental Chemical Engineering, 10, 106423.
Kovalakova, P., Cizmas, L., McDonald, T. J., Marsalek, B., Feng, M., & Sharma, V. K. (2020). Occurrence and toxicity of antibiotics in the aquatic environment: A review. Chemosphere, 251, 126351.
Hu, J., Xu, Q., Li, X., Wang, D., Zhong, Y., Zhao, J., Zhang, D., Yang, Q., & Zeng, G. (2018). Sulfamethazine (SMZ) affects fermentative short-chain fatty acids production from waste activated sludge. Science of the Total Environment, 639, 1471–1479.
Ding, L., Cheng, J., Qiao, D., Yue, L., Li, Y.-Y., Zhou, J., & Cen, K. (2017). Investigating hydrothermal pretreatment of food waste for two-stage fermentative hydrogen and methane co-production. Bioresource Technology, 241, 491–499.
Yi, Q., Gao, Y., Zhang, H., Zhang, H., Zhang, Y., & Yang, M. (2016). Establishment of a pretreatment method for tetracycline production wastewater using enhanced hydrolysis. Chemical Engineering Journal, 300, 139–145.
Tang, M., Li, F., Yang, M., & Zhang, Y. (2020). Degradation of kanamycin from production wastewater with high-concentration organic matrices by hydrothermal treatment. Journal of Environmental Sciences, 97, 11–18.
Gong, P., Liu, H., Wang, M., Dai, X., & Yao, J. (2020). Characteristics of hydrothermal treatment for the disintegration of oxytetracycline fermentation residue and inactivation of residual antibiotics. Chemical Engineering Journal, 402, 126011.
APHA. (2015). Standard Methods for the Examination of Water and Wastewater, 21st Ed. American Public Health Association.
Awad, M., Tian, Z., Zhang, Y., Yang, M., Yin, W., & Dong, L. (2020). Hydrothermal pretreatment of oxytetracycline fermentation residue: Removal of oxytetracycline and increasing the potential for anaerobic digestion. Environ. Eng. Res., 26, 200258–200250.
Wang, Y., Wang, Y., Zhang, Z., Turap, Y., Wang, Y., Wang, I., Wang, Z., & Wang, W. (2021). Combined hydrothermal treatment, pyrolysis, and anaerobic digestion for removal of antibiotic resistance genes and energy recovery from antibiotic fermentation residues. Bioresource Technology, 337, 125413.
Lolo, M., Pedreira, S., Miranda, J. M., Vazquez, B. I., Franco, C. M., Cepeda, A., & Fente, C. (2006). Effect of cooking on enrofloxacin residues in chicken tissue. Food Addit & Contam., 23, 988–993.
Gaballah, M. S., Guo, J., Hassanein, A., Sobhi, M., Zheng, Y., Philbert, M., Li, B., Sun, H., & Dong, R. (2023). Removal performance and inhibitory effects of combined tetracycline, oxytetracycline, sulfadiazine, and norfloxacin on anaerobic digestion process treating swine manure. Science of the Total Environment, 857, 159536.
Cheng, D., Ngo, H. H., Guo, W., Chang, S. W., Nguyen, D. D., Liu, Y., Shan, X., Nghiem, L. D., & Nguyen, L. N. (2020). Removal process of antibiotics during anaerobic treatment of swine wastewater. Bioresource Technology, 300, 122707.
Gartiser, S., Urich, E., Alexy, R., & Kümmerer, K. (2007). Anaerobic inhibition and biodegradation of antibiotics in ISO test schemes. Chemosphere, 66, 1839–1848.
Zhi, S., Li, Q., Yang, F., Yang, Z., & Zhang, K. (2019). How methane yield, crucial parameters and microbial communities respond to the stimulating effect of antibiotics during high solid anaerobic digestion. Bioresource Technology, 283, 286–296.
