Abstract
Colon cancer is the most prevalent cancer and causes the highest cancer-associated mortality in both men and women globally. It has a high incidence and fatality rate, which places a significant burden on the healthcare system. The current work was performed to understand the beneficial roles of nerolidol on the viability and cytotoxic mechanisms in the colon cancer HCT-116 cells. The MTT cytotoxicity assay was done to investigate the effect of nerolidol at different doses (5–100 µM) on the HCT-116 cell viability. The impacts of nerolidol on ROS accumulation and apoptosis were investigated using DCFH-DA, DAPI, and dual staining assays, respectively. The flow cytometry analysis was performed to study the influence of nerolidol on the cell cycle arrest in the HCT-116 cells. The outcomes of the MTT assay demonstrated that nerolidol at different doses (5–100 µM) substantially inhibited the HCT-116 cell viability with an IC50 level of 25 µM. The treatment with nerolidol appreciably boosted the ROS level in the HCT-116 cells. The findings of DAPI and dual staining revealed higher apoptotic incidences in the nerolidol-exposed HCT-116 cells, which supports its ability to stimulate apoptosis. The flow cytometry analysis demonstrated the considerable inhibition in cell cycle at the G0/G1 phase in the nerolidol-exposed HCT-116 cells. Our research showed that nerolidol can inhibit the cell cycle, increase ROS accumulation, and activate apoptosis in HCT-116 cells. In light of this, it may prove to be a potent and salutary candidate to treat colon cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cancer is becoming one of the most serious diseases affecting human health. Colon cancer is the most widespread and serious type of cancer, with high rates of occurrence and fatality rates even in developed countries [1, 2]. Colon cancer is the third most deadly cancer globally, with a projected 900,000 mortalities per year in both men and women by the year 2020 [3]. This places a significant burden on the healthcare system. More than 50% of colon cancer patients are treated by surgery along with chemotherapy; however, 40–50% of these patients still develop a recurrence, and the chances of a complete recovery are really poor. The possibility of a patient surviving this illness is increased by early identification and treatment, which highlights the significance of effective and specific medical care in cancer management [4]. Currently, chemotherapy, radiation, and surgery are the methods used to treat people with colon cancer. The 5-year survival rate of colon cancer patients remains low at 64%, even though there have been advances in treatment [5]. Therefore, patients with colon cancer need access to new and more potent anticancer drug candidates to enhance treatment results and decrease side effects.
Tumor cells specifically modify their biological functions during cancer progression to favor rapid growth, migration, and resistance to genotoxic and metabolic stressors [6]. Apoptosis is a genetically regulated process that eliminates damaged cells to preserve the tissue’s cellular equilibrium. It is believed to be a crucial part of several cellular mechanisms. Defective apoptotic signals may eventually encourage the development of cancer [7]. Inhibition of apoptosis by certain pro-survival proteins is crucial to the maintenance of tumor phenotypes in order to evade the cell death enforced by oncogenic stress during tumor formation. As a result, many crucial mechanisms regulating apoptosis and cell survival are modified to cancer [8]. To avoid apoptosis, cancer cells can develop a number of different defense mechanisms [9]. Apoptosis induction in tumor cells is a major strategy used by anticancer therapies like radiation, chemotherapy, and targeted therapies [10].
The resistance to chemotherapy is a significant issue because it is one of the first-line treatment options for treating aggressive types of colon cancer [11]. The several chemotherapy drugs are being used to treat colon cancer and decrease death rates; however, the clinical usage of these medications is restricted, and the successful recovery of patients is poor due to the long-term side effects such as diarrhea, nausea/vomiting, and mucositis [12]. Therefore, improved therapy options are required for the effective treatment of colon cancer. Natural substances are being investigated as promising cancer therapies. It was reported that 87% of all identified human diseases are being treated with natural substances, and 74% of the most effective life-saving medications are made with active components obtained from plants [13]. Hence, the development of drug substitutes from natural sources may be a beneficial strategy for treating colon cancer.
