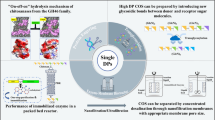
Abstract
Biological activities of chitosan oligosaccharides (COS) are well documented, and numerous reports of COS production using specific and non-specific enzymes are available. However, strategies for improving the overall yield by making it monomer free need to be developed. Continuous enzymatic production from chitosan derived from marine wastes is desirable and is cost-effective. Isolation of potential microbes showing chitosanase activity from various ecological niches, gene cloning, enzyme immobilization, and fractionation/purification of COS are some areas, where lot of work is in progress. This review covers recent measures to improve monomer-free COS production using chitosanase/non-specific enzymes and purification/fractionation of these molecules using ultrafiltration and column chromatographic techniques. Various bioprocess strategies, gene cloning for enhanced chitosanase enzyme production, and other measures for COS yield improvements have also been covered in this review. COS derivative preparation as well as COS-coated nanoparticles for efficient drug delivery are being focused in recent studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chitooligosaccharides (COS) based on their biological activities have numerous applications in various fields like medicine, cosmetics, food and drug delivery, etc. [1], but their uses in various sectors are limited due to commercial non-availability. Chemical or enzymatic hydrolysis method is used for production of biologically active COS from chitosan, which is a natural unbranched co-polymer and possess various functional properties. Chitosan structure involves randomly distributed glucosamine (D-GlcN) and N-acetyl-D-glucosamine (GlcNAc) residues in the linear chain which are linked to each other by β-1,4 bonds. Sequence and percentage of these two molecules determine the biological properties, and bioactivity of these molecules is attributed to the presence of various functional groups like amino/acetamido at C-2 positions and primary/secondary hydroxyl groups at C-3 and C-6 positions, respectively. Although acid hydrolysis is used for large-scale production of COS, it is not preferred due to lower yield (due to presence of monomers, i.e., glucosamine) and risk associated with environmental pollution. COS produced as a result of acid hydrolysis requires multiple protection and deprotection steps [2] and is not considered as bioactive material due to chances of contamination and toxicity, which makes them unsuitable for use in food and pharmaceutical industries. However, in vitro bioactivity of COS (produced chemically) has been reported in some of the studies [3]. Enzymatic production of COS ensures higher yield and high degree of polymerization as controllability of reactions is easier as compared to chemical use. Highly specific enzyme production [4–8] depends upon the presence or absence of acetyl group on either sides of the bond [9, 10] and shows numbers of biological activities useful for food, cosmetics, agriculture, and pharmaceutical industries. Both chitosan specific, i.e., chitosanase, and non-specific enzymes like proteases, cellulases, carbohydrates, lipases, etc. have been used in free and immobilized form for COS production and have been reported from various microbial sources (fungi, bacteria, and actinomycetes). Structure of glycosidic bonds (A–A, A–D, D–D, D–A) present in the differently deacetylated chitosan varies, which affects the enzymatic hydrolysis process. Chitosanases are known for hydrolyzing chitosan specifically and are common in soil microbes [10]. Although microbial chitosanases that show excellent performance in COS production (in terms of yield) are not available commercially for large-scale exochitosanase (EC 3.2.1.132) and endochitosanase (EC 3.2.1.165), enzymes have been reported from various microbial sources separately, but in most of the cases, both these enzymes are secreted and are present in the crude form. Exo-enzyme also called exo-β-D-glucosaminidase cleaves chitosan/COS from non-reducing end and produces glucosamine residues, while endochitosanase enzyme attacks β-1,4 bonds between glucosamine residues in the partly or fully acetylated chitosan chain. It attacks on reducing end of the chitosan chain and produce COS (dimer onwards). Even though both enzymes are desirable separately for industrial production of different molecules (glucosamine and N-acetyl-D-glucosamine by exochitosanase and COS by endochitosanase), presence of exochitosanase with endochitosanase for COS production leads to lower yield due to presence of monomers along with COS in product mixture. Though efforts are made to produce larger oligomers by controlling the reaction, as bioactivity has been correlated with oligomer size, presence of smaller oligomers is almost unavoidable in the process. Removal of smaller oligomers like dimers, trimers, etc. are handled during the downstream processing and are considered as a daunting task. Microbial chitosanase having high specific activity and good yields of COS is selected for industrial production; potential microbial sources of chitosanase enzymes have been reported from diverse ecological samples [9, and fermentative production has been studied in solid and submerged conditions. Medium optimization and various inducers for enhanced production of chitosanase enzymes have been already studied and summarized. Chitosanase gene cloning for overexpression and as a result hyperproduction of endochitosanase enzymes have been reported widely; on the other hand, immobilization of chitosanase enzymes for repeated and strategic uses with various reactor designs and biochemical processes have been done to enhance the process efficiency. Purification and/or fractionation of COS from reaction mixture and their future applications have also been covered in this review. A graphical abstract of this review has been presented in Fig. 1.
Monomer-Free COS Production and Yield Improvement
Bioprocess Strategies
Acid hydrolysis using HCl is commonly used for industrial-scale COS production; however, the presence of larger amount of monomer, i.e., glucosamine units, leads to lower yield of COS [11]. They are also not considered as bioactive materials due to the possibility of contamination by toxic chemical compounds that lead to a lot of environmental pollution. Product profile has been compared after HCl hydrolysis and enzymatic degradation of chitosan (degree of acetylation 12 %, 89.2 kDa) using commercial pectinex ultra [12]. Shorter fragments were produced as a result of enzymatic degradation, and fractions of fully deacetylated COS were higher as compared to chemical hydrolysis; however, COS of up to degree of polymerization (DP) 16 were produced as a result of chemical hydrolysis, and proportion of monoacetylated COS was found more.
