Abstract
Fungal endophytes are living inside plants without any harmful effects; the prospecting about them is increased day by day because they can produce bioactive compounds which can be used in different applications. Herein, the current study was aimed to isolate the endophytic fungi from the Ocimum basilicum plant as safe microorganisms and evaluate their biological activities. The results illustrated that three endophytic fungal strains were isolated and identified morphologically and genetically as Aspergillus nidulans, Aspergillus fumigatus, and Aspergillus flavus and deposited in gene bank under accession numbers MZ045561, MZ045562, and MZ045563 respectively. Moreover, cell-free filtrates of endophytic fungal strains were extracted using ethyl acetate, where these crude extracts exhibited promising antimicrobial activity against Staphylococcus aureus, Bacillus cereus, Bacillus subtilis, Escherichia coli, Salmonella typhimurium, Pseudomonas aeruginosa, Klebsiella pneumonia, and Candida albicans at a concentration of 1000 µg/mL. Furthermore, these endophytic strains exhibited a potential antioxidant activity where IC50 of the crude extract of A. nidulans, A. fumigatus, and A. flavus were (166.3, 68.4, and 347.1 µg/mL) and (151.2, 77.9, and 246.3 µg/mL) using DPPH and ABTS methods, respectively. Furthermore, the ethyl acetate crude extracts of these endophytic fungi did not exhibit any cytotoxic effect against Vero and Wi 38 normal cells. GC–MS analysis of the crude extract of A. nidulans, A. fumigatus, and A. flavus indicated the presence of 22, 22, and 20 active compounds, respectively. The major compounds in the fungal extracts are belonging to fatty acids, fatty acid esters, tetrahydrofurans, and sterols. In conclusion, the isolated endophytic A. nidulans, A. fumigatus, and A. flavus from Ocimum basilicum are promising sources for bioactive compounds.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ocimum is a huge genus within the family Lamiaceae where this family contains more than 150 species that have a long history of traditional uses [1]. Some species of the genus Ocimum are used to treat different types of diseases, especially the species Ocimum basilicum [2,3,4]. Ocimum basilicum is used as an antibacterial, antifungal, and antioxidant [5]. Fresh leaves are used in case of fevers, abdominal, and poor digestion. Leaf’s decoction is a helpful remedy in the treatment of respiratory disorders and promotes the expulsion of kidney stones.
Fungal endophytes live in healthy plant tissues without harmful effects to their hosts [6]. The symbiotic relationship between fungal endophytes and plants has mutual benefits; host plants supply necessary nutrients to the endophytes. Moreover, fungal endophytes assist host plants by preventing invasion of plant pathogens and/or parasites and improving resistance and tolerance of host plants to major biotic or abiotic factors [7]. Endophytes produce bioactive compounds like those in the host plant including alkaloids, coumarins, flavonoids, glycosides, lignans, terpenoids, phenylpropanoids, saponins, quinones, and xanthones [8]. These metabolites can protect their hosts from biological agents and environmental conditions and produce pharmacologically active compounds [9]. On the other side, secondary metabolites of fungal endophytes from Ocimum basilicum are antibacterial, antifungal, antiviral, antioxidants, and anticancer agents [10,11,12,13,14,15]. Recently, bacterial infections associated with antibiotic-resistant strains remain an issue of severe public health concern. To address this problem, there is a need to search and develop new and highly effective antimicrobial agents. To date, extensive data has been generated on the occurrence of bioactive compounds from medicinal plant extracts, while very little exists on the isolation and characterization of secondary metabolites from endophytic fungi [11]. Therefore, the extraction of antimicrobials from new fungal endophytes is required to combat antimicrobial resistance [16, 17]. Therefore, this study aimed to isolate fungal endophytes from Ocimum basilicum leaves and to analyze their secondary metabolites through phytochemical screening and GC–MS. Moreover, to evaluate their antimicrobial activity against human pathogens, cytotoxicity, as well as antioxidant activity.
to assess the preliminary qualitative phytochemical screening of secondary metabo-
lites.
Materials and Methods
Source of Plant sample
The Ocimum basilicum plant used in this study was collected during July 2020. Healthy plants were collected and brought to the laboratory in sterile polyethylene bags in an icebox (4℃).
Isolation of Endophytic Fungi
Isolation of fungal endophytes was performed according to [7]. Leaves of healthy Ocimum basilicum were washed and sterilized with tap water, 70% ethanol, and 4% NaOCl. The sterilized plant parts were cultivated on potato dextrose agar medium (PDA) (Oxoid) supplemented with chloramphenicol (0.2 g/L). The plates were incubated at 27℃ ± 2℃ for 21 days and were observed daily under a stereomicroscope. Hyphal tips that arise out from the cultivated leaf segments were sub-cultured into a PDA medium.
Phenotypic and Genotypic Identification of Endophytic Fungi
Fungal isolates were identified morphologically according to previous studies [18,19,20,21]. Morphological features were observed, which include the color, texture, and diameter of colonies; in addition, vegetative and reproductive structures of the fungi were also recorded. Then endophytes were characterized using molecular identification technique using ITS genes [22].
Extraction of Active Compounds from the Fungal Filtrate
Endophytic fungi were cultured in potato dextrose broth medium (PDB) (Oxoid) at 27℃ ± 2℃ for 21 days under static conditions. The fermentation broth was subjected to filtration under septic conditions to remove fungal mycelia. Culture filtrates of the isolated fungal endophytes were extracted twice using ethyl acetate (EtOAc) (1:1); 100 mL from each filtrate was mixed with 100 mL of ethyl acetate and placed on a vortex shaker for 10 min and settled down for 5 min until the two clear separate layers were formed. The organic layer (EtOAc) was separated from the aqueous layer by the separating funnel. The collected organic phase was evaporated under reduced pressure at 40–45℃ using a rotary evaporator (Heidolph VV2001, Germany); DMSO at 1 mg/mL of concentration was used to dissolve the fungal crude extract and then stored at − 20℃ until further experiments [23].
