Abstract
Background
The present study aimed to elucidate the potential anticancer activity and mechanism of P. harmala’s alkaloid extract, harmine (HAR), and harmaline (HAL) in HCT-116 colorectal cancer cells.
Methods and results
P. harmala’s alkaloid was extracted from harmala seeds. HCT-116 cells were treated with P. harmala’s alkaloid extract, HAR and HAL. Cytotoxicity was determined by MTT assay, apoptotic activity detected via flow cytometry and acridine orange (AO)/ethidium bromide (EB) dual staining, and cell cycle distribution analyzed with flow cytometry. The mRNA expression of Bcl-2-associated X protein (Bax) and glycogen synthase kinase-3 beta (GSK3β) was measured by real-time PCR. Furthermore, the expression of Bax, Bcl-2, GSK3β and p53 proteins, were determined by western blotting. The findings indicated that, P. harmala’s alkaloids extract, HAR and HAL were significantly cytotoxic toward HCT116 cells after 24 and 48 h of treatment. We showed that P. harmala’s alkaloid extract induce apoptosis and cell cycle arrest at G2 phase in the HCT116 cell line. Downregulation of GSK3β and Bcl-2 and upregulation of Bax and p53 were observed.
Conclusion
The findings of this study indicate that the P. harmala’s alkaloid extract has anticancer activity and may be further investigated to develop future anticancer chemotherapeutic agents.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colon carcinoma is a prevalent and highly aggressive malignant neoplasm, and is the second most common cause of cancer-related deaths worldwide [1]. Standard cancer treatment is generally based on the use of chemotherapy, radiotherapy, surgery, immunotherapy, or a combination of the two [2]. Chemotherapy is used in patients with advanced stage disease, but resistance to medicines reduces its effectiveness. On the other hand, chemical drugs can eliminate cancer cells, but can also damage perfectly healthy cells, which leads to side effects. Therefore, it is not surprising that researchers are looking for more effective anticancer drugs from natural sources to reverse those abnormal characteristics of cancer [3, 4]. According to statistics, herbal medicines are used by approximately 80% of people worldwide to treat different diseases [5]. Peganum harmala L., also referred to as “harmal” is a botanical species widely utilized for its medicinal attributes and belongs to the family Nitrariaceae [6]. The seeds contain 2.5 to 4% mixed harmala alkaloids [7]. The antineoplastic effects of P. harmala seeds on cancerous cell lines have been explained in previous studies [8, 9]. However, the effects and mechanism of action of P. harmala seeds on colorectal cancer are still unclear. The most critical alkaloids in P. harmala that have beneficial effects are harmaline (HAL) and harmine (HAR). HAL and HAR are tricyclic β-carboline alkaloids isolated from harmal seeds. HAR and HAL possess several pharmacological effects, such as antimicrobial, antifungal, antioxidant, and antitumor effects [10, 11]. HAR, which exhibits a variety of biological activities, has been identified as the most potent component of P. harmala [12, 13]. These alkaloids decrease the growth of cancer cells in a dose-dependent manner by inducing apoptosis [14, 15]. Apoptosis is a type of programmed cellular death, making it a highly promising target for anticancer therapies. A broad range of factors, including the overexpression of antiapoptotic proteins and the underexpression of proapoptotic proteins, typically inhibit the apoptotic pathway in cancer. Therapies that target apoptosis activate molecules that promote apoptosis and inhibit the activity of antiapoptotic molecules [16]. The tumor suppressor protein p53 is essential for controlling apoptosis signaling pathways, as it regulates the activity of the BCL-2 family proteins [17]. The Bcl-2 family contains a large number of proteins, including both proapoptotic and antiapoptotic proteins. These molecules play crucial roles in the process of apoptosis [18]. Some of the members to this family induce apoptosis (Bax, Bid, Bim), while others prevent apoptosis (Bcl-2, Bcl-xl, Bcl-w) [18]. The regulation of apoptosis is largely dependent on Bcl-2 and Bax among all of these proteins [19]. The examination of effect of the extract obtained from P. harmala on breast cancer cells (MDA-MB-231) has demonstrated the ability of this extract to stimulate both internal and external processes by increasing the expression of the Bax, Puma, and Caspase8 genes while reducing the expression of Bcl-2 [20]. Glycogen synthase-3 kinase (GSK-3) is a serine-tyrosine kinase with two isoforms (α and β) that is constitutively expressed and involved in the regulation of numerous essential cellular pathways [21]. GSK3β has been found to play both tumor suppressor and promoter roles in various types of cancer [22, 23]. GSK3β is overexpressed in various tumor types, including pancreatic, liver, ovarian, and colon carcinomas [24, 25]. GSK3β inhibitors are anticipated to be therapeutic agents for colorectal cancer [26].
The purpose of the present investigation was to identify the therapeutic effects of P. harmala and its underlying molecular mechanisms in the treatment of colon cancer, serving as a foundation for future research on P. harmala’s as a medicinal product for this disease.
