Abstract
It has been optimized thermal acid hydrolytic pretreatment and enzymatic saccharification (Es) in flask culture of Undaria pinnatifida seaweed, which is a prebiotic. The optimal hydrolytic conditions were a slurry content of 8% (w/v), 180 mM H2SO4, and 121°C for 30 min. Es using Celluclast 1.5 L at 8 U/mL produced 2.7 g/L glucose with an efficiency of 96.2%. The concentration of fucose (a prebiotic) was 0.48 g/L after pretreatment and saccharification. The fucose concentration decreased slightly during fermentation. Monosodium glutamate (MSG) (3%, w/v) and pyridoxal 5′-phosphate (PLP) (30 μM) were added to enhance gamma-aminobutyric acid (GABA) production. To further improve the consumption of mixed monosaccharides, adaptation of Lactobacillus brevis KCL010 to high concentrations of mannitol improved the synbiotic fermentation efficiency of U. pinnatifida hydrolysates.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Brown seaweeds are high in easily degradable carbohydrates, making them suitable for synbiotic fermentation. The main carbohydrates are alginate, laminaran, fucoidan, and mannitol. Mannitol and glucose can be produced by hydrolysis of milled brown seaweed. Various physical, chemical, biological, and enzymatic pretreatments, and combinations thereof, have been used to increase hydrolytic yields [1]. For economic reasons, dilute acid hydrolysis is commonly used to prepare seaweed hydrolysates for enzymatic saccharification (Es) and fermentation.
Brown seaweed, which is a prebiotic, has received much attention in the fields of nutrition and biomedicine given its unique physicochemical properties [2]. Fucoidan is a fucose-based sulfated polysaccharide an biotic derived from brown algae with antibacterial, antiviral, anti-oxidant, anticoagulant, anti-inflammatory, antitumor, antithrombotic, antifibrotic, and immunomodulatory activities [3]. Sulfated polysaccharides include fucoidans (chains of L-fucose with sulfated ester groups) from brown seaweeds, agars, and carrageenans (sulfated galactans), from red seaweeds and ulvans (sulfated glucuronoxylorhamnans), and other sulfated glycans from green seaweeds [4]. Here, the brown seaweed Undaria pinnatifida served as a prebiotic ingredient after synbiotic fermentation.
Synbiotics, which are synergistic mixtures of pre- and pro-biotics, and postbiotics (preparations of inanimate microorganisms and/or their components that confer a health benefit to the host) have been used to treat several clinical conditions and improve health [5,6,7]. Synbiotic and postbiotic fermentation are promising options for preparing functional fermented foods.
Gamma-aminobutyric acid (GABA), which is a non-protein amino acid, is one of the major inhibitory neurotransmitters in the human central nervous system and is also widespread in animals, plants, and microorganisms [8]. However, most studies focused on GABA-producing microorganisms rather than GABA per se. GABA-producing lactic acid bacteria (LAB) include strains of Lactococcus lactis, Lactobacillus (Lb.) brevis, Lb. buchneri, Lb. helveticus, Lb. paracasei, Lb. plantarum, and Streptococcus thermophilus [9,10,11,12,13,14,15]. The biosynthetic production of natural GABA by LAB (for food manufacture) exploits the health-promoting properties of GABA and LAB, which are probiotics. Of the LAB species, Lb. brevis has shown great promise in terms of large-scale fermentation for GABA production [16]. Thus, we selected Lb. brevis as the optimal probiotic for GABA production. To further increase the yield of GABA, the effects of the pyridoxal 5′-phosphate (PLP) cofactor and initial monosodium glutamate (MSG) concentrations on GABA production by Lb. brevis were investigated using U. pinnatifida hydrolysates.
The objective was to optimize pretreatment and Es in terms of GABA production, and assess the synbiotic fermentation of synergistic mixtures of prebiotics and probiotics from U. pinnatifida hydrolysates.