Earlier, enzymatic hydrolysis of chitosan was carried out in batch reactors [13, 14], where chitosanase enzyme from Bacillus pumilus BN-262 was mixed with 1 % chitosan (degree of deacetylation > 89 %) at 45 °C, 5.5 pH, and reaction was carried out for 1 h. Lack of enzyme reusability, lower yield, and getting crude product (COS mixture having various degree of polymerization) forced researchers to develop better methods for chitosan hydrolysis. Using ultrafiltration membrane (UFM) reactor for COS production especially larger one (penta and hexamers) has been the most common practice till date. A continuous flow stirred tank reactor with UFM (2 and 3 kDa) [15, 16] was used for improving the yield of physiologically active COS (pentamer and hexamers) from chitosan (degree of deacetylation (DD) 95 %). Chitosanase from Bacillus pumilus was used for hydrolysis, and after optimization of all the reaction parameters, 52 % yield was achieved. Chitosanase enzyme isolated from Bacillus cereus was isolated in three fractions by column chromatography; concentration of COS was 9.78 mg/ml, which was 48 % of total oligomers (high DP) in final product as compared to 29 % resulted from crude enzyme. Retention time was also varied in the reactor, and yield obtained was recorded to be 61 and 69 %, respectively, with 50 and 33 min of reaction time. Chitosanase enzyme from Streptomyces N174 along with chitinase enzyme from Pyrococcus and Trichoderma was used for hydrolysis of whole cell of Rhizopus oligosporus NRRL2710, cell wall of which is considered to be an abundant source of N-acetyl-D-glucosamine and D-glucosamine. Hydrolyzed products were purified by chromatographic steps; cation exchange as well as gel filtration and NMR and matrix-assisted laser desorption/ionization time of flight (MALDI-TOF) of hydrolyzed products (enzymatic) resulted Glc-GlcNAc, (Glc) 2-GlcNAc, and (GlcN) 2 as product [17]. A bipolar membrane electrodialysis system has been used for COS production from chitosan hydrolysis (100 kDa, DD 96 %) [18], where a three-chamber containing cell was constructed for COS production and purification. First compartment was acidified for making chitosan soluble, second was basified for chitosanase inactivation, while the last or third compartment was diluate for COS demineralization. This method was found to be advantageous as all the three steps are performed in single operation and acids and bases are regenerated in situ. A lower ash content of COS was obtained due to homopolar ion exchange membranes; fouling did not occur in the process, and method was found to be energy efficient. However, yield of COS was not determined in the process.
Use of Immobilized Enzymes
Enzymes in immobilized forms are easy to store, stable, facilitate continuous production, convenient in reactor design, can be used repeatedly, and are cost-effective. These are desirable over soluble enzymes; however, poor biocatalytic efficiency is a limiting factor for large-scale industrial bioprocessing to compete with the traditional chemical processes. Nanomatrices like nanoparticles (NP) and nanofibers have been used for decreasing mass transfer limitation which is high in case of chitosan due to their highly viscous solution [19, 20].
In biochemical production of COS, controlling enzymatic reaction for production of larger oligomers is still a challenging task. However, with immobilized chitosanase (Table 1), this problem has been solved to some extent and larger oligomers have been produced as compared to that of soluble enzymes. Dimer to hexamers has been obtained by immobilizing chitosanase enzyme on agar, polyacrylonitrile nanofibrous membranes (PANNFM), and NP. Chitosanase enzyme immobilized on chitin has been used for improving the reusability and yield of higher COS [23]. Agar or agarose gel particle-bound chitosanase has been used for pentamer and hexamer production in batch reaction condition [22]. Multipoint attachment method was used, and surface enzyme density, particle size, temperature, and initial substrate concentration were found to be factors affecting the yield of desirable products (i.e., COS having higher DP). Easier monitoring and stopping of hydrolytic reaction led to yield improvement of larger COS (penta and hexa) from hydrolysis of viscous solution of chitosan (DD 100 %, 370 kDa) with agar gel-immobilized catalysts. Multipoint attachment method on agar gel coated multidisc impeller and was used in reactor design for efficient control. This device showed improved mass transfer properties, and five batches of reaction could be achieved with 22 % yield [25]. However, method was limited by low affinity of bound enzyme for substrates, and hence, a lower production of COS having desirable chain length was observed as compared to soluble enzymes.
A two-step dual reactor system for continuous production of COS has been proposed [13]. In one of the reactor, immobilized enzyme was packed in the column, and it was attached with UFM reactor. Partially hydrolyzed chitosan (PHC) was prepared from viscous chitosan (DD 89 %) in the first reactor, and COS production took place in the UFM reactor. PHC types varied upon changing the flow rate of chitosan substrate concentration and reduced fouling of membranes (10 kDa). This approach was used to remove the drawback of batch reactors where chitosan is hydrolyzed randomly to produce COS, which are further hydrolyzed to monomer or may reduce the size of COS. Such setting reduces the chances of product inhibition, and high DP COS is possible to be achieved.
Chitooligosaccharide Synthesis by Transglycosylation
Chitosanases and GH-18 chitinases showing glycosyltransfer activity have been used for synthesis of COS. Streptomyces griseus HUT 6037 has been shown to produce two types of chitosanases catalyzing glycosyl transfer reaction in the hydrolysis of COS [26]. Upon hydrolysis of pentamer using this enzyme, tetramer, trimer, and dimer were produced and production of monomer or glucosamine was discouraged. In another study, small yield of chitooctamer was synthesized from mixture consisting of dimer, trimer, tetramer, pentamer, hexamer, and heptamer. Chitosanase from B. cereus NTU FC-4 was used for catalyzing reactions in reverse micellar microreactors formed by bis(2-ethylhexyl) sulfosuccinate (AOT) in isooctane [27]. In all these studies, water content was found to be an important factor in affecting the enzyme reaction, and higher oligomers acted as glycosyl acceptor. Lysozyme and chitinase (Trichoderma reesei KDR-11)-mediated chemo-enzymatic synthesis of COS has also been reported by transglycosylation reaction [28, 29]. Ammonium sulfate (0.85 and 30 %, respectively) has been used in the buffer for chain elongation. DP of COS was 4–9 in case of lysozyme study, and pentamer and hexamers were produced successfully when chitinase enzyme was used. A novel chitinase D enzyme isolated recently from Serratia proteamaculans showed both hydrolytic and transglycosylation (TG) activity [30]. This activity has also been enhanced by mutation strategies and preparing fusion chitinase protein [31, 32]. TG activity was improved by preparing fusion chimeras containing polycystic kidney diseases (PKD) alone and with both PKD and chitin-binding protein (CBP); improvement in activity was measured in terms of higher yield of COS (pentamer and hexamer) [33].
