Abstract
Brewers’ spent grains (BSG) make up to 85% of a brewery’s solid waste, and is either sent to landfill or sold as cheap animal feed supplement. Xylo-oligosaccharides (XOS) obtained from BSG are antioxidants and prebiotics that can be used in food formulations as low-calorie sweeteners and texturisers. The effect of extremely low acid (ELA) catalysis in liquid hot water (LHW) hydrothermal treatment (HTT) was assessed using BSG with dry matter contents of 15% and 25%, achieved by dewatering using a screw press. Batch experiments at low acid loadings of 5, 12.5 and 20 mg/g dry mass and temperatures of 120, 150 and 170 °C significantly affected XOS yield at both levels of dry mass considered. Maximum XOS yields of 76.4% (16.6 g/l) and 65.5% (31.7 g/l) were achieved from raw BSG and screw pressed BSG respectively, both at 170 °C and using 5 mg acid/g dry mass, after 15 min and 5 min, respectively. These XOS yields were obtained with BSG containing up to 63% less water and temperatures more than 20 °C lower than that reported previously. The finding confirms that ELA dosing in LHW HTT allows lowering of the required temperature that can result in a reduction of degradation products, which is especially relevant under high solid conditions. This substantial XOS production intensification through higher solid loadings in HTT not only achieved high product yield, but also provided benefits such as increased product concentrations and decreased process heat requirements.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Breweries produce around 40 million tonnes of brewers’ spent grains (BSG) annually, which constitutes up to 85% of the solid waste produced by a brewery [1]. Due to its high moisture content (about 70 to 85% water) and bioactive organic matter, BSG waste is highly susceptible to decomposing [2, 3], and is either used for animal feed or consigned to landfill [4, 5]. However, stricter regulations on animal feed and a drive to improve resource efficiency have compelled breweries to find alternative applications for BSG [4, 6].
Various alternative applications of BSG have been reported, including conversion to biogas, bio-oil, carboxylic acid, levulinic acid, biobutanol and xylitol [7]. However, BSG from brewing is a food-grade lignocellulosic byproduct that has potential for high-value food product applications. BSG contains a wide range of health-promoting compounds, nutraceuticals and phytochemicals, which can be extracted and reapplied in food and beverage formulations [8,9,10]. The relatively large (>25%) hemicellulose fraction, mainly xylan and arabinan, makes BSG well suited as a raw material for producing health-promoting dietary fibres or prebiotics, in particular xylo-oligosaccharides (XOS) [11,12,13]. The degree of polymerisation (DP) of XOS is reported to be an important factor affecting the biological properties thereof, with short-chain XOS (DP < 10) having the greater bifidogenic or prebiotic effect [14]. Furthermore, short-chain XOS substituted with arabinose, known as arabino-xylo-oligosaccharides (AXOS), in particular, have been found to be highly fermentable in vitro by beneficial Bifidobacterium of the human gut [11, 12]. These XOS dietary fibres are found in functional food products as low-calorie sweeteners, antioxidants, prebiotics and texturisers, and are also marketed as supplements.
Efficient fractionation of XOS from BSG requires selective solubilisation of the hemicellulose-xylan component, which can be achieved with hydrothermal treatment (HTT) technologies, which have been applied to various lignocellulosic biomasses, including BSG [15, 16]. Current processes for oligosaccharide production from BSG hemicellulose rely on autocatalytic liquid hot water (LHW) HTT, pressurised water at elevated temperatures (120 to 250 °C), which achieves solubilisation and partial depolymerisation of xylan by hydrolysis i.e. the catalytic action of water [17]. The rate of solubilisation and depolymerisation of xylan is proportional to H+ concentration and which is increased by acetic acid released by hydrolysis of acetyl groups in xylan structures, thereby creating the autocatalytic effect [18, 19]. Autocatalytic LHW HTT of BSG reported maximum XOS yields ranging between 61 and 77% at around 195 to 200 °C, yet to produce more of the preferred arabinose-substituted XOS (AXOS) temperatures below 180 °C are required [11, 13, 20]. However, rates of solubilisation and depolymerisation are lowered at such reduced temperatures (<180 °C), resulting in lowered (<70%) XOS yields [11].
Additionally, the LHW HTT technologies reported for XOS production from BSG used low solid concentration < 11%, which translates into higher energy requirement per mass of biomass processed [15, 21, 22]. Using higher solid loading (>15% dm) generally causes viscosity-related processing issues in conventional, stirred vessels [23]. The limitations in heat and mass transfer associated with the high solid loading typically have a negative effect on desired product yields and/or qualities [21, 24,25,26]. These factors combined hinders the lignocellulosic HTT by producing more degradation products such as furfural, 5-HMF and lignin fragments [27]. However, higher solid loading can result in higher product concentrations, reduced energy requirements and reduced process equipment size [15, 21, 22]. Additionally, high product yields can be obtained using higher solid loadings in LHW HTT if the negative effects of high solid loading can be mitigated [28, 29]. It is anticipated that the use of ELA dosing in LHW HTT can perhaps achieve this. The ELA dosing allows for high solid processes with increased product yields and concentrations, and reduced water usage.
For most lignocelluloses, supplementing the autocatalytic LHW HTT with extremely low acid (ELA) concentrations i.e. <0.7 wt% H2SO4 could increase rates and improve product yields at lower temperatures [16, 30,31,32]. Liquid hot water HTT with ELA is preferable since (i) the lower temperatures may achieve similar product yields as autocatalysed LHW HTT at higher temperatures, and (ii) may reduce the extent of lignocellulose degradation into byproducts [16, 30]. Therefore, the ELA catalysis of LHW HTT may enable the XOS production at higher solid loadings with acceptable yields, by lowering degradation byproduct formation, mitigating the inherent negative effect accompanying the high solid loading. Combining high solids and ELA HTT technology may have environmental benefits such as reduced energy usage and waste production, to balance the environmental consequences which may be associated with minimal amount of catalyst used (<0.7 wt%) [16, 30]. Yet no such ELA-dosed and high solid LHW studies on XOS production of BSG are reported.
