Abstract
MicroRNAs (miRNAs) serve a crucial role in numerous biological processes, such as acute pancreatitis development. Due to its low abundance and high similarity among homogeneous family members, sensitive and reliable detection of microRNA remains a formidable challenge. By combining the three-way junction-assisted rolling circle amplification (RCA) with the trans-cleavage of Cas12a, we propose a novel fluorescent technique for sensitive miRNA detection. In order to increase the amplification efficiency of RCA-based methods, catalytic hairpin amplification (CHA) is incorporated into the RCA process, playing the roles of specific target recognition and three-way junction formation. Consequently, the method demonstrated a six-orders-of-magnitude detection range and a LOD as low as 27 aM, making it a promising method for the early diagnosis of various diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
For the clinical diagnosis of diseases, the quantitative assessment of disease biomarkers must be sensitive and trustworthy. Being an endogenous short-stranded non-coding RNA, microRNAs (miRNAs) play a significant role in many physiological processes, including the post-transcriptional regulation of mRNAs and acute pancreatitis development [1,2,3]. The aberrant expression level of miRNA is closely associated with the development of pancreatitis and is capable of discriminating tissues with different biological behaviors [4, 5]. For example, several miRNAs are abnormally expressed in patients with pancreatitis and pancreatic tumors, such as miRNA-21-5p [6, 7]. Former researches have illustrated that the miRNA-21-5p is high expressed in patients with pancreatic cancer, which is identified as a novel non-invasive biomarkers for the clinical diagnosis of pancreatic cancer [8]. However, sensitive and reliable detection of miRNAs from serum or other complicated samples remains a huge challenge due to that the low abundance of miRNAs and high similarity between homogenous family members.
Reverse transcription polymerase chain reaction (RT-PCR) [9, 10], northern blotting [11, 12], and miRNA microarrays [13] are currently the common techniques for miRNA detection. These techniques are criticized for a number of shortcomings even though they are frequently used in fundamental research and early clinical applications. For instance, the RT-PCR technique is sensitive and can be used for precise miRNA characterization, but it necessitates complex primer design, cumbersome thermal cycle equipment, and well-educated trainers. Although northern blotting method is relatively simple, this method requires large amount of samples to run the assay. Recently, a variety isothermal methods have been developed, including rolling circle amplification (RCA) [14, 15], catalytic hairpin reaction (CHA) [16,17,18], primer exchange reaction (PER), and loop mediated isothermal amplification (LAMP) [19]. These methods have made some progress to the traditional miRNA detection approaches. Among them, the RCA method has attracted abundant attention due to its high efficiency in amplifying target sequences under constant thermal. Various methods have been constructed on the basis of RCA for sensitive and accurate miRNA analysis. For example, Ruixuan Wang et al. proposed a novel fluorescent miRNA detection approach by integrating the RCA and CRISPR-Cas9 system [20]. Gong Zhang et al. reported a method with enhanced miRNA detection sensitivity by integrating the RCA and trans-cleavage activity of CRISPR-Cas12a [21]. Although these techniques demonstrated good detection performance with a limit of detection at the fM level, the sensitivity for the RCA-based approaches still requires improvement. Many efforts have been made in recent years to improve the detection sensitivity of RCA-based approach, basically by involving enzymes (e.g., DSN enzyme) for signal amplification. However, using enzymes to assist signal amplification may conflict with the RCA process, leading to unstable detection outcomes. Therefore, it is still significant to develop a highly sensitive and reliable approach for quantitative determination of miRNAs.
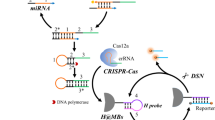
Herein, a novel fluorescent assay is proposed based on three-way junction-assisted RCA integrated with trans-cleavage of Cas12a for the detection of miRNA-21. In this method, a catalytic hairpin reaction that can realize enzyme-free assembly of double-strand DNA (dsDNA) is exploited to assist the cyclized of padlock sequences in RCA. Repeated single-strand DNA sections are transcribed during the RCA process, activating the Cas12a enzyme’s trans-cleavage activity and resulting in the production of signals. Eventually, the proposed approach exhibits a low limit of detection at aM level, making it a promising platform for the early diagnosis of various diseases.