Materials and Methods
Chemicals and Strains
All chemicals used were of analytical grade and were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA) and Duksan Pure Chemical Co. (Gyeonggi-do, Korea). Lb. brevis KCL010 was obtained from the Korean Culture Collection of Probiotics (KCCP, Seongnam, Korea) and was cultured in Difco Lactobacilli de Man, Rogosa, and Sharpe (MRS) broth (Becton, Dickinson and Co., Sparks, MD, USA) at 30℃ and 120 rpm for 18 h. Then, 5% (w/v, 2.8 g dcw/L) inoculates were transferred to 250-mL Erlenmeyer flasks containing 100-mL amounts of U. pinnatifida hydrolysate. To determine cell growth via optical density measurements, the hydrolysate was centrifuged at 8000xg for 15 min to eliminate solids. The supernatant served as the fermentation medium.
Seaweed Preparation
U. pinnatifida (Sea mustard, Miyuk) was obtained from Gijang Local Products Co. Ltd. (Busan, Korea). The seaweed was ground using a roller mill and sieved through a 200-mesh filter before pretreatment. Composition and proximate analyses of U. pinnatifida were performed by the Feed and Foods Nutrition Research Center at Pukyong National University, Korea, using the AOAC method [17]. Then, the levels of carbohydrates and cellulose were employed to calculate the efficiencies of pretreatments and Es.
Thermal Acid Hydrolysis and Enzymatic Saccharification
Thermal acid hydrolytic pretreatment of U. pinnatifida was performed under various conditions, i.e., using seaweed slurry concentrations of 2–12% (w/v), thermal hydrolysis times of 15–120 min, and H2SO4 concentrations of 90–540 mM. The pH of U. pinnatifida hydrolysates was then adjusted to pH 5.0 with 5 N NaOH. The hydrolysates served as fermentation media (with 4% [w/v] MSG) for GABA production. The hydrolysates were fermented at 30°C and 150 rpm with Lb. brevis KCL010. The pretreatment efficiency (Ep, %) was calculated using Eq. (1) as the increase in mannitol and glucose concentrations (ΔSman+glu, g/L) during thermal acid hydrolysis relative to the total carbohydrate content of U. pinnatifida (TC, g/L).
Es of the hydrolysates proceeded using Viscozyme L (β-glucanase, 121 U/mL; Novozymes, Bagsvaerd, Denmark), Celluclast 1.5 L (cellulase, 854 U/mL; Novozymes), and Spirizyme Fuel (a glucoamylase and cellulase blend, 862 U/mL; Novozymes). The three enzymes were diluted to 16 U/mL. Saccharification proceeded at 45°C and 150 rpm for 48 h after thermal acid hydrolysis. To achieve a synergistic effect (compared to single-enzyme treatments), the enzymes were mixed in a 1:1:1 ratio (16 U/mL of each enzyme).
The efficiency of Es (%) was determined as the increase in glucose concentration (ΔSglu, g/L) from the fiber level (F, g/L) during pretreatment, as follows:
Determination of Fucoidan Content
The measurement methods were based on determination of common chemical features (e.g., fucose, sulfated polysaccharides, fuco-oligosaccharides) of fucoidan. The fucoidan content was determined by measuring the amount of fucose released from U. pinnatifida hydrolysates. The fucose was quantified using an enzymatic L-fucose assay kit (K-FUCOSE; Megazyme, Wicklow, Ireland) according to the manufacturer’s instructions. The assay is based on oxidation of L-fucose to L-fucono-1,5-lactone by L-fucose dehydrogenase in the presence of nicotinamide adenine dinucleotide phosphate (NADP+). The amount of L-fucose is equivalent to the level of reduced nicotinamide adenine dinucleotide phosphate (NADPH) produced. The levels of NADPH were measured by optical absorbance. Briefly, 0.1 mL of reaction mix was added to 2.5 mL of mixed reagents (2.0 mL dH2O, 0.4 mL reaction buffer [pH 9.5], 0.1 mL NADP+, and 0.05 mL L-fucose dehydrogenase suspension). The mixture was held at room temperature for 20 min, after which the optical density was measured using a UV–visible spectrophotometer (EMC-18PC-UV; EMCLAB, Duisburg, Germany) at 340 nm. The blank was prepared as above, but 0.1 mL of distilled water was added instead of the sample supernatant (0.1 mL). The fucose concentration was calculated using the following Eq. (3):