Mechanical dewatering is a preferred alternative to thermal dehydration, to reduce process costs [33]. The mechanical dewatering of BSG with a screw press could reduce the water content to a minimum of about 55% [33,34,35]. Screw pressing of biomass is also known to provide a degree of mechanical defibrillation and shortening of the fibres, which may facilitate the HTT [36]. On the other hand, the liquid fraction separated from the BSG by screw press also contains suspended solids that are high in protein (>50%) [35, 37], which creates a value-added co-product. Given the selective removal of protein from the BSG, it is anticipated that it would be advantageous for the HTT step of the BSG. The reduced protein content in the substrate can increase the rate of depolymerisation of hemicellulose by reducing buffering capacity during the HTT [38] and can result in increased oligomer and sugar yields by reduced polysaccharide-protein reaction [39]
It is anticipated that applying a mechanical dewatering of BSG through a screw press, to achieve high solid loadings for HTT, and combining this with ELA catalysis, may provide an opportunity to significantly improve the process for XOS production from BSG with some technical and economic advantage. Therefore, this study investigated the effect of higher solid loading achieved by mechanical dewatering of BSG through screw pressing on XOS production from BSG. Using two BSGs obtained, raw and pressed, the ELA catalysis of LHW HTT was optimised for XOS production in a stirred batch Parr reactor system to reduce acid use, required process temperatures and reduce the degradation product formation (acetic acid, formic acid, furfural and HMF).
Materials and Methods
Raw Material and Screw Press Drying
Fresh BSG was obtained as a 1 m3 batch from a local brewery (Newlands, Cape Town, South Africa); it consisted of a single brew from a Weiss recipe with a 50:50 ratio of malted barley and wheat. A continuous screw press (NEW Eco-tec Verfahrenstechnik GmbH, Mühldorf, Germany) with a 0.3 m long × 0.15 m diameter screen cage and driven by a 2.2-kW 3-phase motor was used to press 400 kg of BSG for dewatering. Samples of the raw BSG (BSG-R) and pressed BSG (BSG-SPD) were aliquoted in sealed vacuum bags and stored frozen at −20 °C. Samples were thawed in a 25 °C water bath before use.
Batch Processing Equipment
A Hastalloy C-276 model 4540 high pressure 1-L bench reactor from Parr Instruments Company (Moline, Illinois, USA) was used for acid catalysed LHW HTT of BSG-R and BSG-SPD. Mixing inside the reactor was controlled with a variable speed motor driving through a magnetic coupling to an impeller with twin six-blade Rushton-type impellers. An external electric band heater was controlled by a proportional-integral-derivative (PID) controller, model 4842 (Parr). An operational procedure described by elsewhere [40, 41] was used, with slight modifications for wet BSG without thermal drying and the acid dosing. Direct acid dosing of the wet BSG was applied in the reactor, instead of soaking dry material, as was suggested earlier [15]. The BSG was loaded into the reactor during mixing and acid was dosed directly, to obtain the required acid loading of between 5 and 20 mg/g dry mass loaded. Choice of H2SO4 acid was supported by simple cost evaluation (Supplementary Table S1). The agitation rate during reactions was adjusted to 40 rpm.
Pre-factorial Screening Experiments
Firstly, the screening runs were conducted to define an acceptable range of process conditions for the ELA-catalysed LHW HTT conducted on the BSG-R (15.3% dm) and BSG-SPD (25% dm). The autocatalysed LHW HTT runs in the screening experiments provided a benchmark for the ELA-catalysed LHW HTT results obtained in this study. Preferred autocatalytic HTT conditions reported for BSG hemicellulose, at 180 °C with no acid loading for maximum XOS [11], and at 120 °C with 100 mg H2SO4/g dry mass loaded for maximum xylose yield [42], were applied (Table 1). Rather than the low solid loadings of less than 9–11% dry matter reported before, these runs were repeated with the BSG-R (15.3% dm) and BSG-SPD (25% dm) higher solid loadings. An additional two concentrations of acid dosage, i.e. 12.5 and 46 mg acid/g dry mass loaded, were also tested at 150 °C and 120 °C. HTT process performances were measured in terms of BSG hemicellulose solubilisation and depolymerisation, and the resulting yields of XOS, xylose and degradation products. The results were used to establish the highest level of acid loading that could be used to obtain mostly XOS for the range of conditions of the subsequent ELA HTT process optimisation through a factorial experimental design.
ELA Factorial Experimental Design
A full factorial design with three centre points was conducted separately for each of the two different BSG feedstocks i.e. BSG-R and BSG-SPD. For each feedstock, 11 batch runs (A1 to A11 and B1 to B11) were performed to evaluate the output variables, while independent variables, namely acid loadings, temperature and residence time, were varied to fit a 23 factorial design (Table 2). Ranges for the values for the variables were selected from literature and from the results of the pre-factorial screening experiments as described in the previous section.
The factorial experimental design runs, A1 to A11 and B1 to B11 for BSG-R and BSG-SPD respectively, were done independently. In the experimental design, the high, mid and low points were coded as −1, 0 and 1 respectively (Table 2). The centre points were done in triplicate to estimate significance of curvature and the experimental reproducibility or error.
A multiple regression analysis was carried out using STATISTICA 13.0 (StatSoft, Inc. Tulsa, USA) to establish the coefficients in Eq. 1 to describe the relationship between the independent variables for four output variables or responses: XOS yield, xylan equivalent yield inhibitors yield (g/100 g dm) and total dissolved solid (TDS) yield. Models were constructed to fit the data according to the following equation for Y, the predicted response for the independent variable:
The coefficients aij are adjustable constants optimised for the model fit and the statistical significance of each was determined (p < 0.05). Results were assessed with analysis of variance (ANOVA) and the degree of fit (R2) to the models was estimated.