Non-Specific Biocatalysts for COS Production
Chitosan is generally susceptible to a number of enzymes and carbohydrases; proteases and lipases [34, 35] have been used for hydrolysis. Four types of randomly distributed glycosidic bonds in chitosan affect hydrolysis process, which could be two N-acetylated units (A–A), acetylated and deacetylated units (A–D), deacetylated and acetylated units (D–A), and two deacetylated units (D–D). The specificity of chitosanases with respect to the cleavage of four different glycosidic linkages in partially N-deacetylated chitosan is determined by the identity of the reducing/non-reducing ends and degree of deacetylation of chitosan. Egg white lysozyme is found to be almost exclusive toward the cleavage of glycosidic linkage between two acetylated units, while chitosanase enzyme from Bacillus sp. is found to be highly specific toward the deacetylated glycosidic linkages [14]. Chitinase enzyme could act on partially N-acetylated chitosan by recognizing GlnAc residues in the chitosan sequence [34]. However, a clear distinction between chitosanase and chitinase for the hydrolysis of differentially deacetylated chitosan is difficult. Moreover, chitosanases from different organisms also differ in their catalytic action, and that is mainly dependent on degree of deacetylation of chitosan [36]. However, it has been generally observed that chitosanases obtained from microbes produce relatively a higher yield of COS compared to chitosanases from other sources. Even though microbial chitosanases have shown to have excellent performances in COS production, they are too expensive to be utilized in large-scale industrial applications. Therefore, other commercial enzymes are utilized under specific conditions to produce COS with relatively low cost [35].
Hydrolysis of chitosan by cellulase enzyme has been well reported and has been summarized [37, 38]. Some bifunctional enzymes having both cellulase and chitosanase activity have also been reported [39, 40] to produce COS from chitosan (DD 93 %, 290 kDa) [20]. Protease, α-amylase, pectinase, and combination of various enzymes for chitosan hydrolysis have been used (Table 2). Chitinase, lysozyme, and cellulase enzyme combination have been used for hydrolysis of two types of chitosan having degree of deacetylation 80 and 92 %. It was observed that enzyme combination having lysozyme and degree of deacetylation 92 % and above was having large molecular weight chitosan as compared to other enzyme and substrate combination, which showed more antibacterial activity against Escherichia coli [14]. Chitosan (DA, 26 %, mol wt 71 ± 2 kDa) hydrolysis was also compared with various proteolytic enzymes like pepsin, papain, and pronase, all of which resulted in low molecular weight chitosan, COS, as well as monomer [44].
Chitosanase Enzyme
Most of the studies of gene cloning and overexpression for chitosanase have been reported from E. coli and Pichia pastoris [9]. Recently, chitosanase gene from marine bacterium Pseudomans QUC1 was inserted and transformed Yarrowia Po1h having two copies of csn gene secreted more enzyme than single copy [45]. Monomer-free COS (DP 2–6) was produced from hydrolysis of chitosan (DD 95 %) with this overly expressed enzyme. Gene expression has also been done in other cases, but very few of them have been studied for monomer-free COS production, like CSNV26 gene from Bacillus subtilis [46] and ctoA gene from Amycolatopsis [47] were cloned in E. coli; improvement in yield along with thermostability was established. However, COS production through these overexpressed enzymes were not determined.
Monomer-free COS (dimer and trimer) production were best compared and studied (concentration of individual COS not determined) when chitosanase gene for Janthinobacterium was expressed in E. coli. Expressed protein was able to hydrolyze chitohexamers to trimer and dimmers, while hexamer substrate conversion by crude enzyme of Janthinobacterium resulted in glucosamine accumulation. This phenomenon can be explained by the fact that organism could be secreting exo-enzyme in addition to endochitosanase [48]. However, overexpressed protein contained only endo-type-secreted enzyme protein, which resulted in the absence of glucosamine from the product. Similarly, when csn gene form Bacillus sp. 168 was overexpressed in E. coli, it hydrolyzed different chitosan substrates (DD 75 %) and produced dimer to hexamer and larger COS [49]. In this study, csn gene was fused to signal peptide sequence OmpA, and secreted enzyme showed more specific activity as well as stability over a wide range of pH. Monomer-free chitotrimer to chitohexamer (chitosan DD, 75–17 %) could also be produced by recombinant expressed chitosanase enzyme from another strain of Bacillus in E. coli [50]. Chitosanase gene from Fusarium sp. was introduced in E. coli using expression vector driven by T7 promoter, which resulted in enzyme overproduction [51], but monomer-free COS production was not determined.
Cloned chitosanase gene isolated from microbes and expressed in another host can be efficiently used for monomer-free COS production. High level of enzyme purification and the absence of unwanted enzyme (exochitosanase, which leads to glucosamine production) in the crude mixture can be used for improving the yield of COS. Overexpression of enzymes also leads to enhanced enzyme activity per unit as well as high specific activity, which, in turn, leads to more product yield. However, very few reports about cloning and overexpression of chitosanase gene for determination of COS are available (Table 3), and most have been done for the purpose of checking purification and homology with other chitosanase enzymes.
Use of marine wastes like squid pen powder, shrimp head powder, etc. has been suggested for cost-effective production of chitosanase enzyme (Table 4). However, most of these studies are limited to use them in growth and culture medium for screening and isolation; some pretreatment processes like autoclaving of substrates like squid pen powder have also been reported for enhancing the desired enzyme production [66]. Autoclaving of squid pen powder medium at 121 °C for 120 min and incubating culture at 25 °C for 5 days led to enhanced chitosanase enzyme by Serratia mercescens TKU011 [64].
Intracellular chitosanase has been reported from Mucor circinelloides, which was found to be bifunctional (lipase and chitosanase) and was able to depolymerize chitosan (DD 66–97, 121–421 kDa) [67]. Liposome prepared by 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC) has been reported to enhance the production of chitosanase enzyme from Streptomyces griseous (2.2 times enhancement of production and release) under the stress condition (heat at 41 °C). Hydrophobicity and net charges on surface of membranes, which changes upon enhanced interaction between cell membrane and liposome, are considered to play a role in releases of chitosanase enzyme from cell membrane [68]. Synthetic boron containing molecule was synthesized and was used for activation of chitosanase enzyme (showed highest activity on 60 % deacetylated chitosan) secreted by Penicillum janthinellum D4 which could be used for industrial processes involved in preparation of COS [69]. The enzyme was treated with the molecule 5 min prior to the addition of the substrates, and a maximum of 160 % activation was recorded. On the contrary, a synthetic boron containing molecule with BF3K side chain was identified as inhibitors in another study; however, it enhanced the growth of B. cereus TKU013 in squid pen powder (SPP) medium [70], which was explained due to enhanced secretion of chitinase enzyme. BioCHOS (a commercial COS mixture), a patented product, is being used against fungal plant pathogens in Norway. It is produced by controlled enzymatic hydrolysis of shrimp shell derived from by-product of shell fish industry (The United Kingdom Patent Application No. 1218954.4.).