Qualitative Screening of Phytochemicals
Qualitative screening of the following phytochemicals was performed according to Sarkar et al. (2020) using the following different standard methods [16, 24].
Test for Alkaloids
Crude extracts of endophytic fungi were tested for alkaloids production using Wagner’s reagent. A fraction of the extract was treated with 3–5 drops of Wagner’s reagent [1.27 g of iodine and 2 g of potassium iodide in 100 mL of water] and observed for the formation of a reddish-brown precipitate (or coloration) [25].
Test for Flavonoids
Crude extracts of endophytic fungi were treated with diluted NaOH, followed by the addition of diluted HCl; solubility and color were noted. A yellow solution with NaOH, which turns colorless with diluted HCl, confirms flavonoids Onwukaeme, Ikuegbvweha, and Asonye [26].
Tests for Glycoside, Steroid, and Terpenoids
Qualitative production of glycoside steroids and terpenoids of crude extracts was performed according to methods used by [27] as follows.
For glycosides, 5 mL of each extract was treated with 2 mL of glacial acetic acid in a test tube and a drop of ferric chloride solution was added to it. This was carefully underlaid with 1 mL of concentrated sulfuric acid. A brown ring at the interface indicated the presence of deoxysugar characteristic of cardenolides. A violet ring may appear below the ring, while in the acetic acid layer, a greenish ring may form.
For steroids, 1 mL of chloroform along with few amounts (1 mL) of the sulfuric acid (98%) was mixed with different extracts (1 mL). The existence of steroids was indicated through the appearance of a brown ring in the tube.
For terpenoids, 1 mL of chloroform was added to 2 mL of each extract, followed by a few drops of concentrated sulfuric acid. A reddish-brown precipitate produced immediately indicated the presence of terpenoids.
Test for Tannin
This test was carried out according to the method used by Auwal et al. [28]. Two milliliters (2 mL) of the aqueous solution of the extract was added to a few drops of 10% Ferric chloride solution (light yellow). The occurrence of the blackish-blue color showed the presence of gallic tannins, and a green-blackish color indicated the presence of catechol tannins.
Test for Saponins
Fungal extract (50 mg) dissolved in 5 mL distilled water up to 20 mL water in the tube and the solution shacked for 15–20 min. The formation of a foam layer up to 2 cm or more confirms the presence of saponins [29].
Test for Reducing Sugars
A 2 mL of fungal extract was treated with Fehling solution and heated over a water bath (5–10 min). Brick red precipitate confirms the presence of reducing sugars.
Gas Chromatography-Mass Spectroscopy (GC–MS) Analysis
The metabolites present in the extracts of the endophytic fungal isolates were analyzed, counted, and identified using GC–MS, as explained by Zothanpuia, Passari, Chandra, Leo, Mishra, Kumar, and Singh [30] with minor modifications. Briefly, crude extract of the strain was dissolved in spectroscopy-grade methanol. GC–MS analysis was performed on Trace GC1310-ISQ mass spectrometer (Thermo Scientific, Austin, Texas, USA), using a direct capillary column (length 30 m, thickness 0.25 µm, internal diameter 25 mm). The oven temperature was started at 50℃ held for 5 min and ramped at 5℃ per min up to 230℃ and held for 2 min; 1 μL of the sample was injected at 250℃ using helium as a carrier gas, split at the ratio of 1:30. The mass spectrometer was run in the electron ionization (EI) mode at 200℃ and 70 eV with a scan range of 40 to 1000 m/z. The spectrum of the detected compounds was compared with the spectrum of the known compounds stored in the WILEY 09 (Wiley, New York, NY, USA) and NIST 11 library. The name, molecular weight, and chemical structure of the detected compounds were also determined.
Antimicrobial Activity
The antimicrobial activity of ethyl acetate crude extracts of fungal isolates was evaluated on Muller Hinton agar (MHA, India) for bacteria and PDA for yeast. Twenty-four hours young culture of Staphylococcus aureus ATCC 6538, Bacillus cereus ATCC 10,987, Bacillus subtilis ATCC 6633, Escherichia coli ATCC 8739, Salmonella typhimurium ATCC14028, Klebsiella pneumonia ATCC 13,883, and Pseudomonas aeruginosa ATCC 9072 and 48 h young culture of Candida albicans ATCC10231 were cultured on the surface of prepared MHA and PDA for bacteria and fungi, respectively. Wells (6 mm) were cut using a sterile cork borer; 100 µl of crude extracts was transferred to each well individually and left for 2 h at 4℃. Chloramphenicol was used as a control for bacterial strain, while fluconazole was used as a control for Candida albicans, and then, the plates were incubated for 24 h at 37℃ for bacteria and 48 h at 28℃ for Candida albicans. After incubation, the inhibition zones were measured and recorded [31,32,33].
Antioxidant activity
DPPH assay
Antioxidant activity of crude extracts of endophytic fungal isolates was carried out using DPPH (2, 2-diphenyl-1-picrylhydrazyl) method according to Khalil, Abdelaziz, Khaleil, and Hashem [34] with minor modifications. Different concentrations of crude extracts (1000 µg/mL, 500 µg/mL, 250 µg/mL, 125 µg/mL, 62.5 µg/mL, 31.25 µg/mL, 15.62 µg/mL, and 7.81 µg/mL) were used to determine the scavenging of DPPH radicals. Antioxidant activity of standard and extracts was determined as DPPH scavenging activity (%): [((control absorbance − extract absorbance)/(control absorbance)) × 100].
ABTS Assay
Another assay for evaluation antioxidant activity is ABTS ABTS (2,2'-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)). The antioxidant activity of all crude extracts was evaluated using ABTs assay according to the method used by Nicoletta [35] with minor modifications.