Additionally, a combined severity function (CSF) was used to aid in the comparison of HTT results in this study with that from literature and between the two BSG feedstocks tested. The autocatalytic HTT severity function for combining contribution of time (t) and temperature (Tr) during HTT was used in the adapted form for the incorporation of the acid catalyst applied with CSF = log R0 – pH, where R0 = t .exp((Tr − 100)/14.75) [43]. Apart from using the resulting pH in the CSF calculation, the resulting pH, converted to mol H+ per gram dry BSG, was also used to compare hydrolysate acidification in the HTT. The resulting ratio, R[H+], of mol H+ per gram dry BSG-SPD relative to mol H+ per gram dry BSG-R, was used for comparison of the HTT results between the two ELA-catalysed LHW HTT factorial runs on the BSG-R and BSG-SPD, as well as the autocatalytic LHW HTT runs from the pre-factorial screening.
Analytical Methods
Standard laboratory analytical procedures (LAPs) of the National Renewable Energy Laboratory (NREL, USA) were used for biomass compositional analysis [44]. A starch kit from Megazyme (K-TSTA, Ireland) was used to determine total and residual starch after ethanol wash of the BSG samples [45]. Amino acids were determined using a Waters Acquity (Milford, USA) ultra performance liquid chromatograph (UPLC) separation with ultraviolet (UV) or fluorescence detection after derivatisation with 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate (AQC). The crude protein content of samples, based on the nitrogen content of protein, was determined using Kjeldahl analysis (DK8 Velp Scientifica, Usmate, Italy), using a factor of N × 6.25 [46]. All samples were analysed, at least, in triplicate.
The concentrations of short-chain oligomers (xylotriose, xylobiose), sugars (glucose, xylose and arabinose) and degradation products (acetic and formic acid, hydroxymethylfurfural (HMF) and furfural) were analysed by high-performance liquid chromatography (HPLC) on a Aminex HPX-87H Ion Exclusion Column equipped with a Cation-H cartridge (Biorad, Johannesburg, South Arica) [47]. Samples from the liquid fraction after HTT were filtered through a 0.2-μm filter before analysis.
To quantify oligosaccharides (glucooligosacharides [GlcOS], XOS and ArOS) in the hydrolysate, liquid samples were subjected to posthydrolysis using 4% wt H2SO4 at 121 °C for 40 min [44]. Oligosaccharides, which included soluble polymers and oligomers, were defined as the difference in the monomeric sugar concentration before and after posthydrolysis of the filtered hydrolysate [11,12,13].
The oligosaccharide (XOS and ArOS) yields were defined as the mass fractions (%) of the initial xylan and arabinan in the dry mass feedstock that was recovered in the hydrolysate liquid after treatment [11,12,13]. The total polysaccharide equivalent (TXeR) weight recovered was calculated by the total mass equivalent of reducing sugars and oligosaccharide recovered in the liquid from the starting polysaccharide after a treatment. The oligosaccharide yield relative to reducing sugars yield (ArOS% and XOS%) was calculated as the mass of oligosaccharide recovered, relative to total equivalent weight polysaccharide recovered.
Results and Discussion
Firstly, reported optimised process conditions for XOS production in autocatalytic LHW HTT (9/11% dm) were applied in the batch Parr reactor system with the BSG feedstocks (15/25% dm) and used as a benchmark for the ELA-catalysed LHW HTT. The BSG hemicellulose solubilisation products XOS, ArOS (Arabinan in XOS), xylobiose, xylotriose and xylose yields and the degradation products were compared under these conditions. A combined severity factor (CSF) was used to evaluate results from the autocatalytic HTT and ELA catalyst HTT treatments on the BSG-R and BSG-SPD.
Screw Press Dewatering of BSG
The dry matter content of the BSG-R (15.3%) was increased by means of the mechanical dewatering using a screw press, resulting in the BSG-SPD with dry matter content of 25%. This was achieved at a throughput rate of 794 kg BSG-R per hour. However, the screw press treatment in this study was relatively moderate, since the maximum of 25% dry mass (dm) content obtained in the present study (Fig. 1) was below the 35 to 40% dm reported [48, 49]. However, the energy requirements reported (40 to 53 kWh/t) for achieving that high solid content [48] is nearly 20 times higher than that used in this study per tonne of wet BSG (2.8 kWh/t = 2.2 kW/0.794 t/h). This is a fraction of the 270 to 320 kWh/t estimated energy requirements for drying by steam to achieve the same moisture reduction [33]. A mass balance revealed that 38.6% of the water fraction from BSG-R and 71.3% of the dry mass was recovered in the BSG-SPD, while the remainder was removed in the press liquid stream (Fig. 1). A considerable reduction in the dissolved solid (DS) fraction of total solids (dry mass) was achieved as a result of the water removal. The dissolved solid fraction in BSG-R reduced from 10.8% (1.6/15.3) to 5.8% (1.5/25) in the BSG-SPD (Fig. 1).
A benefit of screw press dewatering is the selective removal of components such as starch and protein from the BSG-R into the separated press liquid fraction [35, 37, 50]. The screw press dewatering process resulted in the removal of soluble components and suspended materials in the liquid press fraction. The total starch content was reduced from 12.9 to 9.2%, and the crude protein content also decreased from 24.3 to 21.8% (Table 3). Consequently, this translated into 49.1% and 36.0% selective removal of starch and protein respectively, from the BSG-R into the press liquid fraction. These findings were consistent with the selective removal of fine BSG particles (<150 μm) with mechanical screw press dewatering that are reported to be high in starch and protein [35, 37, 50]. Due to the selective removal of proteins and starch by the screw press dewatering process, the BSG contents of cellulose and hemicellulose increased from 10.4% and 18.9%, respectively, in the BSG-R, to 11.8% and 21.8%, respectively, in the BSG-SPD. The increase in fibre fraction and reduction in the interfering components (starch, proteins) of the BSG-SPD may provide a benefit to the subsequent HTT, to counteract the anticipated limitations that may occur as a result of the increased dry matter content. The compositional results of both BSG-R and BSG-SPD (Table 3) are consistent with other compositional values for BSGs reported elsewhere [8], for hemicellulose (19.2 to 41.9%), cellulose (0.3 to 33%), starch (1 to 12%), protein (14.2 to 31%), lignin (11.5 to 27.8%), lipids (3 to 13%) and ash (1.1 to 13%).