While majority of studies related to identifying novel chitosanase enzyme has been revolving around microbial sources, it has also been reported from algal sources as well as plants parts like roots [71] and shoots. Identifying novel sources of industrial enzymes which can grow on cheap substrates are of commercial interest. Bamboo shoot chitosanase enzyme was used for production of low molecular weight chitosan by hydrolysis of crab shell chitosan [72], and cyanobacterial chitosanase has been reported from three strains of Anabaena [11] having antifungal activity. In case of cyanobacterial chitosanase, product profile contained chitodimer and more after hydrolysis of colloidal chitosan which confirmed the endo-nature of purified enzyme.
Fractionation and Purification of COS
Main application of COS is considered to be for human consumption as prebiotics, medicines, and functional foods, which require a high level of product purity [73]. Both chemical and enzymatic method of chitosan hydrolysis gives COS mixture of varied molecular weight. Developing a technology separating COS from reaction mixtures is still a challenging task and requires efficient coupling of chemical engineering knowledge with membrane separation process. Potential applications of COS required enriched fractions of these molecules having defined molecular weight.
Ultrafiltration
COS fractionation using UFM reactor is the most frequent method reported in the literature so far. Operational variables reportedly affecting membrane separation of chitooligosaccharides are types of membranes, operating temperature, pH, and concentration of feed solutions. Enzyme in free and immobilized form has been used for continuous production of COS. COS of high molecular weight as product, prevention of undesirable hydrolysis, and diminished product inhibition are the advantages associated with it as compared to batch hydrolysis. Method optimization for COS fractionation using hollow fiber UFM with 3 and 2 kDa molecular weight cutoff has been reported [16]. Amount of enzyme and chitosan concentration was optimized, and a maximum 52 % yield of COS was achieved when calculated on the basis of fed chitosan concentration in the reactor. Immobilized chitosanase has been used in place of free enzyme for improving the operational stability of membrane bioreactor, and half-life of the productivity of reactor was calculated to be 50 days under the optimized conditions [21].
Combined method of UF and nanofiltration (NF) have also been used for COS separation [16]. Larger molecules like suspended solids and insoluble molecules were removed using 0.22 μm membrane and were followed by UFM (1 kDa). Permeate collected after this process was further separated by NF with the hollow fiber material. Precipitation was done by adding threefold volume of ethanol to the permeate solution after adjusting the pH 8.3. Precipitate was lyophilized after centrifugation and further purified by CM Sephadex C-25 column chromatography.
UF combined with electrodialysis of varying pH has also been used for potentially selective and specific separation of COS (dimer, trimer, and tetramer) from each other [74]. UFM with 10 kDa cutoff was used, and pH of the medium was varied for different rate of electromigration and was optimized further. Configuration of electrodialysis UFM affected the separation, and migration was observed in only one configuration (anode-AEM1-UFM-AEM2-cathode). Rate of migration was dependent on pH of the medium and processing time. Highest rate of migration was observed between pH 4.0 to 6.0. Protonation and deprotonation of amine group of COS varied with change in pH of the medium, which, in turn, affects the electrophoretic mobility of each oligomer and consequently retardation of other oligomers. Yield of dimer was found to be best at pH 7.0 after 2 h.
Column Chromatography
Charcoal-celite as column chromatography material has been used to separate mixture of monosaccharides, disaccharides, trisaccharides, and tetrasaccharides of chitosan and chitin oligosaccharides [75, 76]. Mixture was readily separated into individual component when eluted with ethanol-water mixture, and for recovering particular fraction, different combinations were used. Toyopearl HW-40S column (5 × 68 cm), CM Sephadex C-25 column, and Dowex (H+) ion exchange chromatography have been used for separation of COS having DP (2–12) and DP (2–6), respectively [3, 77].
Size exclusion chromatography like three XK26 column packed with SuperdexTM30 coupled in a series having overall dimension of 2.6 × 180 cm with ammonium acetate buffer as mobile phase [4] has been used for COS separation. In each run, 100 mg of chitosan hydrolyzates was used with RI detector for signal detection. Fractions (3 to 12 mer) were collected and identified by MALDI-TOF MS. Salt of fractions was removed by dialysis and stored after lyophilization.
Immobilized metal affinity chromatography (IMAC) on the basis of differences in the interactions of chelated copper(II) ions has also been used for separation of COS [78]. Chromatographic support polyhydroxylic in nature was functionalized by iminodiacetate (IDA), carboxymethyl aspartate (CM-AsP), and tris(carboxymethyl) ethylenediamine (Tris TED). Enrichment and separation of COS was found to be up to 95 % for dimer and trimer and 90 % for tetramer; yield variation was found to be 60–95 %. Recently, a mixed mode chromatography using hydrophilic interaction and weak cation exchange column has also been used [79]. A weak cation exchanger WCX was used for separation, while hydrophilic interaction liquid chromatography (HILIC) column has been used for separation on the basis of hydrophobic interaction. Mixed column method provided better resolution than any of the method alone, and COS separated were trimer, tetramer, and pentamer in nature.
Membrane fouling problem is associated with UFM and ion exchange columns, so other methods like solvent extraction and encapsulation technology have also been used for COS filtration. Need of expensive equipment and lack of large-scale preparations are drawbacks associated with size exclusion and ultrafiltration membranes, and COS up to tetramer only could be isolated with metal affinity chromatography.
Solvent Fractionation
Selective fractionation of COS in methanol/water has been reported [12, 80]. Neutralized fraction of COS is precipitated with methanol in the concentration of 70, 80, and 90 % (v/v), and each precipitate was centrifuged, washed with corresponding methanol fraction, and was dried under vacuum. Concentration of supernatant is done under reduced pressure. Hydrolysate treatment with acetone-water was used to improve separation of COS of DP below and above 6. Two fractions were separated after treatment with acetone: one with DP 3 to 5 and the other from 6 to 11. Their yield was calculated around 30 and 55 %, respectively. COS fractionation in aqueous solutions containing various ratios of methanol has been used for recovering COS of different molecular weight (TW200528465(A)-2005-09-01, LIN CHIA-WEN).
Applications of COS
Uses of bioactive COS in various sectors have been updated from time to time [1, 81]. Applications of COS for growth of plants like corn (US document identifier US 20130079225 A1), soybean (US document identifier US 20130090236 A1), and other plants by treating seeds with COS during germination (US document identifier US 20130079224 A1) or by initiating early flowering or by increasing number of buds (US document identifier US 20070027032 A1) and other agricultural purposes have been patented. COS of molecular weight (1–3, 3–5, and 5–10 KDa) has also been patented as an active ingredient in anti-ageing cosmetic composition (US document identifier US 20130345168A1) which protects skin against UV damage. Recently, chemical modification and replacement of active functional groups have provided immense material to be used in various fields as biological properties of modified COS depend on their molecular weight and functional groups. Interesting biological properties from these derivatives of COS have been reported over unmodified molecules [82].