In-vitro Cytotoxicity
The cytotoxicity of crude extracts of the endophytic fungal strains at concentrations 500 µg/mL and 1000 µg/mL was determined using the MTT protocol [36] with minor modification against normal Vero and Wi 38 cell lines which collected from ATCC. The viability and inhibition percentages were calculated, as shown in Eqs. 1 and 2, as follows:
Statistical Analysis
The data were expressed as the mean ± SDEV value, which was calculated by using Minitab 18 software extended with a statistical package and Microsoft Excel 365.
Results and Discussion
Isolation and Identification of Endophytic Fungi
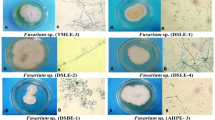
Three different fungal isolates were obtained from surface-sterilized Ocimum basilicum plant parts and coded as S-1, S-2, and S-4. Morphological identification illustrated that fungal isolates S-1 were identified as Aspergillus nidulans where colony diameter after 7 days at 28℃ on PDA about 35 mm, blue-gray with the heavy conidial formation and few Cleistothecia, hyaline reverse deeper, and with abundant Cleistothecia surrounded by dull yellow or buff Hull cells. In addition, fungal isolate S-2 was identified morphologically as A. fumigatus, where colonies grow rapidly, reaching 30–50 mm diameter in 4 days at 28℃, on (PDA), smoky gray-green, reverse faint yellow. Furthermore, fungal isolate S-4 was identified as Aspergillus flavus where colonies grow rapidly, reaching 30–50 mm diameter in 4 days at 28℃, on PDA, dark yellowish-green colonies (powdery) – reverse creamy, pale brown, as shown in Fig. 1.
Phylogenetic trees revealed that three fungal strains were identified genetically as A. nidulans, A. fumigatus, and A. flavus which are related to fungal strains A. nidulans MG518452.1, A. fumigatus MT529212.1, and A. flavus KY964055.1 that were deposited in the NCBI database with similarity percentages of 97.08%, 90.86%, and 94.41%, respectively (Fig. 2). Three fungal strains A. nidulans, A. fumigatus, and A. flavus were recorded in gene bank with accession numbers MZ045561, MZ045562, and MZ045563, respectively.
Phytochemical Analysis
The presence of secondary metabolites indicates the importance of natural products as therapeutic agents. Fungal endophytes are a rich source for many other secondary metabolites such as antibacterial, antifungal, anticancer, and antiparasitic compounds. Different endophytic aspergilli have the ability to produce promising secondary metabolites including alkaloids, terpenoids, ρ-terphenyls, diphenyl ether cytochalasins, xanthones, phenalenones, sterols, and anthraquinone derivatives with different biological activities [37]. The endophytic fungi A. nidulans, A. fumigatus, and A. flavus exhibited antimicrobial activity due to the production of several secondary metabolites. The fungal endophytes from leaves of Ocimum basilicum have promising antimicrobial activity against Gram-positive, Gram-negative bacteria and C. albicans. The results of the preliminary phytochemical study were given in Table 1 and indicated some secondary metabolites found in ethyl acetate fungal extract. Glycosides play an important role against predation by microorganisms, insects, and herbivores [38]. Steroids are known to have antibacterial properties; steroidal compounds specifically interact with membrane lipid and causing leakages of liposomes [39]. Moreover, terpenoids are a cell wall inhibitors class of phytochemicals with antimicrobial properties which cause membrane disruption [40]. Eventually, saponins can affect bacterial growth is due to their ability to cause leakage of proteins and certain enzymes from the cell [41].
GC–MS
GC–MS analysis gives a representative spectral output of each one of the compounds found in the analyzed samples. So, in the past few years, GC–MS has become well recognized as a major technology platform for describing the secondary metabolites in both plant and non-plant species [42, 43]. The GC–MS chromatograms (Fig. 3) of ethyl acetate crude extracts of the fungal strains and the data listed in Table 2 illustrated the presence of 22, 22, and 20 compounds in the extracts of A. nidulans, A. fumigatus, and A. flavus, respectively. The results also revealed the existence of 16 compounds with the same name, molecular weight, and chemical structure at the same retention time (RT) with different peak area % in the extracts of the three fungal strains. The major compounds in the fungal extracts are bisabolol oxide B; hexadecanoic acid methyl ester; 9,12-octadecadienoic acid (Z, Z)- methyl ester; 9-octadecenoic acid (Z)- methyl ester; linoelaidic acid; trans-13-octadecenoic acid; hexadecanoic acid 2-hydroxy-1-(hydroxymethyl)ethyl ester; glycidyl palmitate; 9,12-octadecadienoic acid (Z, Z)- 2-hydroxy-1-(hydroxymethyl)ethyl ester; 9,17-octadecadienal, (Z); ethyl (9z,12z)-9,12-octadecadienoate; glycidyl oleate; and linoleoyl chloride. These compounds have different biological activities such as antimutagenic, anti-inflammatory, antiseptic, anticancer, antihistaminic, anti-corona, antiarthritic, anti-eczemic, antioxidant, antimicrobial, antidiarrheal, antiproliferative, nematocidal, pesticide, hypocholesterolemic, hepatoprotective, hemolytic, and immune function modulation.
Most of the detected compounds are belonging to fatty acid, fatty acid esters, tetrahydrofurans, and sterols. Different studies were reported that these compounds were extracted from the basil plant (Ocimum basilicum) [68,69,70,71]. Endophytic fungi living in medicinal plants can make the same pharmacological bioactive secondary metabolites in the same way as their host medicinal plants, which have been used for a long time in traditional medicine and even now are utilized for their health advantages [72,73,74]. In addition, some reports mentioned the presence of these metabolites in the extracts of aspergilli and other endophytic fungi isolated from different plants [58, 75,76,77]. A. fumigatus has been produced twenty-nine bioactive metabolites that have antimicrobial activities against human pathogenic bacteria as E. coli, S. aureus, and fungi as Candida albicans [37]. Furthermore, 5-hydroxy methyl furan-3-carboxylic acid and 5-acetoxy methyl furan-3-carboxylic acid as furan derivatives were isolated from the culture of endophytic A. flavus, that exhibited antibacterial activity against Staphylococcus aureus [78].