Glucooligosacharides of BSG (including soluble starch, maltodextrins and cellubiose) made up the largest fraction (39.6%) of the dissolved solids in the BSG liquid fraction (Table 4). The starch content of the liquid fraction accounted for 30.5% of the total starch in the BSG-R, which was reduced to 20.0% for the BSG-SPD owing to the screw press dewatering. Similarly, the soluble nitrogen content of the liquid fraction of the BSG reduced from 14.8% in the BSG-R to 9.1% in BSG-SPD. The pressed liquid from the BSG-SPD contained suspended solid products of which proteins and starch constituted the largest fractions [35, 37, 50]. Interestingly, the amino acid analysis of the insoluble solid showed a slightly higher content than the crude protein analysis, probably due to the under reporting of the assumed nitrogen conversion factor of 6.25 for this fraction (Table 4).
Pre-factorial Screening Experimental Results
The pre-factorial screening performed showed that high solid loadings in LHW HTT of BSG could lead to higher XOS and xylose product yields and the ELA addition in LHW HTT can result in a reduction in degradation product formation and thereby improving XOS production. Additionally, from the results, a preferred range of process conditions was selected for the subsequent full factorial optimisation of the ELA-catalysed LHW HTT for XOS production. Figure 2 provides the product yields and the degradation product composition of the hydrolysate under the screening conditions for the BSG-R and the BSG-SPD.
Screening results from (A) BSG-R and (B) BSG-SPD: (1) sugar product yields (g/100 g dm) and (2) degradation products/inhibitor yield (g/100 g dm); [grey square] 180 °C 15 min no acid, [red square] 180 °C 5 min no acid, [yellow square] 150 °C 10 min 12.5 mg acid/g dm, [green square] 120 °C 15 min 46 mg acid/g dm, [blue square ] 120 °C 15 min 100 mg acid/g dm (XOS, xylo-oligosaccharide; ArOS, arabino-oligosaccharide; HMF, 5-hydroxymethyl furfural)
Increased Solid Loadings of BSG in HTT Lead to Higher Product Yields
High solid loadings can lead to higher product yields compared to dilute LHW HTT. Results from screening experiments (Fig. 2) showing the highest XOS yields of up to 78.0% (run AH-A3 using 180 °C and 15 min) were obtained with the autocatalytic (no acid added) LHW HTT using solid loadings, of 15 % dry matter content, significantly higher compared to using dilute 9–11% dry matter content in reported LHW HTT optimisation studies with maximum XOS yields of 61% (190 °C and 5 min). Additionally, results showing increasing solid loadings in LHW HTT from 15 to 25% dm using the same process conditions (180 °C and 5 min) maintained similar XOS yields, of 65.0% (run AH-A2) and 65.3% (run AH-B2) when using 15% dry matter content with BSG-R and 25% dm BSG-SPD respectively (Fig. (2A.1), (B.1)). Moreover, xylose yields obtained with the dilute acid (100 mg H2SO4/g dm) HTT of BSG at the optimal condition reported also increased significantly from 57.3% (run DA-A) to 71.8% (run DA-B) with increasing solid loadings from using BSG-R and BSG-SPD. These similar and higher yields were obtained with even higher xylan content in the BSG-SPD hemicellulose as a result of the screw press dewatering (Table 2), which implies increased solubilisation rates.
The results of higher solid loading leading to higher yields is in agreement with reported LHW HTT treatment of bagasse that showed a positive relationship of XOS yield and dry matter content at certain process conditions [28].Therefore, in LHW HTT optimisations, the solid loading (or dry matter content) is an important variable to consider in addition to temperature and time. The results in this study from the increased solid loadings (15 to 25% dm) used in XOS production by HTT of BSG represent a significant HTT technology improvement, since these results were obtained using up to 60% less water. If the negative effects from higher solid loadings can be mitigated, increased dry matter content can lead to a significant HTT process intensification with higher product concentrations and lower water requirements that can result in reduced heat requirements. The ELA dosing is considered as a possible further improvement in LHW HTT in the factorial runs.
ELA Dosing in LHW HTT of BSG for a Reduction of Degradation Products
As can be seen in formation of degradation products, with the Fig. 2(A.2), 2(B.2), runs with BSG-SPD, with the highest solid loading, overall produced more degradation products compared with BSG-R. While both BSGs, at lower temperatures (<150 °C), combined with using higher acid dosing (from 46 mg H2SO4/g dm), resulted in more acetic acid formation, while the higher temperatures (>150 °C) favoured formic acid and furfural. Higher acid loadings (from 46 mg H2SO4/g dm), for both BSGs, accelerated depolymerisation of XOS to the monomeric xylose sugar which for BSG-SPD, in run DA-B, with the highest acid loading (100 mg H2SO4/g dm) resulted in the highest amounts of xylose (11.9 g/100 g BSG) and lowest yield of XOS (0.98 g/100 g BSG). On the other hand, with no acid, the autocatalytic LHW HTT of BSG-SPD at 180 °C (run AH-B2) resulted in highest XOS yield (9.48 g/100 g BSG equivalent to 65.3%); however, the associated degradation products formed were significant (1.49 g/100 g BSG), partly due to the elevated temperatures (>180 °C). These degradation products may present a challenge during XOS product purification. However, the screening results (run ELA-A) show that the ELA-catalysed LHW HTT (12.5 mg H2SO4/g dm at 150 °C for BSG-R) allows for high XOS yields (61.5%) to be obtained with reduced degradation (0.36 g/100 g BSG) products, and at lower temperatures (<180 °C). Therefore, the final range of ELA LHW HTT conditions considered for the factorial experimental design were (i) for temperatures, between 130 and 170 °C; (ii) ELA acid loadings, 5 to 20 mg H2SO4/g dry BSG loaded; and (iii) time, 5 to 15 min (Table 2). These factorial experiments conducted were 11 batch runs each, on the BSG-R (run A-1 to A-11) and BSG-SPD (run B-1 to B-11) samples, with 15% and 25% dm content respectively.