Nerolidol, a sesquiterpene alcohol, occurs in the many essential oils of different plants [14]. The anti-inflammatory, antibacterial, anti-neurodegenerative, and anticancer properties of nerolidol are well documented [15, 16]. Furthermore, nerolidol has been reported to demonstrate neuroprotective [17], hepatoprotective [18], cardioprotective [19], genoprotective [20], and anti-nociceptive [21] properties. It can inhibit HepG2 hepatocellular carcinoma cell growth [22] and improve the cytotoxicity property of doxorubicin against breast cancer [23]. However, its anti-colon cancer properties have not yet been revealed. Therefore, the current research was carried out to demonstrate the salutary roles of nerolidol in the proliferation and cytotoxic mechanisms of colon cancer HCT-116 cells.
Materials and Methods
Chemicals
Nerolidol, 3-(4,5-dimethylthiazoyl-2-yl)-2,5-diphenyltetrazolium bromide (MTT), buffered saline solution, dimethyl sulfoxide (DMSO), and other chemicals were acquired from Sigma-Aldrich, USA. All the reagents utilized in this work were of analytical range.
Cell Collection and Maintenance
The colon cancer HCT-116 cells were obtained from ATCC, USA, and grown on DMEM enriched with 10% FBS in a CO2 (5%) incubator for 24 h. Cells were gathered and used for the subsequent assays once they reached 80% confluency.
MTT Cytotoxicity Assay
By using the MTT assay, the impact of nerolidol on the HCT-116 cell viability was assessed. On a 96-well plate, cells were cultured at a population of 5 × 103 for 24 h. Following that, cells received nerolidol (5, 10, 25, 50, 75, and 100 µM) treatment for 24 h. Following the treatment, each well received a mixture of 20 µl of MTT reagent and 100 µl of DMEM, which was then maintained for 4 h. After dissolving the developed formazan stones with DMSO (100 µl), the absorbance was measured at 570 nm using ELISA reader.
Dual Staining
By using dual staining, the extent of apoptosis in nerolidol-treated HCT-116 cells was investigated. Cells were grown on a 24-well plate at a population of 5 × 105 cells/well and then 25 and 50 µM of nerolidol were treated for 24 h. To evaluate apoptosis in HCT-116 cells, 100 µg/ml of AO/EB stains was added to each well and incubated for 5 min at dark. The intensity of the generated fluorescence was then assessed under a fluorescent microscope.
DCFH-DA Staining
By using the DCFH-DA staining method, the ROS production in both control and nerolidol-treated HCT-116 cells was evaluated. The 24-well plate was used to culture the HCT-116 cells at a population of 5 × 105 cells per well and then nerolidol at 25 and 50 µM concentrations was treated for 24 h. After that, 10 µl of DCFH-DA dye was mixed into each well and then incubated for 1 h at 37 °C. The fluorescent microscope was utilized to investigate the intensity of the developed fluorescence. The increased green fluorescence correlates to the increased level of ROS formation.
DAPI Staining
Using DAPI staining, the altered apoptotic nuclear morphology of HCT-116 cells caused by nerolidol was examined. The HCT-116 cells were added to the 24-well plate, and then nerolidol at dosages of 25 and 50 µM was treated for 24 h. Then, cells were fixed with paraformaldehyde (4%) for 30 min, and then cells were stained with DAPI (200 µg/ml) for 15 min. The changes to the chromatin caused by nerolidol in the HCT-116 cells were then examined using a fluorescent microscope.
Cell Cycle Analysis
The control and nerolidol-treated HCT-116 cells were collected at a population of 5 × 106 cells/ml, treated with 70% ethanol, and then incubated for 12 h. Following cell washing, staining solution (300 µl) including PI (100 µl), proteinase inhibitor (0.08 mg/ml), and RNase (0.5 mg/ml) was mixed, and the solution was incubated for 30 min. Flow cytometry was utilized to determine the DNA-related PI fluorescence. The MultiCycle software was used to calculate the proportions of nuclei in each cell cycle stage, including G1, S, and G2/M (Phoenix Flow Systems, USA). WinMDI 2.9 software was used to detect the percentage of sub-diploid cells (apoptotic cells).