Synbiotic Fermentation
Each synbiotic fermentation was performed using 100 mL of seaweed hydrolysate in a 250-mL Erlenmeyer flask under semi-anaerobic conditions. Es and final pH correction to pH 5.0 were performed after thermal acid hydrolysis. A seed culture of LAB was grown at 30°C and 120 rpm for 18 h in 30 mL of MRS medium and a 5% inoculate (w/v, 2.8 g dcw/L) transferred to a 250-mL Erlenmeyer flask containing 100 mL of U. pinnatifida hydrolysate. Nutrient supplements (2.0 g/L yeast extract, 5 g/L K2HPO4, 0.25 g/L MgSO4, 10 μM PLP and 40 g/L MSG) were added to the hydrolysate and mixed before inoculation. Mannitol adaptation was performed using Lb. brevis KCL010 cultured in MRS medium with 80 g/L mannitol (MRSM) for 48 h. Then, the cells were washed twice with fresh MRS medium and transferred to the U. pinnatifida hydrolysate for synbiotic fermentation, after centrifugation at 1390 × g, 10 min to remove the MRSM medium. The seaweed hydrolysates were fermented at 30°C and 150 rpm with Lb. brevis KCL010. Samples were obtained periodically for determination of cell growth (OD600nm) and GABA, mannitol, glucose, fucose, and MSG concentrations, and stored at –20°C before analysis. The efficiency of GABA conversion was calculated as follows:
where (GABA)inc represents the increase in GABA concentration (g/L) and (MSG)cons is the consumption of MSG (g/L) during LAB fermentation.
Analytical Methods
Cell growth was monitored by measuring OD600 values using a UV–visible spectrophoto-meter (EMC-18PC-UV). Salinity was measured by a digital salinity refractometer (ES-421; ATAGO, Tokyo, Japan).
MSG and GABA contents were determined by pre-column derivatization with 2-hydroxy-naphthaldehyde, followed by HPLC. The well-established derivatization procedure is detailed elsewhere [18].
Glucose and mannitol concentrations were determined using a high-performance liquid chromatography (HPLC) system (Agilent 1200 Series; Agilent Inc., Santa Clara, CA, USA) equipped with a refractive index detector. An Aminex HPX-87H column (300.0 × 7.8 mm; Bio-Rad, Hercules, CA, USA) was maintained at 65℃, and samples were eluted with 5 mM H2SO4 at 0.6 mL/min. The values are the means from triplicate experiments.
Results and Discussion
U. pinnatifida Composition and Thermal Acid Hydrolysis
The composition of U. pinnatifida was analyzed by the AOAC method and was as follows: 48.5% carbohydrate, 3.5% crude fiber, 18.2% crude protein, 1.8% crude lipid, and 28.0% crude ash.
The effects of the various slurry levels were determined at 121°C with a thermal hydrolysis time of 60 min and H2SO4 concentration of 360 mM [Fig. 1A]. The concentrations of monosaccharides, such as mannitol and glucose, increased with increasing slurry level. Notably, the glucose concentration increased only slightly when the slurry level increased to > 12% (w/v). The initial glucose concentration was 0.22 g/L after thermal acid hydrolysis pretreatment; this was considered when evaluating the efficiency of pretreatment (Ep). At a monosaccharide (mannitol and glucose) concentration of 7.59 g/L, the slurry content was 8% (w/v) with an Ep of 19.3% (g/g). However, a high slurry content increased the salinity (%, g/g) of pretreated slurry from 2.9 to 6.4%. GABA production decreased from 2.38 to 1.65 g/L as the slurry content increased from 8 to 12% (w/v). This indicated that high-salt stress of Lb. brevis KCL010 significantly impeded GABA production during the fermentation of thermal acid-hydrolyzed seaweed medium. Similar results were reported by Laroute et al. [19]; cell yield was reduced by growth inhibition at high chloride concentrations and, consequently, GABA production decreased. We thus employed a slurry content of 8% (w/v) in subsequent experiments.