Full Factorial Optimisation of ELA LHW HTT with BSG-R and BSG-SPD for XOS Production
Extremely low acid dosing of H2SO4 in autocatalytic LHW HTT enabled improved hemicellulose solubilisation and hemicellulose product recovery at lower temperatures. Results from the ELA HTT factorial runs A1-11 and B1-11 (Supplementary S2 and S3) were analysed statistically and models were created to determine the significance of the investigated parameters and their optimal ranges for each output variable (XOS yield, degradation product formation, XOS concentration and TDS solids) using STATISTICA 13.0 (Supplementary Tables S4, S5 and S6). An ANOVA on the results from the factorial runs revealed that the effect of acid loading and interactions were significant for XOS yield with a p value below 0.05 for both BSG-R and BSG-SPD (Supplementary Fig. S4A and B). Temperature showed the most significant effect for XOS yield from the BSG-R (Supplementary Fig. S4A), while the interaction of acid loading with temperature was the most significant for BSG-SPD.
ELA Dosing in LHW HTT of BSG for Improved for XOS Yields
ELA dosing in LHW HTT of BSG enables high XOS yields (>70%) at high solid loadings (>15% dm). The ELA catalysis of LHW HTT significantly improved the HTT process through increased hemicellulose solubilisation and hemicellulose product recovery at lowered process temperatures compared to the autocatalysed LHW HTT for both BSG-R and BSG-SPD (Fig. 3a, b). The process intensification of ELA addition in LHW HTT of BSG at high solid loading (dry matter concentration >15% dm) through the screw press dewatering process resulted similar or even higher XOS yields compared to reported autocatalytic LHW HTT optimisations using low 9–11% dry matter content [11, 13, 20]. The ELA-catalysed HTT of BSG with high solid loadings in this study obtained maximum XOS yields of 76.4% (run A-4), and 65.5% (run B-3) at 170 °C for the BSG-R (Fig. 3a) and BSG-SPD (Fig. 3b) respectively. The XOS yields obtained with ELA-catalysed LHW HTT are impressive compared to the 61% (190 °C) and 77% (200 °C) reported for optimised XOS production from BSG (autocatalytic LHW HTT) with lower solid loadings of 9–11% dm and even at 30 °C higher temperatures [11,12,13, 20, 51]. The ELA catalysis when applied with high solid loadings in LHW HTT significantly improved the hydrothermal process for the fractionation of BSG hemicellulose, XOS and xylose by reducing degradation product formation at lowered process temperatures (up to 30 °C less) using less water (>50% reduction).
ELA Dosing in LHW HTT of BSG for the Reduction in Degradation Product Formation
The undesired increased production of degradation products commonly accompanying in with increased solid loading as seen in the autocatalysed LHW HTT can be reduced by the ELA-catalysed LHW HTT process. Degradation products formed at the conditions for maximum XOS yield from the BSG-SPD (run B-3) was 1.14 g/100 g dm, which was higher than the 0.71 g/100 g dm produced from the BSG-R at the same conditions (run A-3) as shown in Table 5. Nevertheless, these degradation product amounts obtained with ELA dosing in LHW HTT are still significantly lower than (1.49 g/100 g dm) that produced when using no acid at 180 °C and 5 min (AH-B2). Therefore, the ELA HTT results show a substantial process intensification of the HTT technology in hemicellulose fractionation for XOS production from BSG since similar or higher yields were obtained using at least 20 °C lower temperatures, at least 20% lower degradation product formation and up to 60% less water. The increased effect of the ELA dosing and its interactions of the screw press dewatered BSG-SPD can be seen in the statistical analysis of the effects.
The Effect of Screw Press Dewatering on Acidification in HTT
The resulting hydrolysate pH from the factorial and autocatalytic pre-screening runs was assessed to investigate the effect of screw pressing dewatering on HTT acidification. In HTT, the rate of solubilisation and depolymerisation of xylan and glucan polysaccharides is related proportionally to H+ concentration in the hydrolysate [18, 19]. Therefore, final hydrolysate H+ concentrations were determined and compared to establish possible effects of changes in BSG composition, water content and buffering capacity by the screw press dewatering on acidification in HTT (mol H+ per gram dry BSG).
Apparent Acidification in Autocatalytic LHW HTT Proportional to Water Reduction
In autocatalytic HTT, the screw press dewatering of BSG can be consistent with a moisture reduction step. Acidification results from autocatalytic LHW HTT shown in Fig. 4 for raw BSG-R (5.5 g water/g dm) and screw pressed BSG-SPD (3.0 g water/g dm) resulted in comparable apparent acidification (mol H+ produced per gram BSG dry) for each temperature 150 °C and 180 °C. The resulting pH and H+ concentrations obtained are equivalent to a constant HTT acidification, mol H+ produced per gram BSG dry, for both 15% and 25% dm BSG in autocatalytic LHW HTT, at temperature 150 °C (ca 2.49 × 10−7 mol H+/g dry BSG) and 180 °C (ca 8.35 × 10−7 mol H+/g dry BSG). Additional acidifying effects, as a result of the screw press dewatering of BSG-R, resulted in <20% increase in mol H+ released per gram of BSG in autocatalytic LHW HTT in the range of process conditions investigated (Table 6). The resulting ratio, R[H+], of mol H+ per gram BSG-SPD, relative to BSG-R, in autocatalytic HTT was 1.16 (±0.18) and 1.09 (±0.06) for 150 °C 10 min (run AH-A1/B1) and 180 °C 5 min (run AH-A2/B2) respectively (Table 6). Therefore, during autocatalytic LHW HTT in the Parr, the fractionation of selective compounds, including ash, starch and protein, with the screw press dewatering of BSG-R resulting in BSG-SPD did not significantly change the H+ per gram dry BSG released. In autocatalytic LHW HTT, compared to screw press dewatering, temperature is a more significant factor in the BSG hydrolysate acidification. Results show the moisture reduction of 47% between raw (BSG-R) to screw pressed BSG (BSG-SPD) produced only ca 100% increased H+ concentration in LHW HTT; however, a 9% increased temperature (150 to 180 °C) resulted in a significantly increased, > 230% in H+ concentration (Fig. 4). Observed differences in the H+ concentrations were proportional to the differences in the water content. However, for ELA-dosed LHW HTT, the screw press dewatering had a much greater increased acidification.
