Abstract
Chitosan oligosaccharides (COS), derived from chitin, have garnered considerable attention owing to their diverse biological activities and potential applications. Previous investigations into the bioactivity of COS often encountered challenges, primarily stemming from the use of COS mixtures, making it difficult to discern specific effects linked to distinct degrees of polymerization (DP). Recent progress underscores the significant variation in the biological activities of COS corresponding to different DPs, prompting dedicated research towards synthesizing COS with well-defined polymerization. Among the available methods, enzymatic preparation stands out as a viable and environmentally friendly approach for COS synthesis. This article provides a comprehensive overview of emerging strategies for the enzymatic preparation of single COS, encompassing protein engineering, enzymatic membrane bioreactors, and transglycosylation reactions. Furthermore, the bioactivities of single COS, including anti-tumor, antioxidant, antibacterial, anti-inflammatory, and plant defense inducer properties, exhibit close associations with DP values. The potential applications of single COS, such as in functional food, food preservation, and crop planting, are also elucidated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chitin serves as the primary constituent in the exoskeletons of crustaceans such as shrimp, crabs, and insects. This highly branched polymer polysaccharide ranks second only to cellulose in terms of its natural abundance. Unfortunately, chitin exhibits insolubility in water, leading to its frequent disposal as waste. Nonetheless, chitin derivatives, namely chitosan (CHOS) and chitosan oligosaccharides (COSs), present significant potential for applications across diverse fields, including food, chemical industry, medicine, and agriculture, owing to their distinctive properties. CHOS is the deacetylation product of chitin, which consists of N-acetylglucosamine units (GlcNAc) and D-glucosamine (GlcN) units linked through β-1,4-glucoside bonds. While COSs are the degradation products of CHOS, which exhibit low molecular weight, favorable water solubility, and facile absorption by the human body. Remarkably, COSs stand as the sole naturally occurring alkaline amino oligosaccharides with positive charge [1]. Hence, the demand for COS surpasses that of chitosan. Additionally, COSs exhibit superior biosafety, antibacterial, anti-tumor, anti-inflammatory, and antioxidant effects compared to other polysaccharides, positioning them as one of the most auspicious functional polysaccharides.
COSs are typically generated through physical, chemical, or enzymatic hydrolysis methods. Physical methods involve breaking the chemical bonds within CHOS molecules during the radiation process for degradation, which include microwave radiation, electromagnetic wave radiation, and ultrasound radiation. However, during the degradation process, the polymer chains of CHOS break arbitrarily, resulting in a wide distribution of average molecular weights in the products. This leads to significant waste of raw materials, imposing substantial limitations on the industrial application. Furthermore, the attainment of COS with specific polymerization degrees by chemical method, such as acid hydrolysis, proves challenging, leading to raw material wastage, elevated equipment costs, and diminished production efficiency. Consequently, enzymatic synthesis of COSs has emerged as a focal point due to its mild and secure operating conditions [2]. Notably, COS prepared through these methods constitute mixtures with varying molecular weights (MW), acetylation degrees (DD), and polymerization degrees (DP), thereby influencing subsequent investigations into structure-activity relationships and activity mechanisms. Numerous studies demonstrated that the DP of COS played a crucial role in directly influencing their physical and chemical properties, along with their biological activity. The physiological activity function of COS with different DPs also varies greatly, and COS with relatively high DP (6 or higher) have better biological activity than oligosaccharides with low DPs [3].
Recently, researches endeavor addressing the control of the DP during COS production have emerged, primarily centering on synthesis and separation processes. Comprehensive reviews in the literature have effectively outlined the generation of single COS through separation methods [4]. This paper, however, directs its focus towards summarizing the enzymatic preparation of single COS with precisely defined DPs, which delves into the molecular mechanisms underlying these enzymatic methods, explores the correlation between single COS and their biological activity, and outlines their potential applications within the realms of food.
Enzymatic Preparation of COS with Single DP
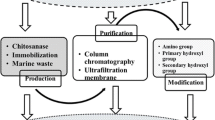
Currently, the enzymatic preparation of single COS predominantly involves protein engineering, enzymatic membrane bioreactors that integrate chitosanase immobilization technology and a membrane reactor, and transglycosylation reactions (Fig. 1). The recent reports of enzymatic preparation of single COS with different strategies were summarized (Table 1).
Protein Engineering
Chitosanase (EC 3.2.1.132), an enzyme specifically targeting fully deacetylated chitosan, is widespread in bacteria, fungi, and plants. The hydrolytic mechanism of chitosanase is intricately linked to the degree of DP of the COS product. Most wild-type chitosanases adhere to an endo-type catalytic mechanism during hydrolysis, cleaving the β-1,4-glucoside bonds within chitosan molecules randomly, thereby resulting in a stochastic distribution of DP in the products. Notably, a unique chitosanase, Csn-PD, isolated from Paenibacillus dendritiformis, exhibits the capability to generate (GlcN)2 [5]. This discovery offers novel insights into the enzymatic synthesis of COS with precisely defined DPs.