Statistical Analysis
Values are revealed as the mean ± SD of three different assays after the results were examined using the GraphPad Prism software. One-way ANOVA and DMRT were performed to investigate changes in the values of the treatment groups, and a significance at p < 0.05 was used.
Results
Effect of Nerolidol on the Viability of HCT-116 Cells
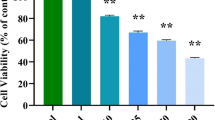
Figure 1 demonstrates the viability of control and nerolidol-treated HCT-116 cells. Our findings showed that, when compared to the control, the nerolidol treatment at 5–100 µM concentrations substantially reduced the viability of HCT-116 cells. The IC50 level of nerolidol was found at 25 µM, which inhibits 50% of the viability. As a result, the 25 and 50 µM of nerolidol were chosen for the subsequent analyses as IC50 and high-dose treatments, respectively.
Effect of nerolidol on the viability of HCT-116 cells. The results of MTT assay revealed that the nerolidol at different doses (5–100 µM) substantially inhibited the viability of colon cancer HCT-116 cells with IC50 level at 25 µM. The results were expressed as the mean ± SD of three individual experiments. GraphPad Prism software was used to analyze the final data using one-way ANOVA and Duncan's multiple range test (DMRT). Values does not share common superscript and significantly differed from the control at p < 0.05
Effect of Nerolidol on the ROS Level in the HCT-116 Cells
The impact of nerolidol on the generation of ROS in HCT-116 cells is revealed in Fig. 2. The control cells exhibited low green fluorescence, whereas the HCT-116 cells exposed to 25 and 50 µM nerolidol demonstrated a strong green fluorescence, which indicates that the nerolidol treatment significantly enhanced ROS accumulation in the HCT-116 cells. These outcomes demonstrate that nerolidol facilitates oxidative stress–mediated damage to the HCT-116 cells by increasing the generation of intracellular ROS.
Effect of Nerolidol on the Apoptotic Cell Nuclear Morphology in the HCT-116 Cells
DAPI staining was done to evaluate the impact of nerolidol on the apoptotic cell nucleus in HCT-116 cells, and the outcomes are revealed in Fig. 3. DAPI staining results showed that the 25 and 50 µM nerolidol-treated HCT-116 cells had distinct changes in nuclear morphology, such as damages, shrinkage, and the formation of apoptotic bodies, which reveals that nerolidol increases apoptosis in HCT-116 cells.
Effect of nerolidol on the apoptotic cell nuclear morphology in the HCT-116 cells. The HCT-116 cells treated with 25 and 50 µM of the nerolidol displayed clear changes such as damaged cell morphology, shrinkage, and formation of apoptotic bodies when compared to the control, revealing the occurrences of apoptotic cell death
Effect of Nerolidol on the Apoptotic Cell Death in the HCT-116 Cells
Figure 4 depicts how nerolidol influenced the apoptosis in HCT-116 cells, which was investigated by dual staining. The HCT-116 cells exposed to 25 and 50 µM nerolidol showed a bright orange/yellow fluorescence with damaged cell morphology, which is in contrast to the untreated control cells. Therefore, it was evident that nerolidol treatment dramatically increased the apoptosis in the HCT-116 cells.
Effect of Nerolidol on the Cell Cycle Arrest in the HCT-116 Cells
Using flow cytometry, the cell cycle phases in the control and nerolidol-exposed HCT-116 cells were studied (Fig. 5). The nerolidol at the dosages of 25 and 50 µM treated HCT-116 cells, which exhibited a higher percentage of cells in the G0/G1 phase. Also, the percentage of cells in the G2/M phase was lower in the HCT-116 cells after they were exposed to 25 and 50 µM of nerolidol. This showed that the nerolidol treatment inhibited the cell cycle at the G0/G1 phases.