The effects of seaweed, thermal acid hydrolysis for various times, and the acid concentration on pretreatment efficiency: A U. pinnatifida slurry content, B thermal hydrolysis time, and C H2SO4 concentration. After thermal acid hydrolysis and mixed enzyme (C+V, 1:1 ratio; 16 U/mL) pretreatment, the effect of Lb. brevis KCL010 fermentation on GABA production was analyzed. Conditions: 30°C, 150 rpm for 72 h, 4% (w/v) MSG
The effects of thermal acid hydrolysis of 8% (w/v) slurry with 360 mM H2SO4 at 121°C for different times are presented in Fig. 1B. The maximum monosaccharide (including the initial glucose) concentration over a hydrolysis time of 30 min was 7.61 g/L with an Ep of 19.6%. However, the Ep after hydrolysis for 60–120 min was no greater than that after 30 min, possibly because 30 min was adequate to hydrolyze all sugars. Similar results have been reported in that, an extended hydrolysis time or a high temperature could have a negative effect on the monosaccharides yields [20]. Therefore, a hydrolysis time of 30 min was selected for subsequent experiments.
The effects of thermal acid hydrolysis of 8% (w/v) slurry at 121°C for 30 min under various H2SO4 concentrations are shown in Fig. 1C. The control group lacked acid. Thermal acid hydrolysis increased the monosaccharide concentrations at all sulfuric acid concentrations tested, and is optimal for digesting polysaccharides in U. pinnatifida hydrolysates. A further increase in the H2SO4 concentration to > 180 mM did not enhance monosaccharide degradation or GABA production. Redding et al. [21] reported that 0.9% and 1.2% acid showed the most options for different temperature and time combinations. Therefore, 180 mM H2SO4 was selected as a suitable acid level; the final monosaccharide concentration was 7.96 g/L and the Ep was 20.5%. In summary, the optimal thermal acid hydrolysis conditions were 8% (w/v) slurry, 180 mM H2SO4, and 121°C for 30 min.
Enzymatic Saccharification of U. pinnatifida
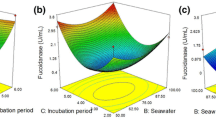
The effects of Es on glucose release from U. pinnatifida hydrolysate were investigated according to enzyme type [Fig. 2A] and dosage [Fig. 2)]. As shown in Fig. 2A, single treatments with Celluclast 1.5 L (C), Viscozyme L (V), and Spirizyme Fuel (S), with consideration of the initial glucose concentration, led to increases of 2.6 g/L glucose with an Es of 93.1%, 2.1 g/L glucose with an Es of 74.1%, and 1.5 g/L glucose with an Es of 53.7% respectively. Mixed enzyme treatments with C+V, V+S, and V+S (1:1 ratio) had no synergistic effects compared to treatment with Celluclast1.5 L alone. Single-enzyme treatment of U. pinnatifida hydrolysate with Celluclast 1.5 L was preferable to mixed enzyme treatments. Next, Es proceeded over 24 h; we aimed to identify the optimal treatment time for obtaining a high glucose concentration. Celluclast 1.5 L was the optimal enzyme after pretreatment of fiber in U. pinnatifida hydrolysate. However, the opposite effect was observed with saccharification of Laminaria japonica hydrolysates. Kim et al. [22] performed simultaneous saccharification and fermentation of an acid hydrolysate of L. japonica using a mixture of commercial enzymes (Celluclast 1.5L, Novoprime B969, and Viscozyme L). The Es process depended greatly on the algal species studied [23].
To determine the optimal dosage of Celluclast 1.5 L, saccharification proceeded with 8–32 U/mL enzyme at 45°C and 150 rpm for 24 h [Fig. 2B]. The optimal enzyme level was 8 U/mL; further increases to 32 U/mL had no significant additional effect on Es. Thus, an enzyme dosage of 8 U/mL was selected for Es of the hydrolysate. In terms of prebiotic production, the fucose concentration was 0.48 g/L, and further increases in the enzyme level to 32 U/mL did not significantly increase it. Similar results were obtained in the seaweed composition analyses. Usov et al. [24] determined the Busov polysaccharide compositions of 17 species, particularly the fucoidan contents (calculated as twice the fucose contents, assuming that the average fucose content is 50% that of fucoidan). Several studies have reported that the brown seaweed Ascophyllum nodosum modulates the composition of the gut microbiota. The seaweed increased the relative abundances of Bacteroidetes and Firmicutes, suggesting potential utility as a functional food to improve human gut health [25, 26]. Therefore, U. pinnatifida hydrolysate may serve as a valuable prebiotic ingredient for synbiotic fermentation.