Screw Press Dewatering Improved Acidification in ELA-Dosed LHW HTT
The screw press dewatering of BSG resulted in significant additional acidifying effects in ELA-catalysed LHW HTT. Additional acidifying effects in ELA-dosed LHW HTT, as a result of the screw press dewatering of BSG-R, increased up to 400% the mol H+ released per dry gram of BSG in BSG-SPD with ELA dosing of only 5 mg H2SO4/g dm at 170 °C for 5 min (Fig. 4). The resulting ratio, R[H+], or mol H+ per gram BSG-SPD relative to BSG-R, after the ELA HTT treatment over the range of process conditions investigated was found between 1.08 and 4.01 (Table 6). Results from ANOVA of effects on R[H+] show indeed the effect of temperature and interactions were significant, with a p value below 0.05 (Fig. 5a). Both the treatment time and its interaction with temperature were found just about significant (Fig. 5a). As shown in Fig. 5b, the increased treatment time in ELA-dosed LHW HTT, especially at high temperatures (170 °C), lead to a decreased acidifying effect with screw pressing; while at lower temperatures (130 °C), the opposite effect was found. The increased acidification in ELA-dosed LHW HTT of BSG-SPD as a result of screw press dewatering lead to increased H+ concentration (up to ca 640%) in the resulting hydrolysates, significantly more than expected by ca 47% moisture reduction from the screw press dewatering of BSG from 15 to 25% dm (Fig. 4). The increased H+ generated with BSG-SPD in ELA-dosed LHW HTT can be a combined effect of reduced water content, reduced buffering capacity and compositional changes caused by screw press dewatering. The increased effect of ELA acid dosing on XOS yield from BSG as a result of the screw press dewatering is confirmed by significant curvature found in statistical analysis of XOS yields from BSG-SPD (Supplementary Fig. S1). An additional quadratic term introduced for acid loading could account for the increased effect of acid loading (Supplementary Fig. S4B).
As shown above, especially with ELA dosing, screw press dewatering can improve the hydrolysate acidification, yet the solid BSG contains a large part of the buffering capacity of BSG that can neutralise HTT acidification. The resulting H+ concentration showed a negative relationship with ELA HTT treatment time as the hydrolysate pH increased for both BSG raw and screw pressed (Table 6) at temperatures of 130 °C in ELA HTT at 5 min (run A5/B-5 with 20 mg H2SO4/g BSG) the pH obtained (pH 2.40/1.84) increase (pH 2.89/1.95) at 15 min.
Screw Press Dewatering and Chemical Compositions in HTT
The changes in chemical composition between BSG-R and BSG-SPD with screw press dewatering did not affect the main HTT mechanisms taking place in solubilisation and depolymerisation of BSG hemicellulose (Fig. 6). No significant difference in monomeric sugars formation relative to oligomers for GlcOS and XOS can be seen when comparing products in hydrolysates from BSG-R and BSG-SPD after ELA-catalysed HTT (Fig. 6). ELA loadings and screw press dewatering had a significant effect on the extent of depolymerisation of the glucan and xylan in the HTT, while the depolymerisation follows proportionally for both glucan and xylan. However, contribution of the screw press dewatering effect to the changes in H+ concentration is less when compared to the ELA loading. Under the same process conditions, the relative monomeric and oligomeric yields were identical for autocatalytic runs for BSG-R and BSG-SPD. However, with equal H2SO4 ELA dosing to both BSG-R and BSG-SPD, the interaction between acid loading and temperature resulted in an increased extent of polysaccharide depolymerisation to monomeric sugars xylose and glucose with the BSG-SPD.
Comparison of HTT yields of oligomeric and reducing sugars: autocatalytic [  ] BSG-R 15% dm and [light blue diamond] BSG-SPD 25% dm and ELA for BSG-R [
] BSG-R 15% dm and [light blue diamond] BSG-SPD 25% dm and ELA for BSG-R [  ] 5 mg and [blue circle] 20 mg acid/g dm and ELA for BSG-SPD [
] 5 mg and [blue circle] 20 mg acid/g dm and ELA for BSG-SPD [  ] 5 mg and [red triangle] 20 mg acid/g dm (enlargement insert for autocatalytic HTT range)
] 5 mg and [red triangle] 20 mg acid/g dm (enlargement insert for autocatalytic HTT range)
Extending Solubilisation and Depolymerisation by Addition of CO2
As a “Green” alternative catalyst, CO2 used with ELA HTT showed increased effective depolymerisation and reduction of XOS to xylose monomeric sugar (Fig. 6). The preliminary test, using BSG-SPD at centre runs of 150 °C and 10 min with additional 20 Bar CO2 (run B-10C), showed that added CO2 resulted in a reduced pH in the hydrolysate, from pH 2.67 (with no CO2) to pH 2.61. The CO2 effectively increased the H+ concentration in the hydrolysate by 15%. The XOS fraction in the xylan recovered decreased from 90.1 to 76.2%, while the total xylan recovered yield reduced only marginally from 63.2 to 61.2% (Table 5). The preliminary test run B-10C had a moderate increase in the degradation products formed from 0.92 to 1.44 g/100 g dm, while the CSF increased from 0.09 to 0.15 compared to run B-10 without CO2 (Table 5). Although literature reports the use of CO2 addition in autocatalytic HTT to aid the autocatalytic HTT process [52,53,54], this novelty shows that CO2 can manipulate the severity in combination with H2SO4 at moderate pressures. Even lower CO2 pressures can be investigated for control on hemicellulose solubilisation and XOS yield.