Effect of nerolidol on the cell cycle arrest in the HCT-116 cells. Representative cell cycle distribution of control and nerolidol at 25 and 50 μM treated colon cancer HCT-116 cells. Propidium iodide (PI)–stained HCT-116 cells were subjected to flow cytometry analysis to determine the cell distribution at each stage of the cell cycle
Discussion
Colon cancer remains the major cause of cancer-associated mortality in both men and women of all age groups globally [24, 25]. Furthermore, resistance to therapies made it more difficult to treat colon cancer [26]. Therefore, there is a need to look for novel, effective, and low-toxic alternatives that could effectively prevent and/or inhibit the progression of colon cancer. In the current work, the anticancer potential of nerolidol against the HCT-116 cells was studied and the results demonstrated the significant beneficial activities of the nerolidol. The uncontrolled and excessive cell proliferation causes the development of cancerous tissues. The tumor cells have a strong ability to replicate themselves and metastasize to other body parts [27]. In order to demonstrate the anticancer effects of nerolidol, we attempted to discover the inhibitory potential of nerolidol on the proliferation of colon cancer HCT-116 cells using the MTT assay. The findings reveal that nerolidol treatment substantially obstructs HCT-116 cell viability. The inhibition of HCT-116 cell growth using natural bioactive compounds was already reported; for example, Lee et al. [28] found that podophyllotoxin effectively decreased HCT-116 cell viability.
ROS are highly reactive substances produced by mitochondria as a metabolic byproduct, which aid in the malignant transition of normal cells into neoplastic precursors [29]. Additionally, ROS are crucial intracellular signaling mediators under normal circumstances, but an abnormal gathering causes oxidative stress in cells and encourages apoptosis [30]. In order to support their fast proliferation, cancer cells produce ROS. However, excessive ROS levels can cause tumor cell death via a number of pathways [31]. Cancer cells are more susceptible to medicines that increase ROS-mediated oxidative stress. In cancer treatment, the stimulation of higher ROS accumulation in the tumor cells is an effective approach [32]. It was evident that several bioactive substances with anticancer potential raised ROS levels, triggering oxidative stress–mediated apoptosis in tumor cells [33]. In the current work, the findings of DCFH-DA staining demonstrated that nerolidol effectively augmented ROS levels in HCT-116 cells, which may facilitate oxidative stress–mediated apoptotic cell death. The increase of ROS production by bioactive compounds was already reported; for instance, Lin et al. [34] found that ferruginol effectively increased the ROS level in HCT-116 cells, thereby facilitating oxidative stress–mediated cell injury, which validates the findings of the current study.
In addition to uncontrolled cell proliferation, defective apoptosis is also a major contributing factor in tumor growth. Apoptosis is a crucial defense mechanism against the progression of tumors. Neoplastic cells undergo oncogenic transformation when genetic and epigenetic changes cause them to become resistant to apoptosis. In tumorigenesis, the defective apoptosis regulation causes an extended life span of tumor cells, growth under stress, tumor angiogenesis, and metastasis. It also adds to therapeutic resistance in tumor cells [35]. The pathophysiology of apoptosis is multifaceted and incorporates extrinsic and intrinsic signal transduction [36]. Tumor cells produce several mechanisms to prevent apoptotic cell death, most notably the overexpression of anti-apoptotic molecules. DNA injury in precancerous lesions can cause apoptosis, which eliminates potentially hazardous cells and prevents tumor growth [37]. Therefore, apoptosis stimulation in cancer cells is considered the most promising approach to obstruct tumor progression. In this study, the results of dual staining exhibited that nerolidol treatment effectively stimulated apoptosis in HCT-116 cells, which evidences that nerolidol can inhibit colon cancer growth by triggering apoptotic cell death. This observation is in agreement with the previous research done by Xiang et al. [38], who found that curcumin triggered apoptosis in HCT-116 cells, which was analyzed by dual staining.
Some apparent morphological abnormalities that occur during apoptosis can be considered indicators of the beginning of the apoptotic process. In summary, apoptosis begins with chromatin condensation in the nucleus, progresses through nuclear fragmentation, and finally results in the formation of apoptotic bodies [39, 40]. The results of DAPI staining also demonstrated increased apoptotic cell nuclear morphology, which is evident by increased damaged cell morphology, nuclear fragmentation, and apoptotic bodies in the nerolidol-exposed HCT-116 cells. Hence, it was clear that nerolidol can be useful to treat colon cancer by encouraging apoptosis. Our present results were supported by the previous research work done by Dangroo et al. [41].