Material and Methods
Materials and Reagents
The oligonucleotides sequences used in this research were adapted from former reports. The details of the oligonucleotides sequences are listed in Table S1. The sequences were bought and purified by Sangon Biotechnology Co., Ltd. (Shanghai, China). TE buffer containing 10 mM Tris and 1 mM EDTA was purchased from Sangon Biotechnology Co., Ltd. (Shanghai, China). The T4 DNA ligase, phi29 enzyme, Cas12a enzyme, and NEB buffer 2 that contains 500 mM NaCl, 100 mM MgCl2, 100 mM Tris–HCl, and 10 mM dithiothreitol (DTT) were obtained from New England Biolabs Inc. (Beijing, China).
Assembly of the Detection Probe and Feasibility of CHA
Assembly of Detection Probe
Ten microliter of Chain i sequences (20 μM) was diluted in 40 μL TE buffer. The solution was annealed 90 °C for 10 min. Then, the solution was gradually cooled to room temperature. Ten microliter of treated Chain i (4 μM) was then mixed with 10 μL Chain ii (4 μM). The mixture was then incubated at 30 °C for 30 min.
Fluorescent Assay to Test the CHA
Ten microliter of FAM-labeled detection probe (4 μM) was mixed with 10 μL miRNA-21 (100 nM) in a tube containing 80 μL NEB buffer 2. After being incubated at 30 °C for 30 min, the fluorescence intensities of the mixture when miRNA-21 existed or not were measured. Ten microliter of FAM-labeled detection probe (4 μM) was mixed with 10 μL miRNA-21 (100 nM) and 10 μL H2 probe (400 nM) in a tube containing 70 μL NEB buffer 2. The mixture was incubated at room temperature for 30 min, and the fluorescence signal was recorded.
miRNA Detection
Ten microliter of detection probe (0.4 μM), 5 μL target miRNA, and 10 μL H2 probe (0.4 μM) were mixed in a tube containing 25 μL NEB buffer 2 and 50 μL DEPC water. The mixture was incubated at room temperature for 20 min. Afterwards, 2 μL T4 DNA ligase was added in the mixture and incubated at 37 °C for 30 min. Then, the mixture was heated to 70 °C to degrade the T4 DNA ligase. Two microliter of phi29 enzyme was then added in the mixture. After being incubated at 37 °C for 60 min, 2 μL Cas12a enzyme, 2 μL sgRNA, and 0.8 μL of 10 μM reporter probe were added to the mixture and incubated at 37 °C for 60 min. Then, fluorescence measurement was performed.
Statistical Analysis
All of the data were presented as means standard deviations. The two-tailed Student’s t test with a significance level of P < 0.05 was used to compare the differences between two groups. To enhance the statistical accuracy, technical and sample replications were conducted.
Results and Discussion
The Working Mechanism of the Established Approach
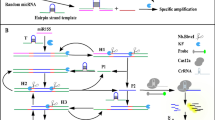
The principle of the proposed approach is illustrated in Fig. 1. In this method, a detection probe is designed to be composed of two chains (Chain i and Chain ii). Chain i hybridizes with Chain ii through the complementary sections (c and c’). Target miRNA can bind with the d section in the detection probe and gradually disassociates the e sequence from the d section. The disassociated e sequence can unfold the H2 probe to induce catalytic hairpin reaction, releasing miRNA for a next signal recycle and forming a dsDNA product with a three-way junction tail. The a’ section in the three-way junction tail can bind with the a section in Chain i to form a cyclized padlock for RCA under the assistance of T4 DNA ligase. With the phi29 enzyme-based chain extension, a long ssDNA product with repeated b’ sections is produced. The b’ sequences in the ssDNA product can be identified by Cas12a enzyme, activating trans-cleavage activity of Cas12a enzyme to cut surrounding ssDNA probes (reporter). Eventually, the reporter probe, whose two terminals were labeled with fluorescent moiety and corresponding quenching moiety, is cut, leading to the re-appearance of fluorescence signals.