Antimicrobial Activity
Bioactive compounds produced from medicinal plants and endophytic microbes have high efficacy against pathogens with low toxicity on cells [79]. Data reported in the Table 3 explained that the endophytic fungal strains exhibited promising antimicrobial activity against all tested microbial strains (S. aureus, B. cereus, B. subtilis, E. coli, S. typhimurium, K. pneumonia and P. aeruginosa, and C. albicans ATCC10231). Generally, the zones of inhibition were ranged from 13 to 26 mm in diameter. The crude extract of A. nidulans and A. flavus exhibited the highest antimicrobial activity against the tested bacteria compared to A. fumigatus which exhibited the lowest inhibition zones among them. With the same behavior, the crude extracts of endophytic A. nidulans and A. flavus exhibited considerable antifungal activity against C. albicans with inhibition zones of 22 mm and 23 mm, respectively, compared to the inhibition zones caused by A. fumigatus (19 mm), as shown in Fig. 4.
This antimicrobial activity may be attributed to several factors as (1) the presence of some major compounds that can inhibit the microbial growth in the crude extract of A. nidulans, A. fumigatus, and A. flavus, according to El-Fayoumy et al. [74]. These compounds included 9-octadecenoic acid (Z)-; methyl ester methyl stearate; 9,12-octadecadienoic acid (Z, Z)-; 2-hydroxy-1-(hydroxymethyl) ethyl ester; and 9,17-octadecadienal, (Z)-, according to GC–MS analysis. (2) The appearance of terpenoids in phytochemical analysis of fungal crude extracts in all crude extracts may also be responsible for antimicrobial activity because it can disrupt the cell membrane of the microbes, as reported by Jasmine et al. [40]. (3) The detection of glycosides in phytochemical analysis of fungal crude extracts that cause cell lysis and disruption of the cytoplasmic membrane leading to loss of membrane selective permeability. This may also share in the antimicrobial activity of the tested crude extracts [80].
Antioxidant Activity
Antioxidants are compounds that defeat reactive oxygen species (ROS) which resulted from biological reactions as a by-product [81]. In addition, antioxidants are capable of holding and balancing the free radicals which cause several diseases [82]. Antioxidants have been considered as therapeutic agents where they possess anti-atherosclerotic, anti-inflammatory, antitumor, anticarcinogenic, antimutagenic, and antimicrobial properties. In our study, the antioxidant activity of crude extracts of endophytic fungal strains A.nidulans, A. fumigatus, and A. flavus at different concentrations (1000–7.81 µg/mL) was performed using DPPH and ABTS methods, as shown in Fig. 5. Results proved that the antioxidant activity of A. fumigatus was the highest among other endophytic fungal strains, followed by A. nidulans and A. flavus. Additionally, IC50 of A. fumigatus was 68.4 µg/mL compared to AA (5.75 µg/mL) in the case of DPPH, while using ABTs IC50 of A. fumigatus and AA were 77.9 µg/mL and 6.6 µg/mL, respectively. On the other hand, IC50 of A. nidulans and A. flavus were 166.3 µg/mL and 347.1 µg/mL and 151.2 µg/mL and 246.3 µg/mL using DPPH and ABTs assays, respectively. Previous studies confirmed the potential antioxidant activity of fungal endophytes [83]. Furthermore, Khalil, Abdelaziz, Khaleil, and Hashem [34] found that A. Ochraceus which was isolated from Avicennia marina has strong antioxidant activity using DPPH free radical method.
Cytotoxicity
In this study, cytotoxicity of ethyl acetate crude extracts of endophytic A. nidulans, A. fumigatus, and A. flavus at concentrations 500 µg/mL and 1000 µg/mL was performed, as shown in Fig. 6. Results showed that endophytic fungal extracts of A. nidulans, A. fumigatus, and A. flavus are safe in use, where cell viability of and Wi 38 normal cell lines was more than 96% after their treatment with the extracts at concentrations from 500 to 1000 µg/mL. Likewise, IC50 of all fungal extracts against Vero and Wi 38 cell lines was greater than 1000 µg/mL; this indicates that these extracts are non-toxic because Ioset, Brun, Wenzler, Kaiser, and Yardley [84] reported that the compound is safe when IC50 is greater than 90 μg/mL. Khalil, Abdelaziz, Khaleil, and Hashem [34] reported that all endophytic fungal strains which isolated from Avicennia marina did not display significant toxicity to Vero normal cell line.
Conclusion
In the current study, three endophytic fungi were isolated and identified morphologically and genetically as A. nidulans, A. fumigatus, and A. flavus. These endophytic fungi have been shown a powerful source of natural compounds with biological activities. Crude extracts of endophytic A. nidulans, A. fumigatus, and A. flavus have shown promising antibacterial activity against Gram-negative and Gram-positive bacteria as well as antifungal activity against unicellular fungi. Likewise, these endophytic fungal compounds revealed antioxidant activity with no cytotoxic effect on normal cell lines. Therefore, these compounds produced by these endophytic fungi may be considered safe and can become alternatives to commercial antimicrobial agents.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code Availability
Not applicable.
References
Yadav, G., & Meena, M. (2021). Bioprospecting of endophytes in medicinal plants of Thar Desert: An attractive resource for biopharmaceuticals. Biotechnology Reports, 30, e00629.