In addition to cell toxicity and apoptosis induction, the impact of nerolidol on the cell cycle arrest in the HCT-116 cells was also investigated in this study. It is crucial to understand that there are various stages in the cell cycle, including gap 1 (G1), gap 2 (G2), and mitosis (M) [42]. A variety of regulatory molecules are involved in the intricate process of the cell cycle, which controls the growth of cancer cells [43]. The checkpoints of the cell cycle are commonly stimulated due to DNA damage and replication stress. Each checkpoint in normal cell division is strictly controlled, and any disruption of those checkpoints causes cells to divide rapidly and uncontrollably [44]. A possible target for cancer therapies is the dysregulation of the cell cycle, which promotes the development of tumors [45]. It was reported that some naturally occurring bioactive compounds, such as hydroxy-G-sanshool, inhibit colon cancer cell growth by inducing the sub-G1 population [46]. According to the current findings, we found that, compared to control cells, HCT-116 cells exposed to nerolidol had a higher sub-G1 population. Therefore, it was clear that the nerolidol caused the inhibition of the cell cycle in the G0/G1 phase and reduced HCT-116 cell growth. Recent studies done by Zhaojun et al. [47] and Kwon et al. [48] found the inhibition of cell cycle at the G0/G1 phase in the HCT-116 cells using bioactive compounds, which supports the results of the present study.
Conclusion
In conclusion, our results show that nerolidol can inhibit viability and accelerate apoptotic cell death in HCT-116 cells by elevating intracellular ROS accumulation and inducing cell cycle arrest. In light of these findings, nerolidol is recommended as a talented and salutary candidate to treat colon cancer in the future. More study will be compulsory in the future to fully comprehend the different molecular pathways that make nerolidol effective against colon cancer as an anticancer drug.
Data Availability
Not applicable.
Abbreviations
- DMOS:
-
Dimethyl sulfoxide
- FBS:
-
Fetal bovine serum
- ROS:
-
Reactive oxygen species
- DAPI:
-
4′,6-Diamidino-2-phenylindole
- DCFH-DA:
-
2′-7′-Dichlorodihydrofluorescein diacetate
References
Siegel, R. L., Miller, K. D., Fuchs, H. E., & Jemal, A. (2021). Cancer statistics, 2021. CA: A Cancer Journal for Clinicians, 71, 7–33.
Pamudurthy, V., Lodhia, N., Konda, V. J., editors. (2020). Advances in endoscopy for colorectal polyp detection and classification. Baylor University Medical Center Proceedings, Taylor & Francis.
Vos, T., Lim, S. S., Abbafati, C., Abbas, K. M., Abbasi, M., Abbasifard, M., et al. (2020). Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet, 396(10258), 1204–1222.
Liu, K. C., Shih, T. Y., Kuo, C. L., Ma, Y. S., Yang, J. L., Wu, P. P., Huang, Y. P., Lai, K. C., & Chung, J. G. (2016). Sulforaphane induces cell death through G2/M phase arrest and triggers apoptosis in HCT 116 human colon cancer cells. American Journal of Chinese Medicine, 44, 1289–1310.
Xie, Y. H., Chen, Y. X., & Fang, J. Y. (2020). Comprehensive review of targeted therapy for colorectal cancer. Signal Transduction and Targeted Therapy, 5, 22.
Chang, J., Jung, H. J., Jeong, S. H., Kim, H. K., Han, J., & Kwon, H. J. (2014). A mutation in the mitochondrial protein UQCRB promotes angiogenesis through the generation of mitochondrial reactive oxygen species. Biochemical and Biophysical Research Communications, 455, 290–297.
Goldar, S., Khaniani, M., Derakhshan, S., & Baradaran, B. (2015). Molecular mechanisms of apoptosis and roles in cancer development and treatment. Asian Pacific Journal of Cancer Prevention, 16, 2129–2144.