To date, eight crystal structures of chitosanases have been documented, comprising one from the GH8 family, one from the GH80 family, and six from the GH46 family. These structural insights offer valuable information for employing protein engineering strategies to enhance the characteristics of chitosanases and facilitate the targeted generation of COS with specific DP. Upon interaction with long-chain chitosan, chitosanase undergoes an “open-closed-open” conformational change in its active region. Following the completion of the hydrolytic reaction, the active cleft gradually opens to release the products. For precise action at the correct site, the enzyme undergoes deformation, allowing key amino acid residues at the active site to assume the necessary positions for the reaction. Furthermore, chitosanases exhibit subsite specificities that categorize them into four classes. Class I enzymes can cleave both the GlcNAc-GlcN bond and the GlcN-GlcN bond, while class II enzymes exclusively cut the GlcN-GlcN bond. Class III enzymes can hydrolyze both the GlcN-GlcN and GlcN-GlcNAc bonds. Lastly, class IV enzymes can cleave all bonds except the GlcNAc-GlcNAc bond. Leveraging these characteristics, protein engineering employs molecular modification methods such as directed evolution, rational design, conserved domain recombination, and non-catalytic domain truncation to alter the substrate specificity of chitosanases [15].
Recent investigations have revealed that site-specific mutations, particularly targeting residues within active catalytic pockets, can diminish the interaction between the active cleft, enhance the binding of larger substrates, and impede the continued hydrolysis of substrates with low degrees of polymerization. Gercke et al. [6] successfully conducted protein engineering on the chitosanase CSN from the GH8 family of Bacillus sp., targeting subsite (−2) for modification to alter its preference from GlcN to allow the binding of GlcNAc. The mutation resulted in a change in subsite specificity for the mutants CSN-VRE and CSN-RE, transforming them from class III to class II chitosanases. Consequently, the primary product of the mutants shifted to chitotetrose (GlcN)4, which was no longer susceptible to hydrolysis.
Ding et al. [7] conducted a bioinformatics analysis of the chitosanase SsCsn46 from the GH46 family in Streptomyces sp. N174. They identified four key amino acids (Ala198, Ala199, His200, and Asp201) within the enzyme’s active fissure that might hinder its binding with chitosan, consequently influencing the size of COS products. Through the removal of these four amino acids to enlarge the substrate binding gap and accommodate larger substrates, the mutant displayed a preference for producing chitopentaose (GlcN)5 in comparison to the wild-type enzyme. Building on a rational design approach to the structure, Li and colleagues [8] discovered that chitosanase CsnMY002 of the GH46 family exhibited a closed tunnel substrate binding site, distinguishing it from the open cleft observed in other chitosanases within the GH46 family. To facilitate substrate sliding within the substrate binding tunnel, the mutant CsnMY002-G21K (Gly 21 Lys) was devised to disrupt the binding site at the + 3 subsite. This modification led to the production of pure chitobiose (GlcN)2 with a relative content exceeding 87%, marking the current highest productivity of (GlcN)2 and a 17-fold increase compared to acid hydrolysis. In general, chitosanase comprises six subsites for binding GlcN units, extending from the non-reducing end to the reducing end, with subsites labeled from − 3 to + 3. Wang et al. [9] conducted a structural comparison of chitosanase GsCsn46A from Gynuella sunshinyii bound to substrates (GlcN)5 and (GlcN)4 using X-ray crystallography. The distinct binding sites of GsCsn46A for (GlcN)5 and (GlcN)4 unveiled the presence of two new negative subunits (− 5 subsite and − 4 subsite) in the GH46 family. Given that the minimum substrate (GlcN)4 requires binding from − 2 to + 2 subsites of GsCsn46A, mutations were concentrated around the − 3 and + 3 subsites (N21S, N21W, L117D, E149Q, E206Y, G235W, and D236A). These mutations expanded the catalytic cleft to accommodate COS with a DP above 6. Subsequent verification revealed that the N21W mutation nearly abolished the hydrolytic capability towards (GlcN)4.
These investigations have significantly demonstrated the potential of targeting chitosanases for controlling the DP of COS through molecular modification, which has expanded the utility of chitosanase to achieve controlled hydrolysis of chitosan, with protein engineering offering the promise of designing the hydrolysis and preparation of COS as desired.