Materials and methods
P. harmala’s alkaloids extraction
Harmala seeds from Shahrekord, Chaharmahal Bakhtiari Province, Iran, were powdered and mixed with 50 mL of glacial acetic acid (30%). After 60 min of stirring, the solution was filtered and washed three times with ethyl acetate: petroleum ether (1:1) to remove organic impurities using a separatory funnel. Then, 10 M sodium hydroxide was added to the collected aqueous layer. The chloroform phase was used to extract the organic component, which contained alkaloids primarily HAR and HAL. Finally, the solvent was removed using a rotary evaporator [8].
HPLC analysis
For high-performance liquid chromatography (HPLC) analysis, Cecil 1100 series was used. A serial dilution (100–1000 μg/mL) of harmine (Sigma, 286,044-1G) and harmaline standard (Sigma, 51,330-1G) were prepared in methanol to draw out a standard curve. P. harmala’s alkaloid extract curve was confirmed by the reference peak of HAL and HAR standard [8].
Fourier Transformation Infrared Spectroscopy (FTIR)
FTIR spectra were measured by Bruker optics. In this technique, a total of 1% (w/w) of P. harmala’s alkaloid extract was mixed with dry KBr. In the wavenumber range of 500–4000 cm−1, each KBr disc was scanned at 2 mm.s−1 at a resolution of 4 cm−1. For various samples, the characteristic peaks were recorded.
Cell line and cell culture
The HCT116 colorectal cancer cell line was purchased from the Iranian Biological Resource Center. The Cells were cultured at 37 °C in 5% CO2 in Dulbecco’s modified Eagle medium supplemented with 10% fetal bovine serum (FBS, Gibco) and 1% penicillin/streptomycin. [27].
MTT assay for cell viability
HCT-116 cells were seeded in a 96-well microplate (3200 cells per well) overnight incubation. Then, the cultured cells were treated with varying concentrations of HAR, HAL, and P. harmala’s alkaloid extract for 24 and 48 h. The control was the untreated cells, and the absorbance was measured using an ELISA plate reader [28].
Fluorescence microscopic studies
The indole alkaloids HAL and HAR are fluorescent [29]. Due to the fluorescent properties of P. harmala, in this study, only the cells were examined with the alkaloid extract without any staining. After 24 h of treatment (IC50), the cells were rinsed with PBS and analyzed using a fluorescence microscope.
Measurement of ROS
Reactive oxygen species (ROS) have been identified as subcellular messengers in various cellular processes, such as apoptosis. To measure the levels of ROS, HCT-116 cells were treated with P. harmala’s alkaloid extract at the IC50 for 48 h. Following this, the cells were rinsed with PBS and treated with DCFH2-DA and PI dye. The alterations in fluorescence intensity were observed using flow cytometry for both the control and treated cells.
Acridine orange (AO)/ethidium bromide (EB) dual staining
To distinguish between normal cells and condensed apoptotic or necrotic nuclei, we used the dual staining technique AO/EB. HCT-116 cells were seeded into a chamber slides and allowed to grow overnight. Then, the cells were exposed to P. harmala’s alkaloid extract (IC50) for 24 h. Control and treated cells were stained with AO/EB (100 μg/mL) for 3 min, and images were immediately collected by fluorescence microscopy.
Cell apoptosis analysis by flow cytometry
The FITC Annexin V/ PI Apoptosis Detection Kit (BioLegend) was used for flow cytometry analysis to determine the percentage of apoptotic cells in untreated and treated HCT-116 cells with P. harmala’s alkaloid extract (IC50, 48 h) [30]. FlowJo software was used for the data analysis.
Cell cycle analysis by flow cytometry
HCT-116 cells were treated with P. harmala’s alkaloid extract at the IC50 for 48 h. The control and treated cells were washed twice with PBS before being fixed in 70% ethanol. Subsequently, the cells were subjected to staining with PI, which included DNase-free RNase, for 2 h [30]. FlowJo software was used for the data analysis.
RNA extraction and cDNA synthesis
Total RNA extracted from both control and treated cells after 48 h using HiPure Isolation Reagent (Roche Applied Sciences), following the manufacturer’s protocol. For cDNA synthesis, a First Strand cDNA Synthesis Kit (Thermo Fisher Scientific, Germany) was used to transcribe 1 μg of total RNA from each sample into cDNA.
Real-time PCR
Real-time PCR was conducted utilizing Rotor-Gen 6000 real-time DNA analysis system (Corbett, Australia) and a SYBR Fast qPCR kit (Ampliqon A/S, Denmark) with appropriate primers (Table 1). The endogenous reference gene (GAPDH) was used for the normalization of the quantitative data. The expression of Bax and GSK3β mRNA was detected 48 h after treatment with P. harmala’s alkaloid extract, HAR, and HAL (at the IC50).