Siddiqui, B. S., Bhatti, H. A., Begum, S., & Perwaiz, S. (2012). Evaluation of the antimycobacterium activity of the constituents from Ocimum basilicum against Mycobacterium tuberculosis. Journal of ethnopharmacology, 144, 220–222.
Fritea, L., Ganea, M., Zdrinca, M., Dobjanschi, L., Antonescu, A., Vicas, S. I., Bodog, F., Sindhu, R. K., & Cavalu, S. (2021). Perspectives on the combined effects of Ocimum basilicum and Trifolium pratense extracts in terms of phytochemical profile and pharmacological effects. Plants, 10, 1390.
Ilić, Z. S., Milenković, L., Šunić, L., Tmušić, N., Mastilović, J., Kevrešan, Ž, Stanojević, L., Danilović, B., & Stanojević, J. (2021). Efficiency of basil essential oil antimicrobial agents under different shading treatments and harvest times. Agronomy, 11, 1574.
Bora, K. S., Arora, S., & Shri, R. (2011). Role of Ocimum basilicum L. in prevention of ischemia and reperfusion-induced cerebral damage, and motor dysfunctions in mice brain. Journal of Ethnopharmacology, 137, 1360–1365.
Powthong, P., Jantrapanukorn, B., Thongmee, A., & Suntornthiticharoen, P. (2018). Screening of antimicrobial activities of the endophytic fungi isolated from Sesbania grandiflora (L.) Pers.
Aldinary, A. M., Abdelaziz, A. M., Farrag, A. A., & Attia, M. S. (2021). Biocontrol of tomato Fusarium wilt disease by a new Moringa endophytic Aspergillus isolates. Materials Today: Proceedings.
Singh, A., Singh, D. K., Kharwar, R. N., White, J. F., & Gond, S. K. (2021). Fungal endophytes as efficient sources of plant-derived bioactive compounds and their prospective applications in natural product drug discovery: Insights, avenues, and challenges. Microorganisms, 9, 197.
Verma, V. C., Kharwar, R. N., & Strobel, G. A. (2009). Chemical and functional diversity of natural products from plant associated endophytic fungi. Natural Product Communications, 4, 1934578X0900401114.
Gunatilaka, A. A. L. (2006). Natural products from plant-associated microorganisms: Distribution, structural diversity, bioactivity, and implications of their occurrence. Journal of Natural Products, 69, 509–526.
Hussain, H., Kliche-Spory, C., Al-Harrasi, A., Al-Rawahi, A., Abbas, G., Green, I. R., Schulz, B., Krohn, K., & Shah, A. (2014). Antimicrobial constituents from three endophytic fungi. Asian Pacific Journal of Tropical Medicine, 7s1, S224–S227.
Meena, K. K., Sorty, A. M., Bitla, U. M., Choudhary, K., Gupta, P., Pareek, A., Singh, D. P., Prabha, R., Sahu, P. K., Gupta, V. K., Singh, H. B., Krishanani, K. K., & Minhas, P. S. (2017). Abiotic stress responses and microbe-mediated mitigation in plants: the omics strategies. Frontiers in Plant Science, 8, 172.
Atiphasaworn, P., Monggoot, S., Gentekaki, E., Brooks, S., & Pripdeevech, P. (2017). antibacterial and antioxidant constituents of extracts of endophytic fungi isolated from Ocimum basilicum var. thyrsiflora Leaves. Current Microbiology, 74, 1185–1193.
Bhattacharjya, D., Adhikari, S., Biswas, A., Bhuimali, A., Ghosh, P., & Saha, S. (2019). Ocimum phytochemicals and their potential impact on human health. Phytochemicals in Human Health.
Techaoei, S., Jirayuthcharoenkul, C., Jarmkom, K., Dumrongphuttidecha, T., & Khobjai, W. (2020). Chemical evaluation and antibacterial activity of novel bioactive compounds from endophytic fungi in Nelumbo nucifera. Saudi Journal of Biological Sciences, 27, 2883–2889.
Firáková, S., Šturdíková, M., & Múčková, M. (2007). Bioactive secondary metabolites produced by microorganisms associated with plants. Biologia, 62, 251–257.
Fadiji, A. E., & Babalola, O. O. (2020). Elucidating mechanisms of endophytes used in plant protection and other bioactivities with multifunctional prospects. Frontiers in Bioengineering and Biotechnology, 8, 467.
Moubasher, A., & Moustafa, A. (1970). A survey of Egyptian soil fungi with special reference to Aspergillus, Pénicillium and Penicillium-related genera. Transactions of the British Mycological Society, 54, 35–44.
Abdel-Azeem, A. M. (2010). The history, fungal biodiversity, conservation, and future perspectives for mycology in Egypt. IMA Fungus, 1, 123–142.
Hasanin, M. S., Hashem, A. H., Abd El-Sayed, E. S., & El-Saied, H. (2020). Green ecofriendly bio-deinking of mixed office waste paper using various enzymes from Rhizopus microsporus AH3: efficiency and characteristics. Cellulose, 1–11.
Hashem, A. H., Suleiman, W. B., Abu-elreesh, G., Shehabeldine, A. M., & Khalil, A. M. A. (2020). Sustainable lipid production from oleaginous fungus Syncephalastrum racemosum using synthetic and watermelon peel waste media. Bioresource Technology Reports, 12, 100569.
Khalil, A. M. A., Hashem, A. H., & Abdelaziz, A. M. (2019). Occurrence of toxigenic Penicillium polonicum in retail green table olives from the Saudi Arabia market. Biocatalysis and Agricultural Biotechnology, 21, 101314.
Supaphon, P., Phongpaichit, S., Rukachaisirikul, V., & Sakayaroj, J. (2013). Antimicrobial potential of endophytic fungi derived from three seagrass species: Cymodocea serrulata Halophila ovalis and Thalassia hemprichii. PloS one, 8, e72520.