Wu, Q., Zou, D., Zheng, X., Liu, F., Li, L., & Xiao, Z. (2022). Effects of antibiotics on anaerobic digestion of sewage sludge: Performance of anaerobic digestion and structure of the microbial community. Science of the Total Environment, 845, 157384.
Delgadillo-Mirquez, L., Gonzalez-Tineo, P., Serrano, D., & Durán, U. (2022). Effects of short-term inhibition of Tetracyclines and Macrolides on specific methanogenic activity and swine biodegradability. Environmental Technology and Innovation, 27, 102574.
Wu, Q., Zou, D., Zheng, X., Liu, F., Li, L., & Xiao, Z. (2022). Effects of antibiotics on anaerobic digestion of sewage sludge: Performance of anaerobic digestion and structure of the microbial community. Science of the Total Environment, 845, 157384.
Hu, J., Xu, Q., Li, X., Wang, D., Zhong, Y., Zhao, J., Zhang, D., Yang, Q., & Zeng, G. (2018). Sulfamethazine (SMZ) affects fermentative short-chain fatty acids production from waste activated sludge. Science of the Total Environment, 639, 1471–1479.
Zhi, S., Li, Q., Yang, F., Yang, Z., & Zhang, K. (2019). How methane yield, crucial parameters and microbial communities respond to the stimulating effect of antibiotics during high solid anaerobic digestion. Bioresource Technology, 283, 286–296.
Balasundaram, G., Vidyarthi, P. K., Gahlot, P., Arora, P., Kumar, V., Kumar, M., Kazmi, A. A., & Tyagi, V. K. (2022). Energy feasibility and life cycle assessment of sludge pretreatment methods for advanced anaerobic digestion. Bioresource Technology, 357, 127345.
Huang, W., Zhao, Z., Yuan, T., Huang, W., Lei, Z., & Zhang, Z. (2017). Low-temperature hydrothermal pretreatment followed by dry anaerobic digestion: A sustainable strategy for manure waste management regarding energy recovery and nutrients availability. Waste Management, 70, 255–262.
Bougrier, C., Albasi, C., Delgenès, J. P., & Carrère, H. (2006). Effect of ultrasonic, thermal and ozone pre-treatments on waste activated sludge solubilisation and anaerobic biodegradability. Chemical Engineering and Processing, 45, 711–718.
Han, L., Zhai, R., Shi, R., Hu, B., Yang, J., Xu, Z., Ma, K., Li, Y., & Li, T. (2024). Impact of cod skin peptide-ι-carrageenan conjugates prepared via the Maillard reaction on the physical and oxidative stability of Antarctic krill oil emulsions. Food Chemistry: X, 21, 101130.
Rafique, R., Poulsen, T. G., Nizami, A., Asam, Z., Murphy, J. D., & Kiely, G. (2010). Effect of thermal, chemical and thermo-chemical pre-treatments to enhance methane production. Energy, 35, 4556–4561.
Ariunbaatar, J., Panico, A., Esposito, G., Pirozzi, F., & Lens, P. N. L. (2014). Pretreatment methods to enhance anaerobic digestion of organic solid waste. Applied Energy, 123, 143–156.
Liu, X., Wang, W., Gao, X., Zhou, Y., & Shen, R. (2012). Effect of thermal pretreatment on the physical and chemical properties of municipal biomass waste. Waste Management, 32, 249–255.
Zhao, R., Yu, K., Zhang, J., Zhang, G., Huang, J., Ma, L., Deng, C., Li, X., & Li, B. (2020). Deciphering the mobility and bacterial hosts of antibiotic resistance genes under antibiotic selection pressure by metagenomic assembly and binning approaches. Water Research, 186, 116318.
Zhao, X., Liu, J., Liu, J., Yang, F., Zhu, W., Yuan, X., Hu, Y., Cui, Z., & Wang, X. (2017). Effect of ensiling and silage additives on biogas production and microbial community dynamics during anaerobic digestion of switchgrass. Bioresource Technology, 241, 349–359.