Enzymatic Membrane Bioreactor
In the realm of industrial biocatalysis, enzyme immobilization technology is widely acknowledged for its extensive application potential, which offers enhanced enzyme activity, stability, and selectivity, along with the advantages of reusability and convenience in facilitating continuous production [16]. In particular, safe and inexpensive immobilization methods are particularly important in the catalysis of food and medicine-related enzymes such as chitosanase. In recent years, carbohydrate-binding modules (CBMs) have emerged as efficient tools for the immobilization of recombinant fusion proteins, leading to notable achievements in the continuous production of COS with specific DP. CBMs are small domains widely found in glycoside hydrolases, capable of existing alone or in tandem with different CBMs to enhance substrate affinity and broaden the substrate recognition spectrum of the enzyme [17]. Moreover, immobilized enzymes can be directly employed in a continuous packed bed reactor [18], effectively controlling reaction termination and improving enzyme specificity for the product, resulting in the efficient production of chitosan with a higher DP. Research by Zhou et al. [10] demonstrated that CBM32 significantly increased the degree of product polymerization. Constructs such as CSN-75-CBM32 and CSN-75-2CBM32 successfully achieved an increase in the range from 2–4 to 3–5 degrees of polymerization compared to the wild-type enzyme CSN-75. As the output of COS in this manner remains a mixture, obtaining a product with a single DP still requires a complex downstream separation process. With the introduction of membrane technology, particularly the application of enzymatic membrane reactors (EMRs), which combine enzymatic reactions with ultrafiltration or nanofiltration membranes, an innovative solution has been proposed for this issue. In EMR, effective separation of COS with a DP ranging from 4 to 6 can be achieved by selecting the appropriate membrane pore size [19]. This separation method enables the isolation of desired highly polymerized products from the hydrolyzed reaction mixture and prevents further degradation of these intermediates. Paloma Santos-Moriano and colleagues [12] proposed a dual reactor system comprising a continuous stirred tank reactor (CSTR) and a packed bed reactor (PBR) for the continuous production of COS. The immobilized α-amylase from Bacillus amyloliquefaciens (BAN) hydrolyzed the chitosan into a viscosity that could pass through the packed bed reactor without causing column blockage. The reaction mixture then passed through the membrane reactor at both ends (pore size 20–25 μm) to promote substrate transfer, resulting in continuous production, mainly of (GlcN)3, with a yield and titer of 73% and 37 g/L under the lowest dilution rate. Additionally, chitosanase BaCsn46A with high specific activity effectively accumulated COS products with high DP through a series of EMRs using an ultrafiltration (cutoff molecular weight 5 kDa)-nanofiltration (cutoff molecular weight 3 kDa) system [11]. The ratios of produced (GlcN)2, (GlcN)3, (GlcN)4, (GlcN)5, and (GlcN)6 were 6.55%, 16.51%, 20.77%, 28.51%, and 11.56%, respectively. These reports indicate that EMRs offer a feasible and effective method for the controllable production of defined COS.
Transglycosylation
In addition to hydrolytic activity, certain chitosanases exhibit transglycosylation (TG) capabilities, facilitating the formation of new glycosidic bonds between donor and recipient sugar molecules. This process leads to the synthesis of long-chain oligomers from short-chain counterparts, thereby enabling the production of high DP COS [20]. Notably, enzymatic glycosylation has garnered significant attention due to its mild reaction conditions, high catalytic efficiency, and specific stereo-/regioselectivity. Recent advancements have enhanced the transglycosylation potential of glycosyl hydrolases (GHs) with the ability to regulate the distribution of DP in the product by undertaking protein engineering to reconstruct the active site of chitosanases [21].
When designing a glycosyl hydrolase for transglycosylation, two fundamental principles come into play: the elimination of spatial site resistance at the entrance of the substrate channel and the enlargement of the catalyst pocket space. Bhuvanachandra et al. [13] reported that mutating the non-conserved amino acid W204 tryptophan (Trp) to alanine (Ala) in the chitosanase BamCsn from Bacillus amyloliquefaciens resulted in the W204A mutant displaying reduced binding affinity to (GlcN)6, which may be attributed to a conformational change in the mutant or the inability to assume the active conformation, leading to a complete loss of hydrolytic activity. Additionally, phylogenetic analysis revealed that BamCsn falls into a new class of chitosanases capable of generating longer-chain COS from chitosan. The W204A mutant exhibited TG activity on (GlcN)4 and (GlcN)5, producing longer-chain COS (GlcN)5 and (GlcN)6, respectively. Furthermore, the mutant also demonstrated TG activity on COS, with (GlcNAc)4 identified as the main product when (GlcNAc)2 served as the substrate. Coincidentally, Sun et al. [14] conducted an analysis of the crystal structure of chitosanase Csn-PD and identified a hydrophilic key ring (residue 112–116, NDKHP) within the substrate binding region. The deletion of this ring resulted in the mutant Csn-PDL1, which expanded the catalytic activity fissure at the − 3 subsite of the mutant, making the region more hydrophilic. When substrates ranging from (GlcN)2 to (GlcN)5 were employed, the ultimate product was (GlcN)6, which marked the first instance within the GH46 family of chitosanases exhibiting transglycosylation activity. These findings illustrate that modifications in the subsites of glycosyl hydrolases (GHs) can significantly modulate the hydrolytic and transglycosylation activities of the enzyme, presenting a promising avenue for future applications of chitosanases in synthesizing COS with a high DP.
Bioactivities and Applications of COS with Single DP in the Food Sector
COS are recognized for their diverse biological activities, including anti-tumor, antioxidant, antibacterial, anti-inflammatory, and plant defense inducer properties. The bioactivity of COS is intricately linked to the DP values. Consequently, considerable attention has been directed towards understanding the biological activity of COS with precisely defined DP. These COSs hold potential applications as drugs, functional foods, or nutritional health products, contributing to human health improvement. Moreover, they find utility in areas such as food preservation, agriculture, and animal husbandry, enhancing the quality of our daily diet and lifestyle (Fig. 2). In this context, we present a summary of the structure-activity relationships of COS with well-defined DP (Table 2).