Western blot assay
HCT116 cells were treated with P. harmala’s alkaloid extract at IC50 concentrations for 48 h. The control and treated cells were lysed with RIPA buffer (Beyotime), supplemented with protease inhibitors (Roche). Subsequently, proteins were extracted and separated based on their molecular weight using SDS-PAGE. After separating the proteins, they were then transferred from the gels onto PVDF membranes. Subsequently, the membranes were subjected to blocking using a bovine serum albumin solution of 3% at room temperature for a duration of 1 h. The membranes were then incubated overnight at 4 °C with a primary antibodies’ solution diluted at a ratio of 1:1000. The primary antibodies included anti-BAX (Santa Cruz Biotechnology, INC, sc-7480), anti-Bcl-2 (Santa Cruz Biotechnology, INC, sc-7382) anti- GSK3β (Santa Cruz Biotechnology, INC, sc-7291) and anti-p53 (Santa Cruz Biotechnology, INC, sc-126). Subsequently, the membranes underwent a series of washes using TBST, with a duration of 5 min for each wash. Following that, the membranes were subjected to incubation with the HRP-conjugated secondary antibody solution (Santa Cruz Biotechnology, INC, sc-2357) at room temperature for 1 h. Ultimately, the blots were washed three times for 5 min each using TBST. Lastly, the chemiluminescent substrate was administered to the blot in accordance with the guidelines provided by the manufacturer. The chemiluminescent signals were then recorded using a CCD camera-based imager (Thermo Fisher Scientific, US). Following that, the signals underwent analysis with ImageJ software, version 1.4.1, and were then normalized to the β-actin bands for the purpose of comparison.
Statistical analysis
The experimental data were analyzed by Graph Pad Prism 8 and are presented as the mean ± SEM of two independent experiments. For the comparison of MTT data, a one-way analysis of variance (ANOVA) followed by Dunnett’s test was utilized. Quantitative real-time PCR and Western blot data were analyzed by an unpaired t- test with Welch’s correction. For the comparison of flow cytometry data, an unpaired t- test was used. A p value less than 0.05 was considered to indicate statistical significance.
Result
Evaluation of alkaloids contents
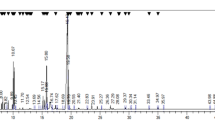
The P. harmala’s alkaloid extract of seeds was analysed with FTIR, and the major β-carboline alkaloids, including HAL and HAR were detected. According to the analogy of infrared absorption of P. harmala’s alkaloid extract with the standards of alkaloids (Fig. 1B), it could be found that in the frequency region of 500–4000 cm−1. The spectrum of P. harmala’s alkaloid extract was consistent with HAR and HAL standards, and the absorptions of P. harmala’s alkaloid extract at different wavenumbers 1076.2, 1153.4, 1365.6, 1531.4 and 1629.8 cm−1 associated with different functional groups (C–N), (C–O), (C–C), (C = C) and (C = O) respectively. The C–H groups were shown in 2856 and 2929 cm−1. In the P. harmala seeds, the major β-carboline alkaloids were quantified by HPLC. The seeds of P. harmala are known for the presence of HAR and HAL alkaloids. The HPLC chromatogram of two standard alkaloids, HAR and HAL, has shown. The HPLC chromatogram of P. harmala’s alkaloid extract has shown two prominent peaks. The peak with the highest abundance is seen at retention time 8.3 (min), and another peak with a significant abundance is found at retention time 10.89 (min), which were very closely comparable to HPLC of known standard alkaloids of HAR and HAL. Hence, it may be concluded that P. harmala’s alkaloid extract contains HAR and HAL alkaloids. The HPLC chromatogram standard alkaloids and P. harmala’s alkaloid extract have been depicted in Fig. 1A.
MTT assay for cell viability
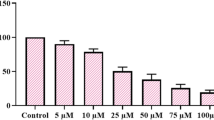
Cell viability was determined by treating HCT116 cells with various concentrations of all substances at different times. It was found that the IC50 value of all treatments for 48 h was more effective with a higher anti-proliferative effect against HCT-116 cells than the IC50 value for 24 h (Table 2). The analysis showed a significant relation between the increase of concentration of the treatments and the increase of cell death (p < 0.001).
Fluorescence microscopic studies
Both of P. harmala’s important beta-carbolines, HAL and HAR, are greatly fluorescent compounds [29]. The HCT-116 cells were treated and analyzed with UV light of a fluorescence microscope. Treated cells emitted blue fluorescence, while untreated cells did not emit blue light (Fig. 2). The HCT-116 cells were treated and analyzed with UV light of a fluorescence microscope compared with untreated.
Measurement of ROS
In this investigation, it was observed that the HCT116 cells treated with P. harmala’s alkaloid extract (IC50, 48 h) showed an increase in the amount of ROS levels compared to the control cells. The mean fluorescent intensity changed from 619 (control) to 757 after 48 h treatment. The intensity of ROS increased by approximately 1.2 folds compared to the control after 48 h of treatment with P. harmala’s alkaloid extract.
Acridine orange (AO)/ethidium bromide (EB) dual staining
AO is a fluorescent cationic dye that can permeate cells and effectively stain nuclear DNA in both living and dead cells. In contrast, the fluorescent dye EB can only stain nuclear DNA in cells that have lost their plasma membrane integrity [31]. Apoptosis was observed in P. harmala’s alkaloid extract treated cells using AO/EB staining, and after 24 h, there were notable morphological changes between treated and control cells (Fig. 3).