The Effects of MSG and PLP on GABA Production
MSG and PLP are required when GAD converts L-glutamate to GABA [27]. The effects of MSG (Fig. 3A) and PLP (Fig. 3B) addition to pretreated U. pinnatifida hydrolysate were investigated; the goal was to increase GABA production by Lb. brevis KCL010 during 72 h of fermentation. Figure 3A shows the GABA production by Lb. brevis KCL010 with and without MSG at 0–5% (w/v). Compared to the control group, GABA production generally increased with the MSG concentration; however, it decreased slightly between MSG addition amounts of 4 to 5% (w/v). The GABA production amount was 2.23 g/L, and the efficiency of GABA conversion (EGC) was 11.86% when Lb. brevis KCL010 grew in the presence of 3% (w/v) MSG; this concentration was optimal. Similar results were obtained for Lactiplantibacillus plantarum FBT215 [28]; GABA production (238.43 μg/mL) was maintained by 200 mM MSG (3.4%) in MRS broth; however, production decreased slightly when the MSG level was 250 mM in modified MRS broth. Villegas et al. [29] reported a gradual increase in GABA yield as the MSG level increased from 0 to 270 mM. Higher MSG concentrations decreased the GABA yield, possibly because an increase in osmotic pressure compromised bacterial metabolism.
Figure 4B shows that the addition of PLP had little effect on GABA production by Lb. brevis KCL010 acting on pretreated U. pinnatifida hydrolysate. GABA production barely increased; however, MSG consumption decreased with increasing PLP concentration. An increase in PLP concentration from 10 to 30 μM increased the EGC from 12.3 to 15.1% but further increases in PLP concentration (up to 50 μM) had no significant additional effect on EGC. Therefore, 30 μM PLP was optimal. Theoretically, PLP addition should increase GAD activity and GABA production: 20 μM PLP significantly enhanced GABA production and GAD activity on S. thermophilus Y2 culture [15]. In contrast, the addition of PLP to the fermentation medium of L. plantarum 90sk did not affect GABA production [30]. It is possible that PLP is degraded in the early phase of growth. Dependence on the timing of PLP addition was previously reported by Yang et al. [15]. Very small quantities of PLP in the culture medium may suffice for evoking glutamate decarboxylase (GAD) activity.
Synbiotic Fermentation with Non-adapted and Adapted Lb. brevis KCL010
Figure 4 shows synbiotic fermentation by Lb. brevis KCL010 adapted and not adapted to high mannitol concentrations. Figure 4A shows that all glucose was consumed within 48 h; however, the mannitol and MSG levels were not completely spent until 120 h. This probably reflects the preference of LAB for glucose compared to other monosaccharides; mannitol consumption is inefficient. This appears to be a strain-specific phenomenon [31]. Growth of Lb. brevis KCL010 with 30 g/L MSG exhibited no lag; the stationary phase was reached by 96 h. The GABA concentration after 120 h of fermentation was 2.37 g/L; the fucose concentration decreased slightly over time. However, an additional treatment is needed to solve the problems posed by carbon catabolite repression (CCR) (Fig. 4A). In the presence of both glucose and mannitol, repression by glucose seems to predominate over induction by mannitol. The current study strongly suggests that glucose represses the mannitol operon of LAB.
To overcome these problems, Lb. brevis KCL010 was adapted to high concentrations of mannitol, as shown in Figure 4B. Glucose was rapidly consumed within 24 h. Mannitol was consumed after 96 h; only 0.64 g/L of mannitol remained in the fermentation medium. Compared to Fig. 4A, Fig. 4 B shows that glucose and mannitol were sequentially used to promote diauxic cell growth. The concentration of GABA was 2.50 g/L after 120 h of fermentation. Choe et al. [32] reported that mannitol operon repressor (MtlR)-histidine phosphocarrier protein (HPr) interactions are the key mechanism by which glucose represses the mannitol operon in E. coli (independent of the mannitol induction mechanism). Similar results were obtained in terms of glucose repression of xylose utilization by Lactococcus lactis IO-1; a mixture of glucose and xylose triggered diauxic cell growth, which was attributable to CCR [33]. L. brevis utilizes glucose and xylose in parallel [34, 35]. Thus, our adapted Lb. brevis KCL010 facilitated efficient utilization of both mannitol and glucose during synbiotic fermentation of U. pinnatifida hydrolysate.