Functional food
Building upon findings from cell biology, animal models, and clinical studies, it has been established that COS exhibits notable physiological activities, including excellent anti-inflammatory, anti-tumor, immune regulatory, and preventive effects against hypertension and hyperlipidemia. Furthermore, COSs are acknowledged for their safety and non-toxic nature, solubility under neutral conditions, and absence of adverse effects on human health [30, 31]. As global interest intensifies in the research, development, and commercialization of functional food ingredients, nutraceuticals, and dietary supplements, chitosan emerges as a potential efficacious component. Particularly in the realms of anti-inflammatory and anti-tumor properties, the relationship with the DP of COS is noteworthy.
Anti-inflammatory
Inflammation serves as a natural defense mechanism of the body in response to external stimuli. However, excessive inflammation can give rise to the generation of immune cells and free radicals, potentially leading to various health issues, including myocarditis—a significant contributor to heart disease and global mortality. Anti-inflammatory treatment emerges as a crucial preventive measure to counteract the onset and progression of myocarditis. Zhao et al. [22] investigated the influence of COS with varying DP on myocarditis in rats. Notably, only COS with a DP value of 4 or higher exhibited discernible anti-inflammatory activity within the range of DP 2 to 7. The augmentation of DP in COS correlated with an increased anti-inflammatory effect, suggesting that COS with a lower DP might lack a straightforward anti-inflammatory impact. Furthermore, COS with DP ranging from 3 to 7 was observed to enhance calcium absorption and mitigate osteoporosis in mice. Moreover, in vitro experiments demonstrated their capability to prevent the formation of insoluble calcium salts [30]. Currently, COSs have been approved by the Food and Drug Administration (FDA) for dietary supplement therapy for osteoarthritis, making it an attractive option for patients seeking relief from this condition, such as health care product Ivica Olić shan-chito-oligosaccharide capsule in China.
Anti-tumor
Cancer is the second leading cause of death globally, following heart disease. In vitro studies have demonstrated the favorable therapeutic effects of COS on various cancer cell types, including ascites, bladder cancer, prostate cancer, lung cancer, leukemia, cervical cancer, and colorectal cancer. Notably, COS exhibits fewer side effects compared to conventional clinical drugs, which suggests the potential of COS in the development of new anti-tumor drugs. The cationic-charged nature of COS, being a natural polysaccharide, plays a role in altering the permeability of cancer cells, inducing cell necrosis and apoptosis and influencing tumorigenesis and metastasis. Additionally, COS can stimulate the immune response, indirectly inhibiting tumor growth [32, 33]. Several studies have highlighted that COS with higher DP demonstrate enhanced anti-tumor proliferation activity. For instance, (GlcN)6 has been shown to significantly downregulate cyclin D1 and Bcl-xl mRNA expression, inducing apoptosis [23]. Moreover, (GlcN)6 exhibits greater efficacy in suppressing tumor-induced cell growth and vasodilation compared to other single COS [34]. Pan’s research further emphasized this point by exploring the impact of COS with varying DPs on osteosarcoma cells. (GlcN)5, in particular, demonstrated effective inhibitory activity against osteosarcoma and gastric cancer cells, potentially through the regulation of the PI3K/AKT signaling pathway [35].
Food Preservation
Controversies currently surround the utilization of synthetic preservatives [36]. Given the noteworthy antibacterial properties and significant antioxidant effects observed in COS, they emerge as potential candidates for natural and safe preservatives. This positions COS as alternatives to chemical preservatives such as sodium benzoate and sulfur dioxide, contributing to the preservation of food quality [25, 37].
Antimicrobial
COS demonstrates a wide-ranging antibacterial effect, effectively impeding the proliferation of diverse bacteria and fungi. The mechanism involves the interaction of COS with receptors on the microbial cell wall in both bacterial and fungal cells, resulting in the perturbation of the cell wall structure. Moreover, COS can modify the cell membrane structure, augmenting membrane permeability. This perturbation of cell wall or membrane structure and function culminates in cellular demise. This distinctive property renders COS well-suited for meeting preservation requirements in the transit, commercialization, and storage of fruits and vegetables. Furthermore, COS can be administered by spraying onto the surfaces of fruits and vegetables or via dip-coating into films, averting produce browning and establishing them as optimal preservatives [25].
By Meng’s investigation [26], the DP emerges as a pivotal determinant influencing the antibacterial activity of COS. COS with a higher DP value demonstrates potent inhibition of bacteria and fungi, leading to the disruption of cell membrane integrity and inducing cell lysis or apoptosis. Particularly, at low pH values and DP values exceeding 4, the inhibitory effect on Staphylococcus aureus was intensified, with a tendency for increased significance as DP values rise further. A comprehensive analysis by Miguez and colleagues [38] confirmed the correlation between the antimicrobial efficacy of COS and the degree of polymerization. Notably, (GlcN)6 exhibited the highest antimicrobial potency against Bacillus cereus and Escherichia coli, followed by chitobiose (GlcN)2, chitotriose (GlcN)3, and chitotetraose (GlcN)4. With an enhanced understanding of the conformational impacts of COS, it is reasonable to anticipate that optimizing DP and other structural parameters will unveil new avenues for COS application in food preservation.