Fluorescent Assays to Test the Assembly of Detection Probe and Feasibility of CHA Process
The assembly of the detection probe is crucial for the specific target recognition and inducing the CHA process. Thus, we firstly tested the assembly of the detection probe via a fluorescent assay. In this fluorescent assay, FAM moiety and BHQ were labeled on the 3’ terminal of Chain i and the 5’ terminal of Chain ii. Before assembly, the FAM signal was high, which would decrease after the assembly of detection probe because the FAM signal was quenched by BHQ, indicating the successful assembly of detection probe (Fig. 2A). To investigate the CHA process, FAM and BHQ are labeled on the terminal of Chain i and Chain ii as illustrated in Fig. 2B. In the present of target miRNA, the recorded fluorescence intensity increased, implying that the hairpin structure of Chain ii was unfold. Upon the addition of H2 probe, the fluorescence signals elevated significantly, suggesting the formation of a signal recycle (Fig. 2C) and performance of the CHA process.
Assembly of detection probe and feasibility of CHA. A Fluorescence spectrum of FAM-labeled Chain ii before and after assembly. B Illustration of the fluorescent assay to test the CHA process. C Fluorescence intensities of the FAM-labeled detection probe when target miRNA and H2 probe exited or not, P < 0.05
Feasibility of the Whole Sensing System
The trans-cleavage activity of Cas12a enzyme was analyzed quantitatively using a fluorescent assay in order to determine the cleavage efficiency of Cas12 enzyme. The trans-cleavage activity of Cas12a enzyme is successfully activated in a time-dependent manner, as shown in Fig. 3A, where the recorded fluorescent signal at 10 min was significantly higher than that in the control group and steadily increased with the increase of incubation time. The detection performance of the established technique was then examined in both the presence and absence of numerous crucial experimental components. T4 DNA ligase and the phi29 enzyme helped the RCA process, which produced a significant amount of ssDNA and caused the Cas12a enzyme to engage in trans-cleavage activity. As a result, when T4 DNA ligase and phi29 enzyme were absent in this system, the observed fluorescence intensity at 520 nm in the spectrum was low. The Cas12 enzyme controls the signal generation in the meanwhile. The result in Fig. 3B demonstrate that when the Cas12a enzyme was absent in this system, the fluorescence intensities at 520 nm in the recorded spectrum were low. A significantly increased fluorescence intensity was only seen when all the necessary experimental components were present in the system, indicating the feasibility of the approach.
Optimization of Experimental Conditions
The ratio of T4 DNA ligase to phi29 enzyme concentration, the amount of Cas12a enzyme, the concentration of H2 probe, and the incubation duration was all modified in order to improve the detection performance. The concentration ratios between T4 DNA ligase and phi29 enzyme were tuned with constant 1U/L T4 DNA ligase. When the concentration ratio was between 0.5:1 and 2:1, the result in Fig. 4A indicates a steadily increasing fluorescence signal, and no additional increments are seen with a higher concentration ratio. Thus, 2: 1 was chosen for the ensuing studies. The amount of Cas12a enzyme is significant for cutting the reporter probe and generating fluorescent signals. As shown in Fig. 4B, the highest florescence value is obtained at the Cas12a concentration of 2 U/L, which was therefore used as the optimal concentration in the following experiments. Another significant element that influences the CHA process of the sensing system is the H2 probe concentration. The H2 probe cannot successfully substitute miRNA to start the entire CHA when its concentration is too low. It will, however, also produce a significant background fluorescence signal if its concentration is too high. As seen in Fig. 4C, the fluorescence intensity increases as the concentration of the H2 probe increases and decreased with more H2 probe. The H2 probe’s concentration was 0.4 μM, at which point the fluorescence intensity peaked. As a result, 0.4 μM was chosen as the ideal concentration. Additionally, the incubation time has a direct impact on the sensing system’s analytical capabilities. The fluorescence signal of the approach progressively rises as the incubation time ranged from 10 to 60 min, as shown in Fig. 4D, and no overt fluorescence rise is noticed. Hence, 60 min was determined to be the ideal duration.