Many factors affect the ability of lactic acid bacteria to produce GABA [14, 30]. Maintenance of high bacterial density may increase GABA yield via the production of GAD [36]. Although the U. pinnatifida hydrolysate is a synthetic medium appropriate for synbiotic production because of its low cost, and the fact that it can serve as a dietary supplement benefiting gut health, further improvements are needed to enhance the productivity of synbiotic fermentation. When both probiotics and prebiotics act together in the gastrointestinal tract, the synergistic effects are optimized. Synergistic interactions between prebiotics and probiotics enhance human health and metabolism [37, 38].
Conclusions
It has been optimized thermal acid hydrolysis pretreatment of the seaweed U. pinnatifida using various slurry levels, thermal hydrolysis times and H2SO4 concentrations. After pretreatment, i.e., during fermentation, high-salt stress of Lb. brevis KCL010 significantly impeded GABA production. The fucose concentration was 0.48 g/L after Es (assuming that the average fucose content is 50% that of fucoidan). Thus, U. pinnatifida hydrolysate may be a useful prebiotic. Various PLP concentrations had no significant effects on GABA production because PLP is degraded in the early phase of growth. Comparison between non-adapted Lb. brevis KCL010 and a strain adapted to high concentrations of mannitol showed that glucose repression could be overcome using adaptive evolution. Therefore, optimization of fermentation parameters may further improve the synbiotic fermentation of pre- and pro-biotics from U. pinnatifida hydrolysates.
References
Agbor, V. B., Cicek, N., Sparling, R., Berlin, A., & Levin, D. B. (2011). Biomass pretreatment: Fundamentals toward application. Biotechnology Advances, 29(6), 675–685.
Lopez-Santamarina, A., Miranda, J. M., Mondragon, A. D. C., Lamas, A., Cardelle-Cobas, A., Franco, C. M., & Cepeda, A. (2020). Potential use of marine seaweeds as prebiotics: A review. Molecules, 25(4), 1004.
Zhu, Y., Liu, L., Sun, Z., Ji, Y., Wang, D., Mei, L., Shen, P., Li, Z., Tang, S., Zhang, H., Zhou, Q., & Deng, J. (2021). Fucoidan as a marine-origin prebiotic modulates the growth and antibacterial ability of Lactobacillus rhamnosus. International Journal of Biological Macromolecules, 180, 599–607.
Charoensiddhi, S., Conlon, M. A., Franco, C. M., & Zhang, W. (2017). The development of seaweed-derived bioactive compounds for use as prebiotics and nutraceuticals using enzyme technologies. Trends in Food Science & Technology, 70, 20–33.
Wegh, C. A., Geerlings, S. Y., Knol, J., Roeselers, G., & Belzer, C. (2019). Postbiotics and their potential applications in early life nutrition and beyond. International Journal of Molecular Sciences, 20(19), 4673.
Salminen, S., Collado, M. C., Endo, A., Hill, C., Lebeer, S., Quigley, E. M. M., Sanders, M. E., Shamir, R., Swann, J. R., Szajewska, H., & Vinderola, G. (2021). The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nature Reviews Gastroenterology & Hepatology, 18(9), 649–667.
Son, S. J., Koh, J. H., Park, M. R., Ryu, S., Lee, W. J., Yun, B., Lee, J. H., Oh, S., & Kim, Y. (2019). Effect of the Lactobacillus rhamnosus strain GG and tagatose as a synbiotic combination in a dextran sulfate sodium-induced colitis murine model. Journal of Dairy Science, 102(4), 2844–2853.
Diana, M., Quílez, J., & Rafecas, M. (2014). Gamma-aminobutyric acid as a bioactive compound in foods: A review. Journal of Functional Foods, 10, 407–420.
Laroute, V., Yasaro, C., Narin, W., Mazzoli, R., Pessione, E., Cocaign-Bousquet, M., & Loubière, P. (2016). GABA production in Lactococcus lactis is enhanced by arginine and co-addition of malate. Frontiers in Microbiology, 7, 1050.
Wang, Q., Liu, X., Fu, J., Wang, S., Chen, Y., Chang, K., & Li, H. (2018). Substrate sustained release-based high efficacy biosynthesis of GABA by Lactobacillus brevis NCL912. Microbial Cell Factories, 17(1), 1–8.