Amino ethyl group has been added at C-2 position (amine group), and hydroxyl group at C-6 position have been replaced; dimethyl amino ethyl and diethyl amino ethyl derivatives have also been synthesized to alter the biological activities. Carboxylated COS (CCOS), derived by introducing carboxyl groups at amino position, were studied for their effect on MMP-9 expression on human fibrosarcoma cells [83]. Angiotensin-converting enzyme (ACE) inhibition studies of synthesized CCOS were done and were found to be beneficial due to enhanced binding ability of COS to the obligatory active site of the enzyme [84]. Gallic acid-conjugated derivatives of COS (G-COS) were prepared and studied for its effect on intracellular free radical generation [85]. Both gallic acid and COS were dissolved in methanol, and solutions were added to each other; precipitate was formed, and freeze dried G-COS was stored. It was found to be a potent scavenger of free radicals that prevented oxidative damages to DNA/cellular biomolecules and increased level of intracellular antioxidant, enzymes in living cells. Phenolic acid-conjugated COS showed strong antioxidant activities, and acids like p-coumaric acids, p-hydroxybenzoic acid, protocatechuic acids were used for synthesis of these derivatives [86]. Different degree of substituted sulfated COS was prepared [87] by dissolving chitosan in formamide and adding chlorosulfonic acid. Protective effect of sulfated COS on H2O2-induced damage was studied on MIN6 cells; enhanced viability of damaged cells has been reported by showing antioxidant effects. Antioxidant effect of sulfated COS has also been reported on Chang cells [88] and H2O2-induced oxidative damaged cells; protective effect on liver and DNA against oxidative stress has also been reported.
Chitosan is positively charged and are known to have mucoadhesive properties due to negative charges present on mucosa. Doxorubicin, a potent anticancer drug, is known for its severe side effects. So, to improve its effectiveness as antitumor drug, solid lipid NP (SLN)-based carrier has been developed for drug delivery. COS shows excellent performance in sustained drug releases and bioavailability properties, thus being seen having a lot of potential as NP drug carrier and for SLN coating [89]. Nanostructure lipid carrier (NLC) carrying flurbiprofen drug coated with COS has been studied for ocular drug delivery [33]. COS has also been shown as potential drug carrier of zidovudine for renal delivery [61].
COS Research: Future Perspective
Despite making a lot of headways in the research related to bioactive COS, getting them in purified form with defined degree of deacetylation and exact known sequence of monomers (acetylated and deacetylated) is still a distant dream. Future research related to COS will tend toward getting highly purified fractions of COS with proper characterization (native and derivatized) to be used in food, pharmaceuticals, and cosmetic industries (downstream processing). Yield improvement using overexpressed chitosanase enzyme, production, along with purification and cost analysis will be explored for COS commercialization. Recent biochemical strategies indicate that enzyme/protein engineering, reactor designing, and use of immobilized novel biocatalysts are the keys. Exploration of bioactivities related to COS will never be stopped (Fig. 2) as industry is looking for novel bioactive molecules and still new activities are being discovered in sectors like food, pharma, and cosmetics. Among future applications, use of COS for drug delivery has shown potential, and lots of studies have been reported recently.
References
Kim, S. K., & Rajapakse, N. (2005). Enzymatic production and biological activities of chitosan oligosaccharides (COS): a review. Carbohydrate Polymer, 62, 357–68.
Kumaya, H., Nakahar, Y., Nukada, T., Ito, Y., & Ogawa, T. (1993). Stereocontrolled synthesis of chitosan dodecamer. Carbohydrate Research, 243, C1–C7.
Li, K., Xing, R., Liu, S., Li, R., Qin, Y., Meng, X., & Li, P. (2012). Separation of chitooligomers with several degrees of polymerisation and study of their antioxidant activity. Carbohydrate Polymer, 88, 896–903.
Wu, H., Aam, B. B., Wang, W., Norberg, A. L., Sorlie, M., Eijsink, V. G. H., & Du, Y. (2012). Inhibition of angiogenesis by chitooligosaccharides with specific degrees of acetylation and polymerisation. Carbohydrate Polymer, 89, 511–518.
Nidheesh, T., Pal, G. K., & Suresh, P. V. (2015). Chitooligomers preparation by chitosanase produced under solid state fermentation using shrimp by product as substarte. Carbohydrate Polymer, 121, 1–9.
Liu, Y. L., Jiang, S., Ke, Z. M., Wu, H. S., Chi, C. W., & Guo, Z. Y. (2009). Recombinant expression of chitosanase and its application in chitooligosaccharides production. Carbohydrate Research, 344(6), 815–819.
Chen, X., Zhai, C., Kang, L., Li, C., Yan, H., Zhou, Y., & Ma, L. (2012). High level expression and characterization of a highly thermostable chitosanase from Aspergillus fumigatus in Pichia pastoris. Biotechnology Letter, 34(4), 689–694.
Zhang, H., Sang, Q., & Zhang, W. (2012). Satatistical optimization of chitosanase production by Aspergillus sp. QD-2 in submerged fermentation. Annals of Microbiology, 62(1), 193–201.
Thadathil, N., & Velappan, S. P. (2014). Recent developments in chitosanase research and its biotechnological applications: a review. Food Chemistry, 150(1), 392–399.
Somashekar, D., & Joseph, R. (1996). Chitosanases: properties and applications. Bioresource Technology, 55(1), 35–45.
Uchida, Y., Izume, M., Ohtakara, A. (1989). Preparation of chitosan oligomers with purified chitosanase and its application. In G. Braek T. Anthonsen, & P. Sandford (Eds.),Chitin and chitosan: Sources, chemistry, biochemistry, physical properties and applications (pp. 372–382). Barking, UK: Elsevier Applied Science 1989.
Cabrera, J. C., & Cutsem, P. V. (2005). Preparation of chitooligosaccharides with degree of polymerization higher than six by acid or enzymatic degradation of chitosan. Stoscheck, CM. Quantitation of Protein. Biochem Engineering Journal, 25, 165–172.
Jeon, Y. J., & Kim, S. K. (2000). Production of chitooligosaccharides using an ultra filtration membrane reactor and their antibacterial activity. Carbohydrate Polymer, 41, 133–141.
Varum, K. M., Holme, H. K., Izume, M., Stokke, B. T., & Smidsrod, O. (1996). Determination of enzymatic hydrolysis specificity of partially N-acetylated chitosans. Biochem et Biophy Acta, 1291, 5–15.