Sarkar, T., Salauddin, M., Pati, S., Sheikh, H. I., & Chakraborty, R. (2021). Application of raw and differently dried pineapple (Ananas comosus) pulp on Rasgulla (sweetened Casein Ball) to enhance its phenolic profile, shelf life, and in-vitro digestibility characteristics. Journal of Food Processing and Preservation, 45, e15233.
Kumar, R. S., Balasubramanian, P., Govindaraj, P., & Krishnaveni, T. (2014). Preliminary studies on phytochemicals and antimicrobial activity of solvent extracts of Coriandrum sativum L. roots (Coriander). Journal of Pharmacognosy and Phytochemistry, 2, 74–78.
Onwukaeme, D., Ikuegbvweha, T., & Asonye, C. (2007). Evaluation of phytochemical constituents, antibacterial activities and effect of exudate of Pycanthus Angolensis Weld Warb (Myristicaceae) on corneal ulcers in rabbits. Tropical Journal of Pharmaceutical Research, 6, 725–730.
Siddiqui, A. A., & Ali, M. (1997). Practical pharmaceutical chemistry. CBS Publishers & Distributors.
Auwal, M. S., Saka, S., Mairiga, I. A., Sanda, K. A., Shuaibu, A., & Ibrahim, A. (2014). Preliminary phytochemical and elemental analysis of aqueous and fractionated pod extracts of Acacia nilotica (Thorn mimosa). Veterinary Research Forum, 5, 95–100.
Raaman, N. (2006). Phytochemical techniques (pp. 19–24). New India Publishing agency.
Zothanpuia, A. K., Passari, P., Chandra, V. V., Leo, V. K., Mishra, B., Kumar, B. P., & Singh. (2017). Singh. Frontiers in Microbiology, 8, 68–68.
Hsueh, P.-R., Ko, W.-C., Wu, J.-J., Lu, J.-J., Wang, F.-D., Wu, H.-Y., Wu, T.-L., & Teng, L.-J. (2010). Consensus statement on the adherence to Clinical and Laboratory Standards Institute (CLSI) Antimicrobial Susceptibility Testing Guidelines (CLSI-2010 and CLSI-2010-update) for Enterobacteriaceae in clinical microbiology laboratories in Taiwan. Journal of Microbiology, Immunology and Infection, 43, 452–455.
Hashem, A. H., Abdelaziz, A. M., Askar, A. A., Fouda, H. M., Khalil, A. M. A., Abd-Elsalam, K. A., & Khaleil, M. M. (2021). Bacillus megaterium-mediated synthesis of selenium nanoparticles and their antifungal activity against Rhizoctonia solani in Faba Bean Plants. Journal of Fungi, 7, 195.
Dacrory, S., Hashem, A. H., & Hasanin, M. (2021). Synthesis of cellulose based amino acid functionalized nano-biocomplex: Characterization, antifungal activity, molecular docking and hemocompatibility. Environmental Nanotechnology, Monitoring & Management, 15, 100453.
Khalil, A., Abdelaziz, A., Khaleil, M., & Hashem, A. (2021). Fungal endophytes from leaves of Avicennia marina growing in semi-arid environment as a promising source for bioactive compounds. Letters in Applied Microbiology, 72, 263–274.
Nicoletta, P. (1999). Screening of dietary carotenoids and carotenoid-rich fruit extracts for antioxidant activities applying 2, 2’-azinobis (3-ethylbenzothiazoline-6-sulfonic acid) radical cation decolorization assay. Methods in Enzymology, 299, 379–389.
Van de Loosdrecht, A., Beelen, R., Ossenkoppele, G., Broekhoven, M., & Langenhuijsen, M. (1994). A tetrazolium-based colorimetric MTT assay to quantitate human monocyte mediated cytotoxicity against leukemic cells from cell lines and patients with acute myeloid leukemia. J Immunol Methods, 174, 311–320.
El-hawary, S. S., Moawad, A. S., Bahr, H. S., Abdelmohsen, U. R., & Mohammed, R. (2020). Natural product diversity from the endophytic fungi of the genus Aspergillus. RSC Advances, 10, 22058–22079.
Wittstock, U., & Gershenzon, J. (2002). Constitutive plant toxins and their role in defense against herbivores and pathogens. Current Opinion in Plant Biology, 5, 300–307.
Epand, R. F., Savage, P. B., & Epand, R. M. (2007). Bacterial lipid composition and the antimicrobial efficacy of cationic steroid compounds (Ceragenins). Biochimica et Biophysica Acta (BBA) Biomembranes, 1768, 2500–2509.
Jasmine, R., Selvakumar, B., & Daisy, P. (2011). Investigating the mechanism of action of terpenoids and the effect of interfering substances on an Indian medicinal plant extract demonstrating antibacterial activity. International Journal of Pharmaceutical Study and Research, 2, 19–24.
Thirumurugan, K., Shihabudeen, M., & Hansi, P. (2010). Antimicrobial activity and phytochemical analysis of selected Indian folk medicinal plants. Steroids, 1, 430–434.
Rukshana, M., Doss, A., & Kumari, P. (2017). Phytochemical screening and GC-MS analysis of leaf extract of Pergularia daemia (Forssk) Chiov. Asian Journal of Plant Science & Research, 7, 9–15.
Kanthal, L. K., Dey, A., Satyavathi, K., & Bhojaraju, P. (2014). GC-MS analysis of bio-active compounds in methanolic extract of Lactuca runcinata DC. Pharmacognosy Research, 6, 58.
Abdelshafy Mohamad, O. A., Ma, J.-B., Liu, Y.-H., Zhang, D., Hua, S., Bhute, S., Hedlund, B. P., Li, W.-J., & Li, L. (2020). Beneficial endophytic bacterial populations associated with medicinal plant Thymus vulgaris alleviate salt stress and confer resistance to Fusarium oxysporum. Frontiers in Plant Science, 11, 47.