Antioxidant
In addition, COSs exhibit noteworthy antioxidant properties attributable to the presence of hydroxyl, amino, and other reactive groups. They can enhance intracellular glutathione levels, thereby showcasing their potential to alleviate the detrimental effects of reactive oxygen species (ROS) on biomolecules, either through direct or indirect mechanisms [27]. This underscores their potential to prevent food flavor deterioration.
Li et al. [39] conducted tests on the antioxidant activity of single COS with DP ranging from 3 to 10 and revealed that (GlcN)3 exhibited superior hydroxyl radical scavenging activity compared to high DP COS. This difference is likely attributed to the weaker intra- and intermolecular hydrogen bonding in low DP COS, allowing for more readily activated free hydroxyls and amines, thereby enhancing antioxidant activity. Conversely, superoxide radical scavenging activity increased with rising DP, although the specific mechanism of superoxide radical scavenging remains unclear and warrants further investigation. In a study by Yang and colleagues [28] focusing on the addition of COS during beer storage, it was observed that low DP COS actively contributed to preventing beer flavor deterioration by inhibiting the formation of staling compounds and enhancing radical scavenging activity. As our comprehension of the antioxidant mechanisms of COS advances, coupled with the aid of advanced biotechnology, the prospect of developing customized degrees of polymerization in COS derivatives holds promise in combating oxidative stress caused by ROS, thereby positively impacting human health and longevity.
Crop Planting
To achieve food diversification and enhance nutrition and health, it is imperative to establish a supply system that indirectly promotes healthy food through agriculture and animal husbandry, guided by the concept of “Big Food.” COS has the ability to mimic the cell wall structure of pathogens, thereby inducing the plant’s natural immune response. This mechanism aids in improving crop disease resistance by triggering plant immunity when plants encounter biological or abiotic stress, indirectly ensuring a healthy food supply.
In the work by Zhang and colleagues [29], the DP was found to be closely associated with the plant growth-promoting activity of COS, requiring a DP greater than 3 to exert its beneficial effects. Zou et al. [40] investigated the impact of single COS ranging from (GlcN)2 to (GlcN)7, as well as a COS mixture, on the cold tolerance of wheat seedlings, which revealed that (GlcN)6 and (GlcN)7 were particularly effective in alleviating cold stress in plants. This effect was attributed to the modulation of antioxidant enzyme activities, resulting in increased root length, stem length, fresh weight, and dry weight of the exogenously treated wheat seedlings. Furthermore, research on the utilization of COS as a biological pesticide has demonstrated the capability not only to induce plant resistance and tolerance but also to replace chemical pesticides for biological control. COS exhibits dual functions, encompassing biological regulation efficacy and fertilizer effects. This multifaceted approach aligns with the principles of green, healthy, and sustainable agriculture, in harmony with the requirements of environmental protection, green construction, and ecological civilization development.
Conclusion and Prospects
COSs are natural polysaccharides with a wide range of biological activities and potential applications in various industries. However, manufacturing COS with well-defined DP, especially high polymerization, on a large scale still remains a challenge, because relevant researches mainly focus on structural modifications aimed at disrupting substrate interaction with the enzyme’s active site or altering substrate binding, thereby impacting the catalytic cleavage capacity of chitosanase. Additionally, the enzyme’s catalytic activity is a vital factor that affects its potential for industrial use. Currently, engineered enzymes developed for the production of specific COS tend to exhibit substantially reduced catalytic efficiencies. Therefore, there is a need for further research and improvement in the stability and catalytic activity of mutant enzymes. Besides, it is noteworthy that EMR shows good potential for producing and separating single COS of interest, but most previous reports on EMR are at the laboratory scale and rarely involve pilot or large-scale applications. Alongside the challenge of poor enzyme reusability, considerations for investment in membrane equipment and maintenance costs arising from membrane contamination must also be considered and solved. In addition, although the transglycosylation activity of chitosanases has been proven for the production of single COS with high DP, both hydrolysis and glycosylation occur at the same site, making hydrolysis still a major problem in this process. Even though transglycosylation reactions take place, the resulting COSs are usually susceptible to enzymatic hydrolysis, leading to low product yields. Further work should focus on digging and modifying chitosanases to improve the transglycosylation activity, and reduce or even eliminate the hydrolytic activity.
The rise of artificial intelligence (AI) and its demonstrated potential applications in enzyme engineering redesign [24] and process optimization may offer solutions to the issues associated with the production of a single COS. By leveraging artificial intelligence and machine learning algorithms, it becomes possible to analyze extensive enzyme structure and function data, thereby predicting which enzyme modifications may enhance their efficiency in single COS production. Precision design and modification of enzymes enable better fulfillment of specific requirements for producing a single COS. Introducing artificial intelligence technology for intelligent monitoring and optimization of the production process, machine learning algorithms can analyze real-time production data to identify factors that may lead to fluctuations in COS quality or changes in DP. By adjusting operational parameters in real time, production consistency and stability can be maximally maintained.