Vogelstein, B., Papadopoulos, N., Velculescu, V. E., Zhou, S., Diaz, L. A., Jr., & Kinzler, K. W. (2013). Cancer genome landscapes. Science, 339(6127), 1546–1558.
Sharma, A., Boise, L. H., & Shanmugam, M. (2019). Cancer metabolism and the evasion of apoptotic cell death. Cancers (Basel), 11(8), 1144.
Abaza, A., Vasavada, A. M., Sadhu, A., Valencia, C., Fatima, H., Nwankwo, I., Anam, M., Maharjan, S., Amjad, Z., & Khan, S. (2022). A systematic review of apoptosis in correlation with cancer: Should apoptosis be the ultimate target for cancer treatment? Cureus, 14(8), e28496.
Morawska, K., Goirand, F., Marceau, L., Devaux, M., Cueff, A., Bertaut, A., Vincent, J., Bengrine-Lefevre, L., Ghiringhelli, F., & Schmitt, A. (2018). 5-FU therapeutic drug monitoring as a valuable option to reduce toxicity in patients with gastrointestinal cancer. Oncotarget, 9, 11559–11571.
Aoullay, Z., Slaoui, M., Razine, R., Er-Raki, A., Meddah, B., & Cherrah, Y. (2020). Therapeutic characteristics, chemotherapy-related toxicities and survivorship in colorectal cancer patients. Ethiopian Journal of Health Sciences, 30(1), 65–74.
Millimouno, F. M., Dong, J., Yang, L., Li, J., & Li, X. (2014). Targeting apoptosis pathways in cancer and perspectives with natural compounds from mother nature. Cancer Prevention Research, 7, 1081–1107.
Zhou, Y., Zeng, L., Liu, X., Gui, J., Mei, X., Fu, X., Dong, F., Tang, J., Zhang, L., & Yang, Z. (2017). Formation of (E)-nerolidol in tea (Camellia sinensis) leaves exposed to multiple stresses during tea manufacturing. Food Chemistry, 231, 78–86.
Chan, W.-K., Tan, L. T., Chan, K.-G., Lee, L.-H., & Goh, B.-H. (2016). Nerolidol: A sesquiterpene alcohol with multi-faceted pharmacological and biological activities. Molecules, 21, 529.
De Carvalho, R. B. F., De Almeida, A. A. C., Campelo, N. B., Lellis, D., & Nunes, L. C. C. (2018). Nerolidol and its pharmacological application in treating neurodegenerative diseases: A review. Recent Patents on Biotechnology, 12, 158–168.
Javed, H., Azimullah, S., Abul Khair, S. B., Ojha, S., & Haque, M. E. (2016). Neuroprotective effect of nerolidol against neuroinflammation and oxidative stress induced by rotenone. BMC Neuroscience, 17, 58.
Baldissera, M. D., Souza, C. F., Grando, T. H., Dolci, G. S., Cossetin, L. F., Moreira, K. L., et al. (2017). Nerolidol-loaded nanospheres prevent hepatic oxidative stress of mice infected by Trypanosoma evansi. Parasitology, 144, 148–157.
Iqubal, A., Sumit, S., Ansari, M. A., Najmi, A. K., Syed, M. A., Ali, J., et al. (2019). Nerolidol attenuates cyclophosphamide-induced cardiac inflammation, apoptosis and fibrosis in Swiss albino mice. European Journal of Pharmacology, 863, 172666.
Thapa, D., Richardson, A. J., Zweifel, B., Wallace, R. J., & Gratz, S. W. (2019). Genoprotective effects of essential oil compounds against oxidative and methylated DNA damage in human colon cancer cells. Journal of Food Science, 84, 1979–1985.
Fonseca, D. V., Salgado, P. R., de Carvalho, F. L., Salvadori, M. G., Penha, A. R., Leite, F. C., et al. (2016). Nerolidol exhibits antinociceptive and anti-inflammatory activity: Involvement of the GABAergic system and proinflammatory cytokines. Fundamental & Clinical Pharmacology, 30, 14–22.