Fu, S. F., Chen, K. Q., Zou, H., Xu, J. X., Zheng, Y., & Wang, Q. F. (2018). Using calcium peroxide (CaO2) as a mediator to accelerate tetracycline removal and improve methane production during co-digestion of corn straw and chicken manure. Energy conversion and management, 172, 588–594.
Ao, T., Xie, Z., Zhou, P., Liu, X., & Li, D. (2021). Comparison of microbial community structures between mesophilic and thermophilic anaerobic digestion of vegetable waste. Bioprocess and Biosystems Engineering, 44, 1–14.
Zhang, X., Tu, B., Dai, L. R., Lawson, P. A., & Zheng, Z. Z. (2018). Petroclostridium xylanilyticum gen. nov., sp. nov., a xylan-degrading bacterium isolated from an oilfield, and reclassification of clostridial cluster III members into four novel genera in a new Hungateiclostridiaceae fam. nov. International Journal of Systematic and Evolutionary Microbiology, 68, 3197–3211.
Li, C., He, P., Hao, L., Lu, F., Shao, L., & Zhang, H. (2022). Diverse acetate-oxidizing syntrophs contributing to biogas production from food waste in full-scale anaerobic digesters in China. Renew. Energ., 193, 240–250.
Yu, Q., Tian, Z., Liu, J., Zhou, J., Yan, Z., Yong, X., Jia, H., Wu, X., & Wei, P. (2018). Biogas production and microbial community dynamics during the anaerobic digestion of rice straw at 39–50°C: A pilot study. Energy & Fuels, 32, 5157–5163.
Zhang, C., Yang, R., Sun, M., Zhang, S., He, M., Tsang, D. C. W., & Luo, G. (2022). Wood waste biochar promoted anaerobic digestion of food waste: Focusing on the characteristics of biochar and microbial community analysis. Biochar, 4, 62.
Lin, Q., De Vrieze, J., Li, C., Li, J., Li, J., Yao, M., Hedenec, P., Li, H., Li, T., Rui, J., Frouz, J., & Li, X. (2017). Temperature regulates deterministic processes and the succession of microbial interactions in anaerobic digestion process. Water Research, 123, 134–143.
Ma, J., Wang, P., Gu, W., Su, Y., Wei, H., & Xie, B. (2021). Does lipid stress affect performance, fate of antibiotic resistance genes and microbial dynamics during anaerobic digestion of food waste? Science of the Total Environment, 756, 143846.
Huang, Q., Liu, Y., & Dhar, B. R. (2021). Pushing the organic loading rate in electrochemically assisted anaerobic digestion of Blackwater at ambient temperature: Insights into microbial community dynamics. Science of the Total Environment, 781, 146694.
Funding
The present work was funded by Natural Science Foundation of Shandong Province (ZR2020QE240). Our thanks should go to the Key Laboratory of Clean Production and Utilization of Renewable Energy (Ministry of Agriculture, P.R. China) for assistance in experimental implementation. All authors are grateful to anonymous reviewers for their constructive and critical comments helping to improve the paper.
Author information
Authors and Affiliations
Contributions
Chunchun Guo: conceptualization, investigation, data curation, writing—original draft. Yanfang Ma: writing—original draft, data curation, formal analysis, visualization. Yitao Li: conceptualization, writing—review and editing. Zhiwu Wang: supervision, review and editing. Shupeng Lin: data curation, investigation. Renjie Dong: conceptualization, methodology. Shan Liu: supervision, conceptualization, writing—review and editing, funding acquisition.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent to Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Guo, C., Ma, Y., Li, Y. et al. Effects of Hydrothermal Pretreatment and Anaerobic Digestion of Pig Manure on the Antibiotic Removal and Methane Production. Appl Biochem Biotechnol (2024). https://doi.org/10.1007/s12010-024-04900-y
Accepted:
Published:
DOI: https://doi.org/10.1007/s12010-024-04900-y