Xylobiose and Xylotriose Yield in XOS
The distribution of degree of polymerisation of the XOS produced in ELA HTT process varies with the ELA HTT process conditions. The short-chain xylan oligomers, xylobiose (X2) and xylotriose (X3) are of special interest for their prebiotic effect. They can be produced [11, 55], and maximised in the XOS fraction. This would minimise or avoid the need for a subsequent post HTT step for the production of the X2 and X3. With ELA-catalysed HTT, the highest combined fractions of X2 and X3 in the XOS were 19.6% and 23.0 for BSG-R and BSG-SPD respectively. Both were obtained at 170 °C with 20 mg H2SO4/g dm acid loading (Fig. 7). Both of these results are higher than the 16.7% reported elsewhere [13], obtained at 190 °C through autocatalytic HTT at their process conditions for maximum XOS yield. For both BSG-R and BSG-SPD, the effect of a rising combined severity factor (CSF) showed increased yield of X2 and X3 in the XOS product (Fig. 7). Therefore, adjusting the CSF with ELA loading can increase the X2 and X3 fractions in XOS, in a single-step process. For BSG-R, the XOS produced at the highest XOS yield (run A-4) contained 11.2% of X2 and X3 combined. However, an increase in acid loadings from 5 to 20 mg at the same process conditions (run A-7) led to a near doubling of the yield (19.6%). Similarly, using BSG-SPD (run B-7), the combined X2 and X3 yields in XOS increased from 10.8 to 23.0%, but at a much lower XOS yield and total xylan equivalent yield.
The preliminary HTT screening of BSG-R and BSG-SPD with more than 40 mg H2SO4 acid loadings increased the combined X2 and X3 yields to more than 30% of the XOS fraction, while the total xylan equivalent yield was above 80% (run A-Z and B-Z in Fig. 7). Incorporating a CO2 catalyst significantly increased the X2 and X3 yields in the XOS produced. In run B-C at 150 °C with BSG-SPD, the addition of 20 Bar CO2 (run B-10C) resulted in a near doubling of the X2 and X3 yields in XOS from 7.2 to 15.6%, while the resulting total xylan equivalent yield was maintained (Fig. 7). Thus, the further use of ELA acid dosage with CO2 could be investigated as a tool for XOS production to optimise short-chain oligomers including X2 and X3. The use of CO2 instead of acids in XOS production is a very interesting idea to experiment.
The Effect of Screw Press Dewatering on the Resulting Hydrolysate from HTT
BSG is heterogeneous and fractionation of BSG hemicellulose in HTT inevitably generates interactions between components that negatively affects the yields and purity of the XOS fraction. Hence, another important factor to improve on in HTT is the fraction of XOS and ArOS in the total dissolved solids (TDS) of the hydrolysate. Protein reduction in BSG for HTT is key since proteinaceous compounds make up the majority of the non-volatile components in the hydrolysate. Protein contributes to the buffering effect in HTT and can react with oligomers and sugars which can reduce their yields. The screw press dewatering of BSG-R had a significant effect on the composition of solubles in the hydrolysate.
XOS Concentration and Total Dissolved Solids from BSG-R and BSG-SPD
The 50% reduction in moisture content through dewatering BSG-R with a screw press had a significant positive effect on the XOS concentration obtained by ELA HTT and increased the purity of the XOS. A XOS concentration of 31.7 g/l was obtained form BSG-SPD with maximum XOS yield (run B-3), almost double the 16.6 g/l obtained from raw BSG-R. The highest xylose concentrations obtained for BSG-R was 7.2 g/l and 14.7 g/l for BSG-SPD both at 170 °C and 20 mg H2SO4 acid dosing for 15 min and 5 min respectively (Sypplementary Tables S1 and S2). Additionally, the hydrolysate of BSG-SPD (run B-4) contained a greater fraction (34.9%) of oligomeric products—XOS and ArOS—in the TDS of the hydrolysate, compared to the 25.1% (run A-4) obtained from raw BSG-R (Table 5). These increased XOS and ArOS concentrations in the TDS of the BSG-SPD hydrolysate were achieved through the increased fibre content by means of screw press dewatering. The screw press dewatering step selectively reduced the ash, GlcOS and proteinaceous compounds, as soluble and insoluble solids. Since it is reported that up to 80% of XOS production cost is due to purification cost, applying a screw press processing step before ELA HTT can decrease overall XOS production costs with the increased XOS concentration and fraction of XOS in TDS of resulting hydrolysate [56].
Protein from Screw Press Dewatering BSG-R
Separation of a high protein fraction with the screw press before HTT is more advantageous since it provides higher protein recovery and less solubilisation in the hydrolysate. Proteinaceous compounds in the BSG-PSD were significantly reduced by screw pressing prior to HTT and produced a valuable, protein-rich (43 wt% total amino acid content) insoluble solid fraction in the press liquid with potential for protein extraction as a co-product to XOS in a multiproduct biorefinery scenario (Supplementary Table S7). The insoluble solid fraction, recovered from the press liquid in this study, contained a lower protein content than previously reported values [35, 37]. This lower protein yield can be as a result of the light pressing applied. Despite the low protein yield in the pressed liquid, the reduction in protein content in BSG-SPD is reflected in the ELA HTT results as the other non-determined dissolved solubles (NDS), including the proteinaceous compounds in the hydrolysate, decreased from 13.2 g/l for BSG-R (run A-4), to 9.0 g/l for BSG-SPD (run B-3), both at their highest XOS yields (Supplementary Tables S1 and S2). These lower NDS values are in agreement with the crude protein recovery achieved using the screw pressed BSG in HTT. Only 45.4% of the crude protein remains in the insoluble solid after ELA HTT of BSG-R at the conditions for the highest XOS yield (run A-4), compared to the 70.9% protein recovered from BSG-SPD (run B-3), when including the crude protein in the screw press liquid (solid compositions given in Supplementary Fig. S7). On the other hand, the ELA HTT can be considered as an enrichment process since the amino acid profiles of the remaining solids showed, for both BSGs, that the basic amino acids were hydrolysed preferentially concentrating essential amino acids in the remaining solids (Supplementary Table S6). Therefore, the separation of a high protein fraction with the screw press together with the residual protein obtained from ELA HTT can be advantageous in a valorisation case for BSG.