Ferreira, F. M., Palmeira, C. M., Oliveira, M. M., Santos, D., Simoes, A. M., Rocha, S. M., Coimbra, M. A., & Peixoto, F. (2012). Nerolidol effects on mitochondrial and cellular energetics. Toxicology in Vitro, 26, 189–196.
Hanusova, V., Caltova, K., Svobodova, H., Ambroz, M., Skarka, A., Murinova, N., Kralova, V., Tomsik, P., & Skalova, L. (2017). The effects of β-caryophyllene oxide and trans-nerolidol on the efficacy of doxorubicin in breast cancer cells and breast tumorbearing mice. Biomedicine & Pharmacotherapy, 95, 828–836.
Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I., Jemal, A., & Bray, F. (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians, 71, 209–249.
Grunwald, V., Karch, A., Schuler, M., Schoffski, P., Kopp, H. G., Bauer, S., et al. (2020). Randomized comparison of pazopanib and doxorubicin as first-line treatment in patients with metastatic soft tissue sarcoma age 60 years or older: Results of a German Intergroup Study. Journal of Clinical Oncology, 38, 3555–3564.
Pietzsch, S., Wohlan, K., Thackeray, J. T., Heimerl, M., Schuchardt, S., Scherr, M., et al. (2021). Anthracycline-free tumor elimination in mice leads to functional and molecular cardiac recovery from cancer-induced alterations in contrast to long-lasting doxorubicin treatment effects. Basic Research in Cardiology, 116, 61.
Fouad, Y. A., & Aanei, C. (2017). Revisiting the hallmarks of cancer. American Journal of Cancer Research, 7(5), 1016–1036.
Lee, S. O., Joo, S. H., Kwak, A. W., Lee, M. H., Seo, J. H., Cho, S. S., Yoon, G., Chae, J. I., & Shim, J. H. (2021). Podophyllotoxin induces ROS-mediated apoptosis and cell cycle arrest in human colorectal cancer cells via p38 MAPK signaling. Biomol Ther (Seoul), 29(6), 658–666.
Porporato, P. E., Filigheddu, N., Bravo-San Pedro, J. M., Kroemer, G., & Galluzzi, L. (2018). Mitochondrial metabolism and cancer. Cell Research, 28, 265–280.
Badrinath, N., & Yoo, S. Y. (2018). Mitochondria in cancer: In the aspects of tumorigenesis and targeted therapy. Carcinogenesis, 39, 1419–1430.
Perillo, B., Di Donato, M., Pezone, A., Di Zazzo, E., Giovannelli, P., Galasso, G., Castoria, G., & Migliaccio, A. (2020). ROS in cancer therapy: The bright side of the moon. Experimental & Molecular Medicine, 52, 192–203.
Liu, Y., Shi, C., He, Z., Zhu, F., Wang, M., He, R., et al. (2021). Inhibition of PI3K/AKT signaling via ROS regulation is involved in Rhein-induced apoptosis and enhancement of oxaliplatin sensitivity in pancreatic cancer cells. International Journal of Biological Sciences, 17, 589–602.
Mi, Y., Xiao, C., Du, Q., Wu, W., Qi, G., & Liu, X. (2016). Momordin Ic couples apoptosis with autophagy in human hepatoblastoma cancer cells by reactive oxygen species (ROS)-mediated PI3K/Akt and MAPK signaling pathways. Free Radical Biology & Medicine, 90, 230–242.
Lin, H. L., Chen, P. R., Mao, C. C., Zheng, W. E., & Wang, J. Q. (2021). Ferruginol-induced apoptosis in Human Colon Cancer Cells (HCT-116) through the mitochondria-mediated apoptotic pathway. Pharmacognosy Magazine, 17, 244–249.
Hanahan, D., & Weinberg, R. A. (2011). Hallmarks of cancer: The next generation. Cell, 144(5), 646–674.
Wong, R. S. (2011). Apoptosis in cancer: From pathogenesis to treatment. Journal of Experimental & Clinical Cancer Research, 30, 87.