Cell apoptosis analysis of HCT116 cells
The apoptotic effect of P. harmala’s alkaloid extract on HCT116 cells was confirmed by using of Annexin V-FITC/PI double staining. The proportion of apoptotic cells was followed in Fig. 4. After HCT116 cells were treated with P. harmala’s alkaloid extract, the early and late apoptotic cells increased from 3.3% (control) to 13% (24 h) and 40.4% (48 h) and viable cells decrease from 95.9% (control) to 82.8% (24 h) and 58.5% (48 h).Observed Necrotic cells were 0.8% (control), 3.7% (24 h) and 1% (48 h).
Cell cycle analysis by flow cytometry
Cell cycle analysis revealed that the percentage of HCT116 cells in the sub G1 phase increased from 3.2% (control) to 12.6% (IC50, 48 h) and the G1 phase increased from 31% (control) to 35.1% (IC50, 48 h), whereas the percentage of cells in the S phase significantly decreased with the corresponding treatment and percentage of HCT116 cells in the G2 phase significantly increased from 7.9% (control) to 25% (IC50) (Fig. 5). These results showed that P. harmala’s alkaloid extract could inhibit the growth of HCT116 cells through an arrest in the G2 phase of the cell cycle.
Real-time PCR
At the IC50 of HAR, HAL and P. harmala’s alkaloid extract treatment, Bax expression was significantly up-regulated about by 4.6, 2.5 and 2.4 fold and GSK3β expression was significantly down-regulated about by 3.2, 4.1 and 2.4 fold (Fig. 6).
Western blot assay
The protein expression levels of Bax, Bcl-2, GSK-3β, and p53 were evaluated using the western blot analysis technique after treatment with P. harmala alkaloid extract. The expression of Bax and p53 proteins showed a significant increase of approximately 5.1 and 1.5 fold, respectively, while the expression of GSK3β and Bcl-2 exhibited a significant decrease of approximately 1.2 and 1.4 fold, respectively (Fig. 7). In the current investigation, the Bax/Bcl-2 ratios showed a notable increase of about 7.3 fold.
Discussion
Researchers worldwide have shown great interest in the powerful anticancer properties of P. harmala, resulting in numerous pharmacological studies investigating this essential impact. The significant cytotoxic effects of the chief beta-carboline alkaloids, HAR and HAL, which are derived from P. harmala, have been observed against different cancer cell lines [14, 32]. The effects of P. harmala seed extracts on different tumor cell lines both in vitro and in vivo, have also been demonstrated [9, 33]. According to research by Jahaniani et al., P. harmala exhibited greater specificity towards cancerous cells compared to commonly used anticancer drugs such as doxorubicin [34]. All of these data suggest that P. harmala and its alkaloids could be used as novel antioxidants in cancer therapy. In the present study, we observed that P. harmala’s alkaloid extract, HAR, and HAL significantly decreased the proliferation of HCT116 colorectal cancer cells in a dose- and time-dependent manner. In the present study, among these substances, HAR was the most effective against colorectal cancer, with the lowest IC50. Furthermore, the alkaloid extract of P. harmala was more effective than HAL. An imbalance between cell proliferation and apoptosis is widely recognized as the underlying cause of oncogenesis [35]. In the present study, we observed that the percentage of apoptotic cells (early and late) among HCT116 cells treated with P. harmala’s alkaloid extract at 24 and 48 h was greater than that among control cells. However, apoptosis was significantly greater for the treated cells at 48 h than treated cells at 24 h. Additionally, we showed that P. harmala’s alkaloid extract inhibited HCT116 cell growth by arresting in the G2 phase of the cell cycle. Zou et al. reported that HAR treatment significantly arrested the cell cycle distribution of B16 melanoma cells in the G2 phase [36]. Wang et al. showed the ability of HAL to induce cell cycle arrest at the G2/M phase and apoptosis in SGC-7901 gastric cancer cells [37]. Our results are in accordance with these studies, however, in previous studies, the effects of HAR and HAL on the cell cycle and the percentage of apoptotic cells were investigated alone, and the effects of P. harmala’s alkaloid extract (HAR and HAL) were not investigated until now. Cancer cells in the colon require a substantial amount of reactive oxygen species (ROS) in order to maintain their cellular functions. However, excessive production of ROS within the cells can disturb the balance of cellular redox, leading to the death of cancer cells. Therefore, there is potential for medicinal plants and their phytochemicals to be used as therapeutic agents by increasing ROS levels within the cells and inducing apoptotic cell death in cancer cells through the manipulation of diverse molecular targets. In the study Bhattacharjee et al. have observed that the alkaloid induced DNA damage and changes in mitochondrial membrane potential in the HeLa (human cervical cancer) cell line [38]. In previous studies it was reported that Cellular damage was associated with the excessive production of ROS caused by anticancer agents [39]. Zhang et al. have documented in their research that harmine derivatives triggered cell apoptosis in human colorectal cancer cell lines. This effect was attributed to the accumulation of ROS and the inhibition of the PI3K/AKT signaling pathway [40]. In this investigation, consistent with prior researches, it was observed that the HCT116 cells treated with an alkaloid extract from P. harmala displayed an increase in the levels of ROS compared to the control cells. The rise in ROS levels following exposure to P. harmala alkaloid extract suggests an indirect DNA damage. However, further investigations into the P. harmala alkaloid extract’s effects on DNA damage were not conducted in this study. It is suggested that future research delve deeper into this aspect for more comprehensive understanding. HAL and HAR are fluorescent indole alkaloids [41]. In the present study, the HCT-116 cells were treated with P. harmala’s alkaloid extract and analyzed via fluorescence microscopy. Normally, to observe the fluorescence under a fluorescence microscope, cells must be stained with fluorescent dyes, but in this study, the cells were only treated with the P. harmala’s alkaloid extract and were not stained with any fluorescent dye. Under the fluorescent microscope, we observed that the cells that were treated emitted a fluorescent blue color, whereas the untreated cells did not exhibit any blue light. The fluorescence characteristics of these alkaloids have been studied, but for the first time, these compounds were studied on cells without staining via fluorescence microscopy. We observed that P. harmala’s alkaloid extract has fluorescent properties and can enter cells. Hence, due to its fluorescing properties, P. harmala’s alkaloid extract represents a promising model substance for further investigations of organic fluorophores. This study revealed that P. harmala’s alkaloid extract, HAR, and HAL standards inhibit the growth of human colorectal cancer cells in vitro in a dose- and time-dependent manner by reducing GSK3β and simultaneously increasing Bax expression.