Kuroiwa, T., Izuta, H., Nabetani, H., Nakajima, M., Sato, S., Mukataka, S., & Ichikawa, S. (2009). Selective and stable production of physiologically active chitosan oligosaccharides using an enzyme membrane bioreactor. Process Biochemistry, 44(3), 283–287.
Lin, Y. W., Hsiao, Y. C., & Chiang, B. H. (2009). Production of high degree polymerized chitooligosaccharides in a membrane reactor using purified chtitosanase from Bacillus cereus. Food Research International, 42, 1355–1361.
Mahata, M., Shinya, S., Masaki, E., Yamamoto, T., Ohnuma, T., & Brezinski, R. (2014). Production of chitooligosaccharides from Rhizopus oligosporus NRRL 2710 cells by chitosanase digestion. Carbohydrate Research, 383, 27–33.
Shee, F. L. T., & Bazinet, L. (2009). Cationic balance and current efficiency of three compartment bipolar membrane electrodialysis system during the preparation of chitosan oligomers. Journal of Membrane Science, 341, 46–50.
Kuroiwa, T., Noguchi, Y., Nakajima, M., Sato, S., Sukekuni, M., & Ichikawa, S. (2008). Production of chitosan oligosaccharides using chitosanase immobilized on amylose-coated magnetic nanoparticles. Process Biochemistry, 43, 62–69.
Sinha, S., Dhakate, S. R., Kumar, P., Mathur, R. B., Tripathi, P., & Chand, S. (2012). Electrospun polyacrylonitrile nanofibrous membranes for chitosanase immobilization and its application in selective production of chitooligosaccharides. Bioresource Technology, 115, 152–157.
Zheng, L. Y., & Xiao, Y. L. (2004). Penicillium sp ZD-Z1 chitosanase immobilized on DEAE cellulose by cross linking reaction. Chemical Engineering, 21(1), 201–205.
Kuroiwa, T., Ichikawa, S., Sato, S., Hiruta, O., Sato, S., & Mukataka, S. (2002). Factors affecting the composition of oligosaccharides produced in chitosan hydrolysis using immobilized chitosanases. Biotechnology Progress, 18, 969–974.
Zeng, J., & Zheng, L. Y. (2002). Studies on Penicillium sp. ZDZ1 chitosanase immobilized on chitin by cross-linking reaction. Process Biochemistry, 38, 531–535.
Song, J. Y., Alnaeeli, M., & Park, J. K. (2014). Effective digestion of chitosan using chitosanase immobilized on silica-gel for the production of multisize chitooligosaccharides. Process Biochemistry, 49(12), 2107–2113.
Montilla, A., Ruiz, M. A., Corzo, N., Giacomini, C., & Irazoqui, C. (2013). Enzymatic generation of chitooligosaccharides from chitosan using soluble and immobilized glycosyltransferase (Branchzyme). Journal of Agriculture and Food Chemistry, 61(43), 10360–10367.
Tanabe, T., Morinaga, K., Fukamizo, T., & Mitsutomi, M. (2003). Novel chitosanase from Streptomyces griseus HUT 6037 with transglycosylation activity. Bioscience Biotechnology Biochemistry, 67(2), 354–364.
Hsiao, Y. C., Lin, Y. W., Su, C. K., & Chiang, B. H. (2008). High degree polymerized chitooligosaccharide synthesis by chitosanase in bulk aqueous system and reverse micellar microreactors. Process Biochemistry, 43, 76–82.
Akiyama, K., Kawazu, K., & Kobayashi, A. (1995). A novel method for chemo-enzymatic synthesis of elicitor active chitosan oligomers and partially N-deacetylated chitin oligomer using N-acylated chitotrioses as substrates in a lysozyme catalyzed transglycosyaltion reaction system. Carbohydrate Research, 279, 151–160.
Usui, T., Matsui, H., & Isobe, K. (1990). Enzymatic synthesis of useful chito-oligosaccharides utilizing transglycosylation by chitinolytic enzyme in a buffer containing ammonium sulphate. Carbohydrate Research, 203, 65–77.
Purushotham, P., & Podile, A. R. (2012). Synthesis of long chain chitooligosaccharides by a hyper transglycosylating processive endochitinase of Serratia proteamaculans 568. Journal of Bacteriology, 194(16), 4260–4271.
Zakariassen, H., Hansen, M. C., Jornali, M., Eijsink, V. G., & Sorlie, M. (2011). Mutational effects on trnasglycosylation activity of family 18 chitinases and construction of a hypertransglycosylating mutant. Biochemistry, 50, 5693–5703.
Sirimontree, P., Suginta, W., Sritho, N., Kanda, Y., Shinya, S., Ohnuma, T., & Fukamizo, T. (2014). Mutation strategies for obtaining chitooligosaccharides with longer chains by transglycosylation reaction of family GH 18 chitinase. Bioscience Biotechnology Biochemistry, 78(12), 2014–2021.
Madhuprakash, J., Gueddari, N. E., Moerschbacher, B. M., & Podile, A. R. (2015). Catalytic efficiency of chitinase-D on insoluble chitinous substrates was improved by fusing auxiliary domains. Plos One. doi:10.1371/journal.pone.0116823.
Aiba, S. (1994). Preparation of N-acetylchitooligosaccharides by lysozymic hydrolysates of partially N-acetylated chitosans. Carbohydrate Research, 261, 297–306.
Zhang, H., Du, Y., Yu, X., Mitsutomi, M., & Aiba, S. (1999). Preparation of chitooligosaccharides from chitosan by a complex enzyme. Carbohydrate Research, 320, 257–260.
Kurita, K. (1998). Chemistry and application of chitin and chitosan. Polymer Degradation and Stability, 59, 117–120.
Xia, W., & Liu, P. (2008). Advance in chitosan hydrolysis by non-specific cellulases. Bioresource Technology, 99(15), 6751–6762.
Xie, U., Hu, J., Wie, Y., & Hong, X. (2009). Preparation of chitooligosaccharides by enzymatic hydrolysis of chitosan. Polymer Degradation and Stability, 94(10), 1895–1899.
Reyes, M. P., & Corona, F. G. (1997). The bifunctional enzyme chitosanase-cellulase produced by the gram negative microorganism Myxobacter sp-AL-1 is highly similar to Bacillus subtilis endoglucanases. Archive of Microbiology, 168, 321–327.
Liu, J., & Xia, W. (2006). Purification and characterization of a bifunctional enzyme with chitosanase and cellulase activity from commercial cellulase. Biochemical Engineering Journal, 30(1), 82–87.