Valentini, E., D’Aguanno, S., Di Martile, M., Montesano, C., Ferraresi, V., Patsilinakos, A., Sabatino, M., Antonini, L., Chiacchiarini, M., Valente, S., Mai, A., Colotti, G., Ragno, R., Trisciuoglio, D., & Bufalo, D. D. (2022). Targeting the anti-apoptotic Bcl-2 family proteins: Machine learning virtual screening and biological evaluation of new small molecules. Theranostics., 12, 2427–2444.
Xiang, L., He, B., Liu, Q., Hu, D., Liao, W., Li, R., Peng, X., Wang, Q., & Zhao, G. (2020). Antitumor effects of curcumin on the proliferation, migration and apoptosis of human colorectal carcinoma HCT-116 cells. Oncology Reports, 44, 1997–2008.
Neophytou, C. M., Trougakos, I. P., Erin, N., & Papageorgis, P. (2021). Apoptosis deregulation and the development of cancer multi-drug resistance. Cancers, 13, 4363.
Elmore, S. (2017). Apoptosis: A review of programmed cell death. Toxicologic Pathology, 35(4), 495–516.
Dangroo, N. A., Singh, J., Ratha, S. K., Gupta, N., Qayum, A., Singh, S., & Sangwana, P. L. (2017). A convergent synthesis of novel alkyne–azide cycloaddition congeners of betulinic acid as potent cytotoxic agent. Steroids, 123, 1–12.
Hanahan, D., & Weinberg, R. A. (2000). The hallmarks of cancer. Cell, 100, 57–70.
Afrin, S., Giampieri, F., Gasparrini, M., Forbes-Hernandez, T. Y., Varela-López, A., Quiles, J. L., et al. (2016). Chemopreventive and therapeutic effects of edible berries: A focus on colon cancer prevention and treatment. Molecules, 21, 169.
Fulda, S., Galluzzi, L., & Kroemer, G. (2010). Targeting mitochondria for cancer therapy. Nature Reviews Drug Discovery, 9, 447–464.
Bajpai, V. K., Khan, I., Shukla, S., Kang, S. M., Aziz, F., Tripathi, K. M., Saini, D., Cho, H. J., Heo, N. S., Sonkar, S. K., et al. (2020). Multifunctional N-P-doped carbon dots for regulation of apoptosis and autophagy in B16F10 melanoma cancer cells and in vitro imaging applications. Theranostic, 10, 7841–7856.
Al-Zharani, M., Nasr, F. A., Alqahtani, A. S., Cordero, M. A. W., Alotaibi, A. A., & Bepari, A. (2021). In vitro cytotoxic evaluation and apoptotic effects of Datura innoxia grown in Saudi Arabia and phytochemical analysis. Applied Sciences, 11, 2864.
Zhaojun, C., Lulin, T., Xin, F., Abdel-nasser, S., Zunguo, L., & Xiong, L. (2022). Hydroxy-g-sanshool from Zanthoxylum bungeanum (prickly ash) induces apoptosis of human colorectal cancer cell by activating P53 and Caspase 8. Frontiers in Nutrition, 9, 914638.
Kwon, M., Oh, T., Jang, M., Kim, G. H., Kim, J. H., Ryu, H. W., Oh, S. R., Jang, J. H., Ahn, J. S., & Ko, S. K. (2022). Kurarinone induced p53-independent G0/G1 cell cycle arrest by degradation of K-RAS via WDR76 in human colorectal cancer cells. European Journal of Pharmacology, 15(923), 174938.
Funding
This project was supported by Researchers Supporting Project number (RSP2023R383), King Saud University, Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Contributions
The authors contributed equally.
Corresponding author
Ethics declarations
Ethics Approval
All work has been done under the guidelines of Institutional Ethics Committee.
Consent to Participate
All authors have their consent to participate.
Consent for Publication
All authors have their consent to publish their work.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhao, X., Chinnathambi, A., Alharbi, S.A. et al. Nerolidol, Bioactive Compound Suppress Growth of HCT-116 Colorectal Cancer Cells Through Cell Cycle Arrest and Induction of Apoptosis. Appl Biochem Biotechnol 196, 1365–1375 (2024). https://doi.org/10.1007/s12010-023-04612-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-023-04612-9