Analytical Performance of the Established Approach
The analytical performance of the established approach for the detection of miRNA-21 in a series of buffer samples was verified under the obtained optimized experimental parameters. As shown in Fig. 5A, the recorded fluorescence signals elevated with the concentration of miRNA-21 increase from 100 aM to 100 pM. A good linear relationship between obtained fluorescence intensities and the logarithm of miRNA-21 concentration is shown in Fig. 5B. The regression equation was Y = 152.5969lgC + 1060.1388 (R2 = 0.9968), where Y is the measured fluorescence intensity value and C is the concentration of miRNA-21. The proposed approach is one of the most sensitive miRNA sensing systems when compared to many existing techniques, with a limit of detection (LOD) of 27 aM. Accurate analysis of target miRNA is also a great challenge due to the high similarity between family members. To investigate the selectivity of the established approach, two interfering miRNAs (let-7b, miRNA-155) and two synthesized miRNAs that have one or two bases mismatched with miRNA-21 (mis-1 and mis-2) at the same concentration were analyzed. The result in Fig. 5C shows that the samples containing target miRNA exhibited the highest fluorescent response. The fluorescence signals produced by the interfering miRNAs were approximately 19% and 15% of those produced from the target, respectively. In addition, the fluorescence responses of mismatched sequences were 34% and 22% of those produced from the target. The above results demonstrated the high selectivity of the proposed approach.
Analytical performance of the established approach. A Fluorescence spectrum of the approach when detecting different concentrations of miRNA-21. B Correlation equation between the recorded fluorescence intensities and the concentrations of target. C Fluorescence intensities of the approach when detecting different miRNAs. P < 0.05 (***)
Analysis of Human Serum Samples
To test the feasibility of the established approach in analyzing target miRNA from clinical samples, the synthesized miRNA-21 was diluted to different concentrations by the commercial serum solutions to prepare samples. The method and RT-PCR method were exploited to detection miRNA-21 in the prepared samples. The result in Fig. 6 shows a high correlation between the calculated miRNA concentrations by the proposed method and by RT-PCR indicating that the sensing system maintained its specific molecular recognition ability for the target gene even in real human serum.
Conclusion
We depict here a novel and sensitive biosensor for the amplified fluorescent detection of miRNA-21 by integrating three-way junction-assisted RCA and trans-cleavage of Cas12a. Based on the CHA, RCA, and attached signal amplification via the trans-cleavage activity of Cas12a enzyme, the method exhibited a wider detection range of six orders of magnitude and a LOD as low as 27 aM. The recorded fluorescence intensity of the approach possessed a good linear relation with the logarithm of the target concentration. Additionally, the detection results from clinical samples by the method were also highly consistent with that detected by RT-PCR. Due to its high sensitivity and selectivity, we believe this biosensor has a great deal of potential for routine monitoring of target genes of interest in a variety of domains, such as clinical diagnosis and biological research.
Data Availability
All data generated and analyzed during this study are included in this article.
References
Du, W., Liu, G., Shi, N., Tang, D., Ferdek, P. E., Jakubowska, M. A., Liu, S., Zhu, X., Zhang, J., Yao, L., Sang, X., Zou, S., Liu, T., Mukherjee, R., Criddle, D. N., Zheng, X., Xia, Q., Berggren, P. O., Huang, W., … Fu, X. (2022). A microRNA checkpoint for Ca(2+) signaling and overload in acute pancreatitis. Molecular Therapy, 30, 1754–1774.
Yang, Y., Huang, Q., Luo, C., Wen, Y., Liu, R., Sun, H., & Tang, L. (2020). MicroRNAs in acute pancreatitis: From pathogenesis to novel diagnosis and therapy. Journal of Cellular Physiology, 235, 1948–1961.
Zhou, W., Dong, S., Chen, Z., Li, X., & Jiang, W. (2022). New challenges for microRNAs in acute pancreatitis: Progress and treatment. Journal of Translational Medicine, 20, 192.
Mohr, A. M., & Mott, J. L. (2015). Overview of microRNA biology. Seminars in Liver Disease, 35, 3–11.
Pozniak, T., Shcharbin, D., & Bryszewska, M. (2022). Circulating microRNAs in medicine. International Journal of Molecular Sciences, 23, 3996.
Qu, K., Zhang, X., Lin, T., Liu, T., Wang, Z., Liu, S., Zhou, L., Wei, J., Chang, H., Li, K., Wang, Z., Liu, C., & Wu, Z. (2017). Circulating miRNA-21-5p as a diagnostic biomarker for pancreatic cancer: Evidence from comprehensive miRNA expression profiling analysis and clinical validation. Science and Reports, 7, 1692.