Additionally, HCT116 cells underwent treatment with P. harmala’s alkaloid extract, showed decrease in the expression of anti-apoptotic proteins Bcl-2 and GSK3β, while increasing the expression of pro-apoptotic proteins Bax and p53. The Western blot assay results corroborated the qRT-PCR findings for BAX and GSK-3β expression levels.
The upregulation of Bax gene and downregulation of Bcl-2 resulted in the induction of cell death in cancer cells. Various herbal drugs can potentially alter Bax and Bcl-2 expression, thereby initiating apoptosis in cancerous cells. Previous studies have shown the presence of anticancer and cytotoxic characteristics in the P. harmala’s alkaloid extract, HAR, and HAL [42,43,44]. Hamsa et al. studied the effect of HAR on B16f-10 melanoma and reported that it affected both intrinsic and extrinsic pathways by upregulating the Bax gene and downregulating Bcl-2 [45]. In another study, DING et al. reported that HAR led to a decrease in the levels of p-Akt and Bcl-2, while simultaneously increasing the expression of Bax. They suggest that HAR promoted apoptosis, prevented the proliferation of breast cancer cell lines, and inhibited tumor growth in vivo [42]. In the present study, the ratios of Bax/Bcl-2 were significantly increased during apoptosis. In this study, another protein that was examined is p53. In the cytoplasm and mitochondrial membrane, p53 plays a crucial role in promoting apoptosis. It does by forming inhibitory complexes with anti-apoptotic proteins BCL-2 and BCL-xL, or activating pro-apoptotic proteins Bak and Bax [46,47,48]. In previous investigations, it has been demonstrated that HAR and the extract from P. harmala’s extract can induce apoptosis in breast cancer cell lines by enhancing the expression of P53 [20, 49]. Another research conducted by Xu et al. revealed that HAL effectively boosts the expression of p53 protein in Hepatocellular cancer cells [50]. In the current study, consistent with the prior findings, an increase in the levels of p53 protein was observed after treatment P. harmala’s alkaloid extract. GSK3β, known to be a survival factor for cancer, is fundamentally activated in colorectal cancer cells. Several studies have shown that the expression level and amount of active forms of GSK3β are significantly elevated in colon cancer cell lines and colon cancer patients compared with those in their normal counterparts [51, 52]. The induction of cytotoxicity in colon cancer cells has been demonstrated by inhibiting GSK3β activity through the use of chemical inhibitors and targeting its expression through RNA interference against GSK3β [52, 53]. Blocking GSK3β with drugs in human colorectal cancer cells was found to activate Bax via a p53-dependent mechanism, ultimately resulting in apoptosis [54]. A previous study showed that the downregulation of GSK3β by natural compounds such as flavonol glycosides has cytotoxic effects on cancerous intestinal cells [55]. The research demonstrated that the alkaloid extract from P. harmala enhances the p53 level and stimulates the production of its downstream protein Bax. Simultaneously, it reduces the expression of the anti-apoptotic protein Bcl-2, thereby facilitating the intrinsic apoptosis pathway. Additionally, it inhibits apoptosis by decreasing the expression of GSK3β. It is suggested that GSK3β gene mRNA and protein decreased by approximately 2.4 and 1.2 fold respectively compared to untreated cells. We have observed that the alkaloid extract of P. harmala has been found to reduce the activity of GSK3β and simultaneously activate p53. It is well-documented that p53 directly triggers the activation of Bax and forms inhibitory complexes with Bcl2 protein. These interactions ultimately lead to the permeabilization of mitochondria and the release of cytochrome c, leading to apoptosis.