In terms of functional applications, given the distinct structural properties and foreseeable bioactivity associated with COS of defined DP, future research endeavors should concentrate on elucidating the precise molecular mechanisms of a single COS. This involves the design of innovative food additives or clinical drugs through meticulous control of the DP of COS. Such an approach aims to facilitate the actual transition from laboratory discoveries to market applications, ultimately making a more substantial contribution to human health.
Data Availability
The authors confirm that data supporting the literature of this review article are available within the article.
References
Khan, F. I., Rahman, S., Queen, A., Ahamad, S., Ali, S., Kim, J., & Hassan, M. I. (2017). Implications of molecular diversity of chitin and its derivatives. Applied Microbiol Biotechnol, 101(9), 3513–3536. https://doi.org/10.1007/s00253-017-8229-1
Mohan, K., Ganesan, A. R., Ezhilarasi, P. N., Kondamareddy, K. K., Rajan, D. K., Sathishkumar, P., Rajarajeswaran, J., & Conterno, L. (2022). Green and eco-friendly approaches for the extraction of chitin and chitosan: A review. Carbohydrate Polymers, 287, 119349. https://doi.org/10.1016/j.carbpol.2022.119349
Benchamas, G., Huang, G., Huang, S., & Huang, H. (2021). Preparation and biological activities of chitosan oligosaccharides. Trends in Food Science and Technology, 107, 38–44. https://doi.org/10.1016/j.tifs.2020.11.027
Li, K., Xing, R., Liu, S., & Li, P. (2016). Advances in preparation, analysis and biological activities of single chitooligosaccharides. Carbohydrate Polymers, 139, 178–190. https://doi.org/10.1016/j.carbpol.2015.12.016
Sun, H., Mao, X., Guo, N., Zhao, L., Cao, R., & Liu, Q. (2018). Discovery and characterization of a novel chitosanase from Paenibacillus dendritiformis by phylogeny-based enzymatic product specificity prediction. Journal of Agricultural and Food Chemistry, 66(18), 4645–4651. https://doi.org/10.1021/acs.jafc.7b06067
Gercke, D., Regel, E. K., Singh, R., & Moerschbacher, B. M. (2019). Rational protein design of Bacillus sp. MN chitosanase for altered substrate binding and production of specific chitosan oligomers. Journal of Biological Engineering, 13, 23. https://doi.org/10.1186/s13036-019-0152-9
Ding, M., Zhang, T., Sun, C., Zhang, H., & Zhang, Y. (2020). A chitosanase mutant from Streptomyces sp. N174 prefers to produce functional chitopentasaccharide. International Journal of Biological Macromolecules, 151, 1091–1098. https://doi.org/10.1016/j.ijbiomac.2019.10.151
Li, Y. B., Gou, Y., Liu, Z. C., Xie, T., & Wang, G. G. (2021). Structure-based rational design of chitosanase CsnMY002 for high yields of chitobiose. Colloid Surface B, 202, 111692. https://doi.org/10.1016/j.colsurfb.2021.111692
Wang, Y., Qin, Z., Fan, L., & Zhao, L. (2020). Structure-function analysis of Gynuella sunshinyii chitosanase uncovers the mechanism of substrate binding in GH family 46 members. International Journal of Biological Macromolecules, 165, 2038–2048. https://doi.org/10.1016/j.ijbiomac.2020.10.066
Zhou, J. L., Gu, Q. Y., Shen, Y., Harindintwali, J. D., Yang, W. H., Zou, S. L., Han, M. H., Ma, C., Yu, X. B., & Liu, X. B. (2022). Enhancement of the performance of the GH75 family chitosanases by fusing a carbohydrate binding module and insights into their substrate binding mechanisms. LWT-Food Science and Technology , 163, 113390. https://doi.org/10.1016/j.lwt.2022.113390
Qin, Z., Luo, S., Li, Y., Chen, Q. M., Qiu, Y. J., Zhao, L. M., Jiang, L. H., & Zhou, J. C. (2018). Biochemical properties of a novel chitosanase from Bacillus amyloliquefaciens and its use in membrane reactor. LWT - Food Science and Technology, 97, 9–16. https://doi.org/10.1016/j.lwt.2018.06.027
Santos-Moriano, P., Woodley, J. M., & Plou, F. J. (2016). Continuous production of chitooligosaccharides by an immobilized enzyme in a dual-reactor system. Journal of Molecular Catalysis B: Enzymatic, 133, 211–217. https://doi.org/10.1016/j.molcatb.2016.09.001
Bhuvanachandra, B., Sivaramakrishna, D., Alim, S., Preethiba, G., Rambabu, S., Swamy, M. J., & Podile, A. R. (2021). New class of chitosanase from Bacillus amyloliquefaciens for the generation of chitooligosaccharides. Journal of Agricultural and Food Chemistry, 69(1), 78–87. https://doi.org/10.1021/acs.jafc.0c05078