Cho, Y. R., Chang, J. Y., & Chang, H. C. (2007). Production of γ-aminobutyric acid (GABA) by Lactobacillus buchneri isolated from Kimchi and its neuroprotective effect on neuronal cells. Journal of Microbiology and Biotechnology, 17(1), 104–109.
Yao, C., Chou, J., Wang, T., Zhao, H., & Zhang, B. (2018). Pantothenic acid, vitamin C, and biotin play important roles in the growth of Lactobacillus helveticus. Frontiers in Microbiology, 9, 1194.
Komatsuzaki, N., Shima, J., Kawamoto, S., Momose, H., & Kimura, T. (2005). Production of γ-aminobutyric acid (GABA) by Lactobacillus paracasei isolated from traditional fermented foods. Food Microbiology, 22(6), 497–504.
Park, S. J., Kim, D. H., Kang, H. J., Shin, M., Yang, S. Y., Yang, J., & Jung, Y. H. (2021). Enhanced production of γ-aminobutyric acid (GABA) using Lactobacillus plantarum EJ2014 with simple medium composition. LWT-Food Science and Technology, 137, 110443.
Yang, S. Y., Lü, F. X., Lu, Z. X., Bie, X. M., Jiao, Y., Sun, L. J., & Yu, B. (2008). Production of γ-aminobutyric acid by Streptococcus salivarius subsp. thermophilus Y2 under submerged fermentation. Amino Acids, 34(3), 473–478.
Li, H., Qiu, T., Huang, G., & Cao, Y. (2010). Production of gamma-aminobutyric acid by Lactobacillus brevis NCL912 using fed-batch fermentation. Microbial Cell Factories, 9(1), 1–7.
Sánchez-Machado, D. I., López-Cervantes, J., Lopez-Hernandez, J., & Paseiro-Losada, P. (2004). Fatty acids, total lipid, protein and ash contents of processed edible seaweeds. Food Chemistry, 85(3), 439–444.
Hayat, A., Jahangir, T. M., Khuhawar, M. Y., Alamgir, M., Siddiqui, A. J., & Musharraf, S. G. (2014). Simultaneous HPLC determination of gamma amino butyric acid (GABA) and lysine in selected Pakistani rice varieties by pre-column derivatization with 2-Hydroxynaphthaldehyde. Journal of Cereal Science, 60(2), 356–360.
Laroute, V., Mazzoli, R., Loubière, P., Pessione, E., & Cocaign-Bousquet, M. (2021). Environmental conditions affecting GABA production in Lactococcus lactis NCDO 2118. Microorganisms, 9(1), 122.
Jeong, G. T., Ra, C. H., Hong, Y. K., Kim, J. K., Kong, I. S., Kim, S. K., & Park, D. H. (2015). Conversion of red-algae Gracilaria verrucose to sugar, levulinic acid and 5-hydroxymethylfurfural. Bioprocess and Biosystems Engineering, 38, 201–217.
Redding, A. P., Wang, Z., Keshwani, D. R., & Cheng, J. J. (2011). High temperature dilute acid pretreatment of coastal Bermuda grass for enzymatic hydrolysis. Bioresource Technology, 102, 1415–1424.
Kim, N. J., Li, H., Jung, K., Chang, H. N., & Lee, P. C. (2011). Ethanol production from marine algal hydrolysates using Escherichia coli KO11. Bioresource Technology, 102(16), 7466–7469.
Hong, I. K., Jeon, H., & Lee, S. B. (2014). Comparison of red, brown and green seaweeds on enzymatic saccharification process. Journal of Industrial and Engineering Chemistry, 20(5), 2687–2691.
Usov, A. I., Smirnova, G. P., & Klochkova, N. G. (2001). Polysaccharides of algae: 55. Polysaccharide composition of several brown algae from Kamchatka. Russian Journal of Bioorganic Chemistry, 27(6), 395–399.
Chen, L., Xu, W., Chen, D., Chen, G., Liu, J., Zeng, X., Shao, R., & Zhu, H. (2018). Digestibility of sulfated polysaccharide from the brown seaweed Ascophyllum nodosum and its effect on the human gut microbiota in vitro. International Journal of Biological Macromolecules, 112, 1055–1061.