Conclusion
The present study demonstrates the anticancer activity of P. harmala’s alkaloid extract, HAR and HAL against human colon cancer cell lines. This suggests that P. harmala’s alkaloid extract could be an effective anticancer agent for both chemoprevention and treatment of colorectal cancer. However, further clinical and pharmacological investigations are necessary to authenticate these finding.
In addition, the limitation of this study is that the effect of the P. harmala’s alkaloid extract on normal cell lines and other colon cancer cell lines has not been investigated, thus further research is needed.
Data availability
The data supporting findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
References
Xi Y, Xu P (2021) Global colorectal cancer burden in 2020 and projections to 2040. Transl Oncol 14(10):101174. https://doi.org/10.1016/j.tranon.2021.101174
Miller K, Nogueria L, Siegel R (2019) Cancer treatment & survivorship facts & figures 2019–2021. American Cancer Society, Atlanta
Zhong L, Li Y, Xiong L, Wang W, Wu M, Yuan T et al (2021) Small molecules in targeted cancer therapy: advances, challenges, and future perspectives. Signal Transduct Target Ther 6(1):201
Ali Abdalla YO, Subramaniam B, Nyamathulla S, Shamsuddin N, Arshad NM, Mun KS et al (2022) Natural products for cancer therapy: a review of their mechanism of actions and toxicity in the past decade. J Trop Med. https://doi.org/10.1155/2022/5794350
Chaachouay N, Douira A, Zidane L (2022) Herbal medicine used in the treatment of human diseases in the Rif, Northern Morocco. Arab J Sci Eng 47(1):131–153
Niroumand MC, Farzaei MH, Amin G (2015) Medicinal properties of Peganum harmala L. in traditional Iranian medicine and modern phytotherapy: a review. J Tradit Chin Med 35(1):104–109
Tarkowská D (2020) A fast and reliable UHPLC–MS/MS-based method for screening selected pharmacologically significant natural plant indole alkaloids. Molecules 25(14):3274
Tehrani Seyed Hassan, S., Hashemi Sheikh Shabani, S., Tahmasebi Enferadi, S., & Rabiei, Z. (2014) Growth inhibitory impact of Peganum harmala L on two breast cancer cell lines. Iran J Biotechnol 12(1):8–14
Sadaf HM, Bibi Y, Ayoubi SA, Safdar N, Sher A, Habib D et al (2022) Extraction, separation and purification of bioactive anticancer components from peganum harmala against six cancer cell lines using spectroscopic techniques. Separations 9(11):355
Burman MD, Bag S, Ghosal S, Karmakar S, Pramanik G, Chinnadurai RK, Bhowmik S (2023) Exploring the structural importance of the C3═ C4 double bond in plant alkaloids harmine and harmaline on their binding interactions with hemoglobin. ACS Omega 8(40):37054–37064
Uddin MJ, Xu S, Crews BC, Aleem AM, Ghebreselasie K, Banerjee S, Marnett LJ (2020) Harmaline analogs as substrate-selective cyclooxygenase-2 inhibitors. ACS Med Chem Lett 11(10):1881–1885
Ayoob I, Hazari YM, Lone SH, Khuroo MA, Fazili KM, Bhat KA (2017) Phytochemical and cytotoxic evaluation of peganum harmala: structure activity relationship studies of harmine. ChemistrySelect 2(10):2965–2968
Cao R, Chen Q, Hou X, Chen H, Guan H, Ma Y et al (2004) Synthesis, acute toxicities, and antitumor effects of novel 9-substituted beta-carboline derivatives. Bioorg Med Chem 12(17):4613–4623. https://doi.org/10.1016/j.bmc.2004.06.038
Yao P, Yao P, Ku X, Yang J (2023) Harmine suppresses the malignant phenotypes and PI3K activity in breast cancer. Anticancer Drugs 34(3):373
Rashidi M, Mahmoudian E, Mirzaei S, Mazloomi SN, Bazi A, Azadeh H, Mozaffari M (2022) Harmaline downregulates angiogenesis markers and suppresses the growth of 4T1 breast cancer cells in vivo and in vitro. Chem Biol Interact 365:110087. https://doi.org/10.1016/j.cbi.2022.110087
Pfeffer CM, Singh ATK (2018) Apoptosis: a target for anticancer therapy. Int J Mol Sci 19(2):448. https://doi.org/10.3390/ijms19020448
Wei H, Wang H, Wang G, Qu L, Jiang L, Dai S et al (2023) Structures of p53/BCL-2 complex suggest a mechanism for p53 to antagonize BCL-2 activity. Nat Commun 14(1):4300
Qian S, Wei Z, Yang W, Huang J, Yang Y, Wang J (2022) The role of BCL-2 family proteins in regulating apoptosis and cancer therapy. Front Oncol 12:985363
Cory S, Adams JM (2005) Killing cancer cells by flipping the Bcl-2/Bax switch. Cancer Cell 8(1):5–6. https://doi.org/10.1016/j.ccr.2005.06.012