Lee, D. X., Xia, W. S., & Zhang, J. L. (2008). Enzymatic preparation of chitooligosaccharides by commercial lipase. Food Chemistry, 111, 291–295.
Lee, Y. S., Yoo, J. S., Chung, S. Y., Lee, Y. C., Cho, Y. S., & Choi, Y. L. (2006). Cloning, purification and characterization of chitosanase from Bacillus sp. DAU 101. Applied Microbiology Biotechnology, 73, 113–121.
Kim, P., Kang, T. H., Chung, K. J., Kim, I. S., & Chung, K. (2004). Purification of a constitutive chitosanase produced by Bacillus sp MET 1299 with cloning and expression of the gene. FEMS Microbioogy Letter, 240, 31–39.
Kumar, A., & Tharanathan, B. V. (2004). A comparative study of depolymerisation of chitosan by proteolytic enzymes. Carbohydrate Polymer, 58, 75–83.
Liu, G. L., Li, Y., Zhou, H. X., Chi, Z. M., & Madzak, C. (2012). Overexpression of bacterial chitosanase gene in Yarrowia lipolytica and chitosan hydrolysis by the recombinant chitosanase. Journal of Molecular Catalysis B: Enzymatic, 83, 100–107.
Feki, O. K., Frikha, F., Zouari, I., & Jaoua, S. (2013). Heterologous expression and secretion of an antifungal Bacillus subtilis chitosanase (CSNV26) in Escherichia coli. Bioprocess Biosystem Engineering, 36(7), 985–92.
Saito, A., Ooya, T., Miyatsuchi, D., Fuchigami, H., Terakado, K., & Nakayama, S. (2009). Molecular characterization and antifungal activity a family 46 chitosanase from Amycolatopsis sp CsO-2. FEMS Microbology Letter, 293, 79–84.
Johnsen, M .G., Hansen, O. C., & Stougaard, P. (2010). Isolation, characterization 497 on and 498 heterologous expression of a novel chitosanase from Janthinobacterium sp. strain 4239. Microbial Cell Factories, 9, 5.
Pechsrichuang, P., Yoohat, K., & Yamabahi, M. (2013). Production of recombinant Bacillus subtilis chitosanase, suitable for biosynthesis of chitosan-oligosaccharides. Bioresource Technology, 127, 407–414.
Yoon, H. G., Yang, S. W., Kim, H. Y., Kim, H. K., Shin, D. H., Hong, B. S., & Cho, H. Y. (2000). Analysis of essential leucine residue for catalytic activity of novel thermostable chitosanase by site-directed mutagenesis. Journal of Protein Chemistry, 19(7), 621–630.
Shimosaka, M., Kumehara, M., Zhang, X. Y., Nogawa, M., & Okazaki, M. (1996). Cloning and characterisation of a chitosanase gene from the plant pathogenic fungus Fusarium solani. Journal of Fermentation and Bioengineering, 82(5), 426–431.
Kang, L., Chen, X. M., Fu, & Ma, L. (2012). Recombinant expression of chitosanase from Bacillus subtilis HD145 in Pichia pastoris. Carbohydrate Research, 352(1), 37–43.
Su, C., Wang, D., Yao, L., & Yu, Z. (2006). Purification, characterization and gene cloning of a chitosanase from Bacillus species strain S65. Journal of Agriculture and Food Chemistry, 54, 4208–4214.
Kimoto, H., Kusaoke, H., Yamamoto, I., Fujii, Y., Onodera, T., & Taketo, A. (2002). Biochemical and genetic properties of Paenibacillus glycosyl hydrolase having chitosanase activity and discoidin domain. Juornal of Biological Chemistry, 277, 14695–14702.
Lee, H. S., Jang, J. S., Choi, S. K., Lee, D. W., Kim, E. J., Jung, H. C., & Pan, J. G. (2007). Identification and expression of GH-8 family chitosanase from several Bacillus thuringiensis subspecies. FEMS Microbiology Letter, 277(2), 133–141.
Choi, Y. J., Kim, E. J., Piao, Z., Yun, Y. C., & Shin, Y. C. (2004). Purification and characterization of chitosanase from Bacillus sp. strain KCTC 0377 BP and its application for the production of chitosan oligosaccharides. Applied Environmental Microbiology, 70(8), 4522–4531.
Shimono, K., Matsuda, H., & Kawamukai, M. (2002). Functional expression of chitinase and chitosanase and their effects on morphologies in the yeast Schizosaccharomyces pombe. Bioscience Biotechnology Biochemistry, 66(5), 1143–1147.
Li, S., Chen, L., Wang, C., & Xia, W. (2008). Expression, purification and characterization of endo-type chitosanase of Aspergillus sp. CJ22-326 from Escherichia coli. Carbohydrate Research, 343(17), 3001–3004.
Jiang, X., Chen, D., Chen, L., Yang, G., & Zou, S. (2012). Purification, characterization and action mode of a chitosanase from Streptomyces roseolus induced by chitin. Carbohydrate Research, 355, 40–44.
Wang, S. L., Tseng, W. N., & Liang, T. W. (2011). Biodegradation of shell fish wastes and production of chitosanase by a squid pen assimilating bacterium Acinetobacter calcoaceticus TKU 024. Biodegradation, 22, 939–948.
Liang, Z., Gong, T., Sun, X., Tang, J. Z., & Zhang, Z. (2012). Chitosan oligomers as drug carrier for renal delivery of zidovudine. Carbohydrate Polymer, 87(3), 2284–2290.
Wang, S. L., Chen, T. R., Liang, T. W., & Wu, P. C. (2009). Conversion and degradation of shellfish wastes by Bacillus cereus TKU018 fermentation for the production of chitosanases and bioactive material. Biochemical Engineering Journal, 48, 111–117.
Wang, S. L., Chen, S. J., & Wang, C. L. (2008). Purification and characterization of chitinases and chitosanases from a new species strain Pseudomonas sp. TKU015 using shrimp shells as a substrate. Carbohydrate Research, 343, 1171–1179.
Wang, S. L., Lin, C. L., Peng, J. H., Liang, T. W., & Liu, K. C. (2008). Purification and characterization of a chitosanase from Serratia mercescens TKU011. Carbohydrate Research, 343, 1316–1323.
Wang, S. L., Liou, J. Y., Liang, T. W., & Liu, K. C. (2009). Conversion of squid pen by using Serratia sp. TKU020 fermentation for the production of enzymes, antioxidants and N-acetylchitooligosaccharides. Process Biochemistry, 44(8), 854–861.
Wang, S. L., Wu, P. C., & Liang, T. W. (2009). Utilization of squid pen for the efficient production of chitosanase and antioxidants through prolonged autoclave treatment. Carbohydrate Research, 344(8), 979–984.