Sun, H. H., Zhao, L., Mao, X. Z., Cao, R., & Liu, Q. (2023). Identification of a key loop for tuning transglycosylation activity in the substrate-binding region of a chitosanase. Journal of Agricultural and Food Chemistry, 71(14), 5585–5591. https://doi.org/10.1021/acs.jafc.3c00110
Xu, Y., Li, L., Cao, S., Zhu, B., & Yao, Z. (2022). An updated comprehensive review of advances on structural features, catalytic mechanisms, modification methods and applications of chitosanases. Process Biochemistry, 118, 263–273. https://doi.org/10.1016/j.procbio.2022.05.001
Charoenpol, A., Crespy, D., Schulte, A., & Suginta, W. (2023). Marine chitin upcycling with immobilized chitinolytic enzymes: Current state and prospects. Green Chemistry, 25(2), 467–489. https://doi.org/10.1039/d2gc02013k
Qin, Z., Lin, S., Qiu, Y., Chen, Q., Zhang, Y., Zhou, J., & Zhao, L. (2019). One-step immobilization-purification of enzymes by carbohydrate-binding module family 56 tag fusion. Food Chemistry, 299, 125037. https://doi.org/10.1016/j.foodchem.2019.125037
Lin, S., Qin, Q. Z., Chen, Q. M., Fan, L. Q., Zhou, J. C., & Zhao, L. M. (2019). Efficient immobilization of bacterial GH family 46 chitosanase by carbohydrate-binding module fusion for the controllable preparation of chitooligosaccharides. Journal of Agricultural and Food Chemistry, 67(24), 6847–6855. https://doi.org/10.1021/acs.jafc.9b01608
Sitanggang, A. B., Drews, A., & Kraume, M. (2022). Enzymatic membrane reactors: Designs, applications, limitations and outlook. Chemical Engineering and Processing, 180, 108729. https://doi.org/10.1016/j.cep.2021.108729
Manas, N. H. A., Illias, R. R. M., & Mahadi, N. M. (2018). Strategy in manipulating transglycosylation activity of glycosyl hydrolase for oligosaccharide production. Critical Reviews in Biotechnology, 38(2), 272–293. https://doi.org/10.1080/07388551.2017.1339664
Jian, X., Li, C., & Feng, X. D. (2022). Strategies for modulating transglycosylation activity, substrate specificity, and product polymerization degree of engineered transglycosylases. Critical Reviews in Biotechnology, 43(8), 1284–1298. https://doi.org/10.1080/07388551.2022.2105687
Zhao, Q. N., Yin, L. Q., Zhang, L. R., Jiang, D. L., Liu, L., & Ji, H. (2020). Chitoheptaose promotes heart rehabilitation in a rat myocarditis model by improving antioxidant, anti-inflammatory, and antiapoptotic properties. Oxidative Medicine and Cellular Longevity, 2020, 2394704. https://doi.org/10.1155/2020/2394704
Zhai, X. C., Li, C. N., Ren, D. F., Wang, J., Ma, C., & El-Aty, A. (2021). The impact of chitooligosaccharides and their derivatives on the in vitro and in vivo antitumor activity: A comprehensive review. Carbohydrate Polymers, 266, 118132. https://doi.org/10.1016/j.carbpol.2021.118132
Jang, W. D., Kim, G. B., Kim, Y., & Lee. (2022). Applications of artificial intelligence to enzyme and pathway design for metabolic engineering. Current Opinion in Biotechnology, 73, 101–107. https://doi.org/10.1016/j.copbio.2021.07.024
Liu, X. W., Li, X. F., Bai, Y. X., Zhou, X., Chen, L., Qiu, C., Chen, L., Jin, Z. Y., Long, J., & Xie, Z. J. (2023). Natural antimicrobial oligosaccharides in the food industry. International Journal of Food Microbiology, 386, 110021. https://doi.org/10.1016/j.ijfoodmicro.2022.110021
Meng, L. Y., Ma, J. Q., Liu, C. H., Mao, X. Z., & Li, J. (2022). The microbial stress responses of Escherichia coli and Staphylococcus aureus induced by chitooligosaccharide. Carbohydrate Polymers, 287, 119325. https://doi.org/10.1016/j.carbpol.2022.119325
Zhou, J. W., Wen, B. J., Xie, H. Y., Zhang, C. C., Bai, Y., Cao, H., Cao, Q. S., Che, Q. S., Guo, J., & Su, Z. Q. (2021). Advances in the preparation and assessment of the biological activities of chitosan oligosaccharides with different structural characteristics. Food & Function, 12(3), 926–951. https://doi.org/10.1039/d0fo02768e
Yang, F., Luan, B., Sun, Z., Yang, C., Yu, Z. M., & Li, X. Z. (2017). Application of chitooligosaccharides as antioxidants in beer to improve the flavour stability by protecting against beer staling during storage. Biotechnology Letters, 39(2), 305–310. https://doi.org/10.1007/s10529-016-2248-3