Shabani SHS, Tehrani SSH, Rabiei Z, Enferadi ST, Vannozzi GP (2015) Peganum harmala L’.s anti-growth effect on a breast cancer cell line. Biotechnol Rep 8:138–143
Ullah A, Ali N, Ahmad S, Rahman S, Alghamdi S, Bannunah A et al (2021) Glycogen synthase kinase-3 (GSK-3) a magic enzyme it’s role in diabetes mellitus and glucose homeostasis, interactions with fluroquionlones: a mini-review. Brazil J Biol. https://doi.org/10.1590/1519-6984.250179
Naito S, Bilim V, Yuuki K, Ugolkov A, Motoyama T, Nagaoka A et al (2010) Glycogen synthase kinase-3β: a prognostic marker and a potential therapeutic target in human bladder cancerglycogen synthase kinase-3β in bladder cancer. Clin Cancer Res 16(21):5124–5132
Tang QL, Xie XB, Wang J, Chen Q, Han AJ, Zou CY et al (2012) Glycogen synthase kinase-3beta, NF-kappaB signaling, and tumorigenesis of human osteosarcoma. J Natl Cancer Inst 104(10):749–763. https://doi.org/10.1093/jnci/djs210
Vidri RJ, Fitzgerald TL (2020) GSK-3: an important kinase in colon and pancreatic cancers. Biochim Biophys Acta (BBA)-Mol Cell Res 1867(4):118–626
Ugolkov AV, Matsangou M, Taxter TJ, O’halloran, T. V., Cryns, V. L., Giles, F. J., & Mazar, A. P. (2018) Aberrant expression of glycogen synthase kinase-3β in human breast and head and neck cancer. Oncol Lett 16(5):6437–6444
Thapa R, Gupta G, Bhat AA, Almalki WH, Alzarea SI, Kazmi I et al (2023) A review of Glycogen Synthase Kinase-3 (GSK3) inhibitors for cancers therapies. Int J Biol Macromol. https://doi.org/10.1016/j.ijbiomac.2023.127375
Bondarian F, Ebrahimi A, Mahjoubi F, Hervan EM, Gonbad RA (2019) Evaluation of phytochemical content of white tea clone 100 and changes the expression of tumor suppressor genes on colorectal cancer cell line HCT116. Pharmacognosy Res 11(3):224
Malmir S, Ebrahimi A, Mahjoubi F (2020) Effect of ginger extracts on colorectal cancer HCT-116 cell line in the expression of MMP-2 and KRAS. Gene Rep 21:100824
Lewerenz L, Hijazin T, Abouzeid S, Hänsch R, Selmar D (2020) Pilot study on the uptake and modification of harmaline in acceptor plants: An innovative approach to visualize the interspecific transfer of natural products. Phytochemistry 174:112362
Shabani S, Mahjoubi F, Moosavi MA (2019) A siRNA-based method for efficient silencing of PYROXD1 gene expression in the colon cancer cell line HCT116. J Cell Biochem 120(12):19310–19317. https://doi.org/10.1002/jcb.26858
Baskic D, Popovic S, Ristic P, Arsenijevic NN (2006) Analysis of cycloheximide-induced apoptosis in human leukocytes: fluorescence microscopy using annexin V/propidium iodide versus acridin orange/ethidium bromide. Cell Biol Int 30(11):924–932. https://doi.org/10.1016/j.cellbi.2006.06.016
Vahedi MM, Shahini A, Mottahedi M, Garousi S, Shariat Razavi SA, Pouyamanesh G et al (2023) Harmaline exerts potentially anti-cancer effects on U-87 human malignant glioblastoma cells in vitro. Mol Biol Rep 50(5):4357–4366
Moloudizargari M, Mikaili P, Aghajanshakeri S, Asghari MH, Shayegh J (2013) Pharmacological and therapeutic effects of Peganum harmala and its main alkaloids. Pharmacogn Rev 7(14):199–212. https://doi.org/10.4103/0973-7847.120524
Jahaniani F, Ebrahimi SA, Rahbar-Roshandel N, Mahmoudian M (2005) Xanthomicrol is the main cytotoxic component of Dracocephalum kotschyii and a potential anti-cancer agent. Phytochemistry 66(13):1581–1592
Letai A (2017) Apoptosis and cancer. Annu Rev Cancer Biol 1:275–294
Zou N, Wei Y, Li F, Yang Y, Cheng X, Wang C (2017) The inhibitory effects of compound Muniziqi granule against B16 cells and harmine induced autophagy and apoptosis by inhibiting Akt/mTOR pathway. BMC Complement Altern Med 17(1):517. https://doi.org/10.1186/s12906-017-2017-4
Wang Y, Wang C, Jiang C, Zeng H, He X (2015) Novel mechanism of harmaline on inducing G2/M cell cycle arrest and apoptosis by up-regulating Fas/FasL in SGC-7901 cells. Sci Rep 5(1):18613. https://doi.org/10.1038/srep18613
Bhattacharjee P, Sarkar S, Ghosh T, Bhadra K (2018) Therapeutic potential of harmaline, a novel alkaloid, against cervical cancer cells in vitro: apoptotic induction and DNA interaction study. J Appl Biol Biotechnol 6(4):1–8
Serrano J, Palmeira C, Kuehl D, Wallace K (1999) Cardioselective and cumulative oxidation of mitochondrial DNA following subchronic doxorubicin administration. Biochim Biophys Acta (BBA)-Bioenerg 1411(1):201–205