Afzal, A., Oriqat, G., Akram Khan, M., Jose, J., & Afzal, M. (2013). Chemistry and biochemistry of terpenoids from curcuma and related species. Journal of Biologically Active Products from Nature, 3, 1–55.
Reddy, G. J., Reddy, K. B., & Reddy, G. V. S. (2020). In vivo anti-diabetic and anti-hyperlipidemic activities of ethyl acetate/methanol fractions of Feronia elephantum fruit in type 2 diabetic rats: Via α-amylase and PPAR-γ by using in silico Approach. Indian Journal of Pharmaceutical Education and Research, 54, 761–770.
Tyagi, T., & Agarwal, M. (2017). Phytochemical screening and GC-MS analysis of bioactive constituents in the ethanolic extract of Pistia stratiotes L. and Eichhornia crassipes (Mart) solms. Journal of Pharmacognosy and Phytochemistry, 6, 195–206.
Kim, B. R., Kim, H. M., & Jin, C. H. (2020). Composition and antioxidant activities of volatile organic compounds in radiation-bred coreopsis cultivars, 9.
Chenniappan, J., Sankaranarayanan, A., & Arjunan, S. (2020). Evaluation of antimicrobial activity of Cissus quadrangularis L. stem extracts against avian pathogens and determination of its bioactive constituents using GC-MS. Journal of Scientific Research, 64.
El-Fayoumy, E. A., Shanab, S. M. M., Gaballa, H. S., Tantawy, M. A., & Shalaby, E. A. (2021). Evaluation of antioxidant and anticancer activity of crude extract and different fractions of Chlorella vulgaris axenic culture grown under various concentrations of copper ions. 21, 51.
Pratama, O. A., Tunjung, W. A. S., Sutikno, S., & Daryono, B. S. (2019). Bioactive compound profile of melon leaf extract (Cucumis melo L.‘Hikapel’) infected by downy mildew. Biodiversitas Journal of Biological Diversity, 20.
Revathi, P., Jeyaseelansenthinath, T., & Thirumalaikolundhusubramaian, P. (2015). Preliminary phytochemical screening and GC-MS analysis of ethanolic extract of mangrove plant-bruguiera cylindrica (Rhizho) L. International Journal of Pharmacognosy and Phytochemical Research, 6, 729–740.
Karunanithi, A., & Venkatachalam, S. (2019). Optimization of ultrasound-assisted extraction of phenolic compounds from wood apple pulp: Identification of phytochemicals using GC-MS. Chemical Industry and Chemical Engineering Quarterly, 14–14.
Duraisamy, M., & Selvaraju, R. Analysis of chemical compounds by using gas chromatography and mass spectrum analysis, in vitro antioxidant and antibacterial activity of methanolic extracts of seaweed ulva flexuosa wulfen (green algae).
Akbari, S., Abdurahman, N. H., Yunus, R. M., Alara, O. R., & Abayomi, O. O. (2019). extraction, characterization and antioxidant activity of fenugreek (Trigonella-Foenum Graecum) seed oil. Materials Science for Energy Technologies, 2, 349–355.
Fadzir, U. A., Mokhtar, K. I., Mustafa, B. E., & Darnis, D. S. (2018). Evaluation of bioactive compounds on different extracts of linum usitatissimum and its antimicrobial properties against selected oral pathogens. Makara Journal of Health Research.
Amudha, P., Jayalakshmi, M., Pushpabharathi, N., & Vanitha, V. (2018). Identification of bioactive components in Enhalus acoroides seagrass extract by gas chromatography–mass spectrometry. Asian Journal of Pharmaceutical and Clinical Research, 11, 131–137.
Anisha, C., & Radhakrishnan, E. K. (2017). Metabolite analysis of endophytic fungi from cultivars of Zingiber officinale Rosc. identifies myriad of bioactive compounds including tyrosol. 3 Biotech, 7, 146–146.
Mangamuri, U., Muvva, V., Poda, S., Naragani, K., Munaganti, R. K., Chitturi, B., & Yenamandra, V. (2016). Bioactive metabolites produced by Streptomyces Cheonanensis VUK-A from Coringa mangrove sediments: Isolation, structure elucidation and bioactivity. 3 Biotech, 6, 63.
Habib, M. R., & Karim, M. R. (2009). Antimicrobial and cytotoxic activity of di-(2-ethylhexyl) phthalate and anhydrosophoradiol-3-acetate isolated from Calotropis gigantea (Linn.) Flower. Mycobiology, 37, 31–36.
Walters, D., Raynor, L., Mitchell, A., Walker, R., & Walker, K. (2004). Antifungal activities of four fatty acids against plant pathogenic fungi. Mycopathologia, 157, 87–90.
Degenkolb, T., & Vilcinskas, A. (2016). Metabolites from nematophagous fungi and nematicidal natural products from fungi as an alternative for biological control. Part I: Metabolites from nematophagous ascomycetes. Applied Microbiology and Biotechnology, 100, 3799–3812.
Kaur, N., Chaudhary, J., Jain, A., & Kishore, L. (2011). Stigmasterol: A comprehensive review. International Journal of Pharmaceutical Sciences and Research, 2, 2259.
Saeidnia, S., Manayi, A., Gohari, A. R., & Abdollahi, M. (2014). The story of beta-sitosterol – A review. European Journal of Medicinal Plants, 4, 590–609.
Hadi, M. Y., Mohammed, G. J., & Hameed, I. H. (2016). Analysis of bioactive chemical compounds of Nigella sativa using gas chromatography-mass spectrometry. Journal of Pharmacognosy and Phytotherapy, 8, 8–24.
Gu, R., Wang, Y., Long, B., Kennelly, E., Wu, S., Liu, B., Li, P., & Long, C. (2014). Prospecting for bioactive constituents from traditional medicinal plants through ethnobotanical approaches. Biological & pharmaceutical bulletin, 37, 903–915.