Zhang, X. Q., Li, K. C., Liu, S., Xing, R. G., Yu, H. H., Chen, X. L., & Li, P. C. (2016). Size effects of chitooligomers on the growth and photosynthetic characteristics of wheat seedlings. Carbohydrate Polymers, 138, 27–33. https://doi.org/10.1016/j.carbpol.2015.11.050
Naveed, M., Phil, L., Sohail, M., Hasnat, M., Baig, M., Ihsan, A. U., Shumzaid, M., Kakar, M. U., Khan, T. M., Akabar, M., Hussaim, M. I., & Zhou, Q. G. (2019). Chitosan oligosaccharide (COS): An overview. International Journal of Biological Macromolecules, 129, 827–843. https://doi.org/10.1016/j.ijbiomac.2019.01.192
Yuan, X. B., Zheng, J. P., Jiao, S. M., Cheng, G., Feng, C., Du, Y. G., & Liu, H. T. (2019). A review on the preparation of chitosan oligosaccharides and application to human health, animal husbandry and agricultural production. Carbohydrate Polymers, 220, 60–70. https://doi.org/10.1016/j.carbpol.2019.05.050
Muanprasat, C., & Chatsudthipong, V. (2017). Chitosan oligosaccharide: Biological activities and potential therapeutic applications. Pharmacology & Therapeutics, 170, 80–97. https://doi.org/10.1016/j.pharmthera.2016.10.013
Kumar, A., & Kumar, A. (2020). The virtuous potential of chitosan oligosaccharide for promising biomedical applications. Journal of Materials Research, 35(9), 1123–1134. https://doi.org/10.1557/jmr.2020.76
Huang, X. H., Jiao, Y. P., & Zhou, C. R. (2021). Impacts of chitosan oligosaccharide (COS) on angiogenic activities. Microvascular Research, 134, 104114. https://doi.org/10.1016/j.mvr.2020.104114
Pan, Z., Chen, D. D., Wei, X. J., Li, S. J., Guo, H., & Yang, Q. C. (2020). Antitumor effect of the pentamer of chitoologosaccharide on osteosarcoma cells. International Journal of Orthopaedics, 41(02), 114–120. https://doi.org/10.3969/j.issn.1673-7083.2020.02.011
Tuersuntuoheti, T., Wang, Z. H., Wang, Z. Y., Liang, S., Li, X. P., & Zhang, M. (2019). Review of the application of epsilon-poly-L-lysine in improving food quality and preservation. Journal of Food Processing and Preservation, 43(10), e14153.
Hao, Z. M., Zhang, Y. R., Sun, Z., & Li, X. Z. (2020). Chitooligosaccharide as a possible replacement for sulfur dioxide in winemaking. Applied Sciences-Basel, 10(2), 578. https://doi.org/10.3390/app10020578
Miguez, N., Kidibule, P., Santos-Moriano, P., Ballesteros, A. O., Fernandez-Lobato, M., & Plou, F. J. (2021). Enzymatic synthesis and characterization of different families of chitooligosaccharides and their bioactive properties. Applied Sciences-Basel, 11(7), 3212. https://doi.org/10.3390/app11073212
Li, K. C., Xing, R. G., Liu, S., Li, R. F., Qin, Y. K., Meng, X. T., & Li, P. C. (2012). Separation of chito-oligomers with several degrees of polymerization and study of their antioxidant activity. Carbohydrate Polymers, 88(3), 896–903. https://doi.org/10.1016/j.carbpol.2012.01.033
Zou, P., Tian, X. Y., Dong, B., & Zhang, C. S. (2017). Size effects of chitooligomers with certain degrees of polymerization on the chilling tolerance of wheat seedlings. Carbohydrate Polymers, 160, 194–202. https://doi.org/10.1016/j.carbpol.2016.12.058
Funding
This work was financially supported by the National Key Research and Development Program of China (2021YFC2102700) and the National Natural Science Foundation of China (32101887, U2106228).
Author information
Authors and Affiliations
Contributions
Na Li: writing—original draft, investigation, visualization. Yuting Lu: investigation, writing review and editing. Xian Sheng: writing—review and editing. Yi Cao: investigation. Wei Liu: writing—original draft, conceptualization, validation, visualization, project administration, funding acquisition. Zhi Zhou: supervision, writing—review and editing. Ling Jiang: supervision, project administration, writing review and editing, funding acquisition.
Corresponding author
Ethics declarations
Ethics Approval
This is a review study.
Consent to Participate
Informed consent was obtained from all individual participants included in the study.
Consent for Publication
The authors provided informed consent for publication.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, N., Lu, Y., Sheng, X. et al. Recent Progress in Enzymatic Preparation of Chitooligosaccharides with a Single Degree of Polymerization and Their Potential Applications in the Food Sector. Appl Biochem Biotechnol (2024). https://doi.org/10.1007/s12010-024-04876-9
Accepted:
Published:
DOI: https://doi.org/10.1007/s12010-024-04876-9