Okolie, C. L., Rajendran, S. R., Udenigwe, C. C., Aryee, A. N., & Mason, B. (2017). Prospects of brown seaweed polysaccharides (BSP) as prebiotics and potential immunomodulators. Journal of Food Biochemistry, 41(5), e12392.
Ham, S., Bhatia, S. K., Gurav, R., Choi, Y. K., Jeon, J. M., Yoon, J. J., Choi, K. Y., Ahn, J. O., Kim, H. T., & Yang, Y. H. (2022). Gamma aminobutyric acid (GABA) production in Escherichia coli with pyridoxal kinase (pdxY) based regeneration system. Enzyme and Microbial Technology, 155, 109994.
Kim, J., Lee, M. H., Kim, M. S., Kim, G. H., & Yoon, S. S. (2022). Probiotic properties and optimization of gamma-aminobutyric acid production by Lactiplantibacillus plantarum FBT215. Journal of Microbiology and Biotechnology, 32(6), 783–791.
Villegas, J. M., Brown, L., de Giori, G. S., & Hebert, E. M. (2016). Optimization of batch culture conditions for GABA production by Lactobacillus brevis CRL 1942, isolated from quinoa sourdough. LWT-Food Science and Technology, 67, 22–26.
Yunes, R. A., Poluektova, E. U., Dyachkova, M. S., Klimina, K. M., Kovtun, A. S., Averina, O. V., Orlova, V. S., & Danilenko, V. N. (2016). GABA production and structure of gadB/gadC genes in Lactobacillus and Bifidobacterium strains from human microbiota. Anaerobe, 42, 197–204.
Kim, J. H., Shoemaker, S. P., & Mills, D. A. (2009). Relaxed control of sugar utilization in Lactobacillus brevis. Microbiology, 155(4), 1351–1359.
Choe, M., Min, H., Park, Y. H., Kim, Y. R., Woo, J. S., & Seok, Y. J. (2019). Structural insight into glucose repression of the mannitol operon. Scientific Reports, 9(1), 1–11.
Kanagachandran, K., Stanbury, P. F., Hall, S. J., & Ishizaki, A. (1997). Glucose repression of xylose utilisation by Lactococcus lactis IO-1. Biotechnology Letters, 19(9), 923–925.
Zhang, Y., & Vadlani, P. V. (2015). Lactic acid production from biomass-derived sugars via co-fermentation of Lactobacillus brevis and Lactobacillus plantarum. Journal of Bioscience and Bioengineering, 119(6), 694–699.
Cui, F., Li, Y., & Wan, C. (2011). Lactic acid production from corn stover using mixed cultures of Lactobacillus rhamnosus and Lactobacillus brevis. Bioresource Technology, 102(2), 1831–1836.
Shan, Y., Man, C. X., Han, X., Li, L., Guo, Y., Deng, Y., Li, T., Zhang, L. W., & Jiang, Y. J. (2015). Evaluation of improved γ-aminobutyric acid production in yogurt using Lactobacillus plantarum NDC75017. Journal of Dairy Science, 98(4), 2138–2149.
Kerry, R. G., Patra, J. K., Gouda, S., Park, Y., Shin, H. S., & Das, G. (2018). Benefaction of probiotics for human health: A review. Journal of Food and Drug Analysis, 26(3), 927–939.
Kumar, D., Lal, M. K., Dutt, S., Raigond, P., Changan, S. S., Tiwari, R. K., Chourasia, K. N., Mangal, V., & Singh, B. (2022). Functional fermented probiotics, prebiotics, and synbiotics from non-dairy products: A perspective from nutraceutical. Molecular Nutrition & Food Research, 66(14), 2101059.
Funding
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No.2022R1F1A1074594).
Author information
Authors and Affiliations
Contributions
Na Yeon Kim and Ji Min Kim designed the study and carried out the experimental part. Jong-Youn Son critically read and revised the manuscript. Chae Hun Ra supervised the work and experimental designs, and approved the final manuscript for submission. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Na Yeon Kim and Ji Min Kim are co-first authors.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kim, N.Y., Kim, J.M., Son, JY. et al. Synbiotic Fermentation of Undaria pinnatifida and Lactobacillus brevis to Produce Prebiotics and Probiotics. Appl Biochem Biotechnol 195, 6321–6333 (2023). https://doi.org/10.1007/s12010-023-04415-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-023-04415-y