Malathi, K., & Ramaiah, S. (2017). Ethyl iso-allocholate from a medicinal rice Karungkavuni inhibits dihydropteroate synthase in Escherichia coli: A molecular docking and dynamics study. Indian Journal of Pharmaceutical Sciences, 78, 780–788.
Nour, A. H., Elhussein, S. A., & Osman, N. (2009). Characterization and chemical composition of the fixed oil of fourteen basil (Ocimum basilicum L.) accessions grown in Sudan. International Journal of Chemical Technology, 1, 52–58.
Tarchoune, I., Baâtour, O., Harrathi, J., Hamdaoui, G., Lachaâl, M., Ouerghi, Z., & Marzouk, B. (2013). Effects of two sodium salts on fatty acid and essential oil composition of basil (Ocimum basilicum L.) leaves. Acta Physiologiae Plantarum, 35, 2365–2372.
Srivastava, H., Shukla, P., Tripathi, S., & Shanker, B. (2014). Antioxidant and antimicrobial activities of sweet basil oils. International Journal of Pharmaceutical Sciences and Research, 5, 279.
Idris, A. A., Nour, A. H., Ali, M. M., Erwa, I. Y., Ishag, O. A. O., & Nour, A. H. (2020). Physicochemical properties and fatty acid composition of Ocimum basilicum L seed oil. Asian Journal of Physical and Chemical Sciences, 8, 1–12.
Kaul, S., Gupta, S., Ahmed, M., & Dhar, M. K. (2012). Endophytic fungi from medicinal plants: A treasure hunt for bioactive metabolites. Phytochemistry Reviews, 11, 487–505.
Venieraki, A., Dimou, M., & Katinakis, P. (2017). Endophytic fungi residing in medicinal plants have the ability to produce the same or similar pharmacologically active secondary metabolites as their hosts. Hellenic Plant Protection Journal, 10, 51–66.
Abo Nahas, H. H. (2019). Endophytic fungi: A gold mine of antioxidants. Microbial Biosystems, 4, 58–79.
Gao, H., Hong, K., Zhang, X., Liu, H. W., Wang, N. L., Zhuang, L., & Yao, X. S. (2007). New steryl esters of fatty acids from the mangrove fungus Aspergillus awamori. Helvetica Chimica Acta, 90, 1165–1178.
Pohl, C. H., Kock, J. L., & Thibane, V. S. (2011). Antifungal free fatty acids: A review. Science against microbial pathogens: Communicating current research and technological advances, 3, 61–71.
Kanjana, M., Kanimozhi, G., Udayakumar, R., & Panneerselvam, A. (2019). GC-MS analysis of bioactive compounds of endophytic fungi Chaetomium globosum, Cladosporium tenuissimum and Penicillium janthinellum. International Journal of Biomedical and Pharmaceutical Sciences, 2, 2.
Liu, Z., Zhao, J.-Y., Sun, S.-F., Li, Y., Qu, J., Liu, H.-T., & Liu, Y.-B. (2019). Sesquiterpenes from an Endophytic Aspergillus flavus. Journal of Natural Products, 82, 1063–1071.
Lotlikar, G., & Naik-Samant, S. (2020). Bactericidal activity of endophytic bacteria isolated from Acanthus ilicifolius: A mangrove plant of Divar Island, Goa, against human pathogenic bacteria (pp. 293–301). Elsevier.
Tagousop, C. N., Ekom, S. E., Ngnokam, D., & Voutquenne-Nazabadioko, L. (2018). Antimicrobial activities of flavonoid glycosides from Graptophyllum grandulosum and their mechanism of antibacterial action. BMC Complementary and Alternative Medicine, 18, 1–10.
Kurutas, E. B. (2016). The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: Current state. Nutrition Journal, 15, 71–71.
Pham-Huy, L. A., He, H., & Pham-Huy, C. (2008). Free radicals, antioxidants in disease and health. International Journal of Biomedical Sciences, 4, 89–96.
Ibrahim, M., Oyebanji, E., Fowora, M., Aiyeolemi, A., Orabuchi, C., Akinnawo, B., & Adekunle, A. A. (2021). Extracts of endophytic fungi from leaves of selected Nigerian ethnomedicinal plants exhibited antioxidant activity. BMC Complementary Medicine and Therapies, 21, 98.
Ioset, J.-R., Brun, R., Wenzler, T., Kaiser, M., & Yardley, V. (2009). Drug screening for kinetoplastids diseases. A Training Manual for Screening in Neglected Diseases.
Acknowledgements
The authors express their sincere thanks to the Faculty of Science, Al-Azhar University, Egypt, for support of this research work.
Author information
Authors and Affiliations
Contributions
Mohamed H. Sharaf: conceptualization, methodology, formal analysis and investigation, writing – original draft preparation, writing – review and editing, resources, software; Amer M. Abdelaziz: conceptualization, methodology, formal analysis and investigation, writing – original draft preparation, writing – review and editing, resources, software; Mohamed H. Kalaba: conceptualization, methodology, formal analysis and investigation, writing – original draft preparation, writing – review and editing, resources, software; Ahmed A. Radwan: methodology, formal analysis and investigation, writing – original draft preparation, writing – review and editing, resources, software; Amr H. Hashem: conceptualization, methodology, formal analysis and investigation, writing – original draft preparation, writing – review and editing, resources, software.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflicts of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sharaf, M.H., Abdelaziz, A.M., Kalaba, M.H. et al. Antimicrobial, Antioxidant, Cytotoxic Activities and Phytochemical Analysis of Fungal Endophytes Isolated from Ocimum Basilicum. Appl Biochem Biotechnol 194, 1271–1289 (2022). https://doi.org/10.1007/s12010-021-03702-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-021-03702-w