Zhang X-F, Sun R-Q, Jia Y-F, Chen Q, Tu R-F, Li K-K et al (2016) Synthesis and mechanisms of action of novel harmine derivatives as potential antitumor agents. Sci Rep 6(1):33204
Pardo A, Reyman D, Poyato J, Medina F (1992) Some β-carboline derivatives as fluorescence standards. J Lumin 51(5):269–274
Ding Y, He J, Huang J, Yu T, Shi X, Zhang T et al (2019) Harmine induces anticancer activity in breast cancer cells via targeting TAZ. Int J Oncol 54(6):1995–2004. https://doi.org/10.3892/ijo.2019.4777
Zhang Y, Shi X, Xie X, Laster KV, Pang M, Liu K et al (2021) Harmaline isolated from Peganum harmala suppresses growth of esophageal squamous cell carcinoma through targeting mTOR. Phytother Res 35(11):6377–6388
Bournine L, Bensalem S, Fatmi S, Bedjou F, Mathieu V, Iguer-Ouada M et al (2017) Evaluation of the cytotoxic and cytostatic activities of alkaloid extracts from different parts of Peganum harmala L. (Zygophyllaceae). Eur J Integr Med 9:91–96
Hamsa TP, Kuttan G (2011) Harmine activates intrinsic and extrinsic pathways of apoptosis in B16F–10 melanoma. Chin Med 6(1):1–8
Mihara M, Erster S, Zaika A, Petrenko O, Chittenden T, Pancoska P, Moll UM (2003) p53 has a direct apoptogenic role at the mitochondria. Mol Cell 11(3):577–590
Chipuk JE, Kuwana T, Bouchier-Hayes L, Droin NM, Newmeyer DD, Schuler M, Green DR (2004) Direct activation of Bax by p53 mediates mitochondrial membrane permeabilization and apoptosis. Science 303(5660):1010–1014
Leu JI-J, Dumont P, Hafey M, Murphy ME, George DL (2004) Mitochondrial p53 activates Bak and causes disruption of a Bak–Mcl1 complex. Nat Cell Biol 6(5):443–450
Roshankhah S, Rodsari BA, Jalili C, Salahshoor MR (2020) The role of harmine in up-regulating p53 gene expression and inducing apoptosis in MCF-7 cell line. Middle East J Cancer 11(1):34
Xu B, Li M, Yu Y, He J, Hu S, Pan M et al (2018) Effects of harmaline on cell growth of human liver cancer through the p53/p21 and Fas/FasL signaling pathways. Oncol Lett 15(2):1931–1936
Ghatak S, Mehrabi SF, Mehdawi LM, Satapathy SR, Sjölander A (2022) Identification of a novel five-gene signature as a prognostic and diagnostic biomarker in colorectal cancers. Int J Mol Sci 23(2):793
Shakoori A, Ougolkov A, Yu ZW, Zhang B, Modarressi MH, Billadeau DD et al (2005) Deregulated GSK3beta activity in colorectal cancer: its association with tumor cell survival and proliferation. Biochem Biophys Res Commun 334(4):1365–1373. https://doi.org/10.1016/j.bbrc.2005.07.041
Shakoori A, Mai W, Miyashita K, Yasumoto K, Takahashi Y, Ooi A et al (2007) Inhibition of GSK-3β activity attenuates proliferation of human colon cancer cells in rodents. Cancer Sci 98(9):1388–1393
Tan J, Zhuang L, Leong H-S, Iyer NG, Liu ET, Yu Q (2005) Pharmacologic modulation of glycogen synthase kinase-3β promotes p53-dependent apoptosis through a direct Bax-mediated mitochondrial pathway in colorectal cancer cells. Can Res 65(19):9012–9020
Nasri I, Chawech R, Girardi C, Mas E, Ferrand A, Vergnolle N et al (2017) Anti-inflammatory and anticancer effects of flavonol glycosides from Diplotaxis harra through GSK3β regulation in intestinal cells. Pharm Biol 55(1):124–131
Acknowledgements
The authors express their gratitude to the National Institute of Genetic Engineering and Biotechnology (NIGEB) for providing financial support to this research program.
Funding
This study was fully supported by National Institute for Genetic Engineering and Biotechnology (NIGEB).
Author information
Authors and Affiliations
Contributions
ZS: Design of the work, Data analysis, Experimental phase, Interpretation of data for the work, Writing—review & editing. STE: Design of the work, Editing, Data analysis. TM: Writing & Editing. FM: Corresponding author, Design of the work, Editing, funding, Interpretation of data for the work. All authors have read and agreed to the published version of the manuscript. All Authors read and confirmed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors have no conflicts of interest to declare.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Salimizadeh, Z., Enferadi, S.T., Majidizadeh, T. et al. Cytotoxicity of alkaloids isolated from Peganum harmala seeds on HCT116 human colon cancer cells. Mol Biol Rep 51, 732 (2024). https://doi.org/10.1007/s11033-024-09655-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11033-024-09655-7