Struszczyk, K., Antczak, M. S., Walczak, M., & Pomianowska, E. (2009). Isolation and purification of Mucor circinelloides intracellular chitosanolytic enzymes. Carbohydrate Polymer, 78, 16–24.
Ngo, D. N., Qian, Z. J., Je, J. Y., Kim, M. M., & Kim, S. K. (2008). Aminoethyl chitooligosaccharides inhibit the activity of angiotensin converting enzyme. Process Biochemistry, 43, 119–123.
Nguyen, A. D., Huang, C. C., Liang, T. W., Nguyen, V. B., Pan, P. S., & Wang, S. L. (2014). Production and purification of fungal chitosanase and chitooligomers from Penicillium janthinellum D4 and discovery of the enzyme activators. Carbohydrate Polymer, 108, 331–337.
Liang, T. W., Chen, Y., Pan, P. S., & Wang, S. L. (2014). Purification of chitinase/chitosanase from Bacillus cereus and discovery of an enzyme inhibitor. International Journal of Biological Macromolecule, 63, 8–14.
Sinha, S., Tripathi, P., & Chand, S. (2012). A new bifunctional chitosanase enzyme from Streptomyces sp. and its application in production of antioxidant chitooligosaccharides. Applied Biochemistry Biotechnology, 167, 1029–1039.
Chang, Y. M., Lee, Y. J., Liao, J. W., Jhan, J. K., Chang, C. T., & Chung, Y. C. (2014). In vitro and in vivo safety evaluation of low molecular weight chitosan prepared by hydrolyzing crab shell chitosans with bamboo shoot chitosanase. Food and Chemical Toxicology, 71, 10–16.
Pinelo, M., Jonsson, G., & Meyer, A. S. (2009). Membrane technology for purification of enzymatically produced oligosaccharides: molecular and operational features affecting performance. Separation and Purification Technology, 70, 1–11.
Aider, M., Brunet, S., & Bazinet, L. (2008). Effect of pH and cell configuration on the selective and specific electrodialytic separation of chitosan oligomers. Separation and Purification Technology, 63(3), 612–619.
Singh, S., Packwood, J., Samuel, C. J., Critchley, P., & Crout, D. H. G. (1995). Glycosidase-catalyzed oligosaccharides synthesis: preparation of N-acetyl chitooligosaccharides using the β-N acetylhexosaminidase of Aspergillus oryzae. Carbohydrate Research, 279, 293–305.
Rupley, J. A. (1964). The hydrolysis of chitin by concentrated hydrochloric acid, and preparation of low molecular weight substrates for lysozyme. Biochimica et Biophysica Acta, 83, 245–248.
Gao, X. A., Zhang, Y. F., Park, R. D., Huang, X., Zhao, X. Y., Xie, J., & Jin, R. D. (2012). Preparation of chitooligosaccharides from chitosan using crude enzyme of Bacillus cereus D-11. Journal of Applied Biological Chemistry, 55(1), 13–17.
Devedec, F. L., Bazinet, L., Furtos, A., Venne, K., Brunet, S., & Mateescu, M. A. (2008). Separation of chitosan oligomers by immobilized metal affinity chromatography. Journal of Chromatography, 1194, 165–171.
Dong, X., Shen, A., Gou, Z., Chen, D., & Liang, X. (2012). Hydrophilic interaction/weak cation exchange mixed mode chromatography for chitooligosaccharides separation. Carbohydrate Research, 361, 195–199.
Lee, M. Y., Var, F., Shin, Y. Y., Kajiuchi, T., & Wang, J. W. (1999). Optimum condition for precipitation of chitosan oligomers with DP 5–7 in concentrated hydrochloric acid at low temperature. Process Biochemistry, 34(5), 493–500.
Xia, W., Liu, P., Zhang, J., & Chen, J. (2011). Biological activities of chitosan and chitooligosaccharides. Food Hydrocolloids, 25(2), 170–179.
Lodhi, G., Kim, Y.S., Hwang, J.W., Kim, S. K., Jeon, Y. J., Je, J. Y., Ahn, C. B.,& Moon, S.(2014). Chitooligosaccharides and its derivatives: preparation and biological applications. Biomedical Research International doi:10.2014/2014/654913.
Rajapakse, N., Kim, M. M., Mendis, E., Huang, R., & Kim, S. K. (2006). Carboxylated chitooligosaccharides (CCOS) inhibit MMP-9 expression in human fibrosarcoma cells via downregulation of AP-1. Biochimica et Biophysica Acta, 1760(12), 1780–1788.
Huang, R., Mendis, E., & Kim, S. K. (2005). Improvement of ACE inhibitory activity of chitooligosaccharides (COS) by carboxyl modification. Bioorganic and Medicinal Chemistry, 13(11), 3649–3655.
Ngo, D. H., Qian, Z. J., Ngo, D. N., Vo, T. S., Wijesekara, I., & Kim, S. K. (2011). Gallyl chitooligosaccharides inhibit intracellular free radical-mediated oxidation. Food Chemistry, 128(4), 974–981.
Eom, T. K., Senevirathne, M., & Kim, S. K. (2012). Synthesis of phenolic acid conjugated chitooligosaccharides and evaluation of their antioxidant activity. Environmental Toxicology and Pharma, 34(2), 519–527.
Lu, X., Guo, H., Sun, L., Zhang, L., & Zhang, Y. (2013). Protective effect of sulfated chitooligosaccharides with different degree of substitution in MIN6 cells. International Journal of Biological Macromolecule, 52, 92–98.
Lee, S. J., Kim, E. K., & Hwang, J. W. (2009). Antioxidative effect of sulfated chitooligosaccharides on oxidative injury. Journal of Chitin and Chitosan Science, 14(4), 192–196.
Ying, X. Y., Cui, D., Yu, L., & Du, Y. Z. (2011). Solid lipid nanoparticles modified with chitosan oligosaccharides for controlled releases of doxorubicin. Carbohydrate Polymer, 84, 1357–1364.
Acknowledgment
Sujata Sinha is thankful to Department of Science and Technology (DST), Government of India for providing fellowship in the form of WOS-A (DST NO. SR/WOS-A/LS-129/2009).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sinha, S., Chand, S. & Tripathi, P. Recent Progress in Chitosanase Production of Monomer-Free Chitooligosaccharides: Bioprocess Strategies and Future Applications. Appl Biochem Biotechnol 180, 883–899 (2016). https://doi.org/10.1007/s12010-016-2140-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-016-2140-6