Muhlberg, L., Kuhnemuth, B., Costello, E., Shaw, V., Sipos, B., Huber, M., Griesmann, H., Krug, S., Schober, M., Gress, T. M., & Michl, P. (2016). miRNA dynamics in tumor-infiltrating myeloid cells modulating tumor progression in pancreatic cancer. Oncoimmunology, 5, e1160181.
Tang, J., Li, X., Cheng, T., & Wu, J. (2021). miR-21-5p/SMAD7 axis promotes the progress of lung cancer. Thorac Cancer, 12, 2307–2313.
Juan, D., Gangyi, C., Wei, W., Xin, H., Huipan, P., Qinlin, P., Feng, D., Xin, C., Yun, D., & Zhuo, T. (2018). Colorimetric PCR-based microRNA detection method based on small organic dye and single enzyme. Analytical Chemistry, 90, 7107–7111.
Androvic, P., Valihrach, L., Elling, J., Sjoback, R., & Mikael, K. (2017). Two-tailed RT-qPCR: A novel method for highly accurate miRNA quantification. Nucleic Acids Res, 45, 144.
Pall, G. S., & Hamilton, A. J. (2008). Improved northern blot method for enhanced detection of small RNA. Nature Protocols, 3, 1077.
Várallyay, É., Burgyán, J., & Havelda, Z. (2008). MicroRNA detection by northern blotting using locked nucleic acid probes. Nature Protocols, 3, 190–196.
Li, W., & Ruan, K. (2009). MicroRNA detection by microarray. Analytical and Bioanalytical Chemistry B, 394, 1117–1124.
Johne, R., Muller, H., Rector, A., van Ranst, M., & Stevens, H. (2009). Rolling-circle amplification of viral DNA genomes using phi29 polymerase. Trends in Microbiology, 17, 205–211.
Xu, L., Duan, J., Chen, J., Ding, S., & Cheng, W. (2021). Recent advances in rolling circle amplification-based biosensing strategies-A review. Analytica Chimica Acta, 1148, 238187.
Song, W., Zhu, K., Cao, Z., Lau, C., & Lu, J. (2012). Hybridization chain reaction-based aptameric system for the highly selective and sensitive detection of protein. The Analyst, 137, 1396–1401.
Wu, J., Tian, Y., He, L., Zhang, J., Huang, Z., Luo, Z., & Duan, Y. (2021). An efficient localized catalytic hairpin assembly-based DNA nanomachine for miRNA-21 imaging in living cells. The Analyst, 146, 3041–3051.
Zhao, L., Mao, J., Hu, L., Zhang, S., & Yang, X. (2021). Self-replicating catalyzed hairpin assembly for rapid aflatoxin B1 detection. Analytical Methods, 13, 222–226.
Zhao, Y., Chen, F., Li, Q., Wang, L., & Fan, C. (2015). Isothermal amplification of nucleic acids. Chemical Reviews, 115, 12491–12545.
Wang, R., Zhao, X., Chen, X., Qiu, X., Qing, G., Zhang, H., Zhang, L., Hu, X., He, Z., Zhong, D., Wang, Y., & Luo, Y. (2020). Rolling circular amplification (RCA)-assisted CRISPR/Cas9 cleavage (RACE) for highly specific detection of multiple extracellular vesicle MicroRNAs. Analytical Chemistry, 92, 2176–2185.
Zhang, G., Zhang, L., Tong, J., Zhao, X., & Ren, J. (2020). CRISPR-Cas12a enhanced rolling circle amplification method for ultrasensitive miRNA detection. Microchemical Journal, 158, 105239.
Acknowledgements
We appreciate the financial support from the Chongqing Medical University. We also thank the director of central laboratory for providing essential equipment.
Author information
Authors and Affiliations
Contributions
Shuqi Zhao designed and wrote the manuscript; Zhiquan Wu performed experiments and analyzed obtained data.
Corresponding author
Ethics declarations
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wu, Z., Zhao, S. Three-Way Junction-Assisted Rolling Circle Amplification Integrated with trans-Cleavage of Cas12a for Sensitive and Reliable Detection of miRNA. Appl Biochem Biotechnol 196, 3115–3125 (2024). https://doi.org/10.1007/s12010-023-04691-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-023-04691-8