CSF Effect on Xylan Recovery and XOS Yield
ELA dosing in LHW HTT increased accumulation of XOS in the recovered xylan equivalent (XOS%) compared to autocatalytic LHW HTT. ELA HTT resulted in more accumulation of XOS in the recovered xylan equivalent (XOS%) of 91.7% (BSG-R run A-4 at 170 °C) and 93.0% (BSG-SPD run B-3 at 170 °C), compared to reported autocatalytic LHW HTT results of 86.5% (190 °C and CSF = −0.62) as shown in Fig. 8a. The higher XOS% were obtained at higher CSF’s (−0.55 and −0.13) as a result of the ELA addition. This XOS accumulation in the hydrolysate from ELA HTT occurred due to the increased H+ concentration that led to an increased rate coefficient for xylan solubilisation to XOS, compensating for the negative effect of lower temperatures (170 °C compared to 190 °C). At the lower temperatures, XOS depolymerisation to xylose and furfural formation was also reduced [18, 31].
Comparison of ELA and autocatalytic HTT a XOS% yield (highest XOS yields of each are indicated) and b xylan recovery using CSF ([light blue triangle] 11% dm autocatalytic HTT [13], [orange diamond] 15% dm BSG-R ELA (this study), [red circle] 25% dm BSG-SPD ELA (this study), [  ] 28% dm rice straw ELA (Kapoor et al 2017)). The lines only show trends
] 28% dm rice straw ELA (Kapoor et al 2017)). The lines only show trends
ELA-dosed HTT of BSG-R (15% dm) resulted in a higher xylan equivalent yield of >85% compared to the reported autocatalytic HTT data (11% dm) for BSG (xylan equivalent yield of <66%), as shown in Fig. 8b, which is described as a direct result of the acid catalyst [31]. However, mass and heat transfer limitations inherent with high solid loadings from ELA HTT of BSG-SPD in the Parr system resulted in a drop in xylan equivalent yield at higher CSF (<70%) compared to the more dilute and homogeneous slurry of BSG-R. A comparison with a pilot-scale ELA HTT of rice straw conducted at >24% dry matter content in a high solid loading screw reactor [32], showing high xylan recovery (>78%), can be obtained at increased CSFs when using high solid loading processing equipment (Fig. 8b). This suggests that for BSG-SPD, higher xylan recovery and increased XOS yields can be targeted in similar screw type reactor vessels or steam explosion reactors that are more suitable for high solid loading HTT’s. This highlights the applicability of ELA dosing and the use of bench-scale BSG ELA HTT data for scale up applications [57].
Conclusions
Lack of efficient processing technologies of BSG hinders the large-scale valorisation of BSG. XOS produced from BSG have high-value applications in novel food, beverage and health product formulations. This study demonstrated a HTT process intensification for production of XOS from raw (15.3% dm) and screw pressed (25% dm) BSG using ELA dosing in LHW HTT. With the use of ELA dosing, the increased production of degradation products as a result of increased solid loading could be circumvented. Similar and even higher XOS yields (61.4–76.4%), compared to reported autocatalytic LHW HTT process (9–11% dm) yields (61% at 190 °C), were obtained even at lower temperature requirements (150–170 °C) together with a reduction in degradation products. The screw press dewatering process demonstrated effective moisture reduction in the BSG for process intensification in biorefining. Additional to the moisture content reduction, the screw press dewatering step also increased XOS purity in the hydrolysate from 25.3 to 34.1% by increasing the fibre fraction in the BSG. This increase was due to the selective separation of dissolved solids, ash, starch and protein. Screw press dewatering of BSG enhanced the autocatalytic LHW HTT by increasing H+ concentrations such that the hydrolysate pH were found to approximate the reduction in water; on the other hand, the ELA dosing further enhanced acidifying effects in ELA-dosed LHW HTT with up to 400% increased mol H+ released per gram of BSG in the range investigated. Additionally, as “Green Chemistry”, CO2 can be used at moderate pressure and temperatures to replace acid usage in XOS production. The process improvements demonstrated in the study is a significant process intensification in HTT technology that can support BSG valorisation concepts for breweries around the world.
Data Availability
The data supporting this research is provided in the supplementary material.
References
Mussatto, S. I., Dragone, G., & Roberto, I. C. (2006). Brewers’ spent grain: generation, characteristics and potential applications. Journal of Cereal Science, 43(1), 1–14. https://doi.org/10.1016/j.jcs.2005.06.001.
Robertson, J. A., IAnson, K. J. A., Brocklehurst, T. F., Faulds, C. B., & Waldron, K. W. (2010). Effect of storage conditions on the microbial ecology and biochemical stability of cell wall components in brewers spent grain. Journal of Agricultural and Food Chemistry, 58(12), 7266–7272. https://doi.org/10.1021/jf1001099.
Wang, B., Luo, Y., Myung, K. H., & Liu, J. X. (2014). Effects of storage duration and temperature on the chemical composition, microorganism density, and in vitro rumen fermentation of wet brewers grains. Asian-Australasian Journal of Animal Sciences, 27(6), 832–840. https://doi.org/10.5713/ajas.2013.13668.
Kerby, C., & Vriesekoop, F. (2017). An Overview of the utilisation of brewery by-products as generated by British craft breweries. Beverages, 3(24), 1–12. https://doi.org/10.3390/beverages3020024.
Rosa, M., & Beloborodko, A. (2015). A decision support method for development of industrial synergies : case studies of Latvian brewery and wood-processing industries. Journal of Cleaner Production, 105, 461–470. https://doi.org/10.1016/j.jclepro.2014.09.061.
Thomas, K. R., & Rahman, P. K. S. M. (2006). Brewery wastes . Strategies for sustainability. a review. Aspects of Applied Biology, 80, 147–153.
Skendi, A., Harasym, J., & Galanakis, C. M. (2018). 7 – Recovery of high added-value compounds from brewing and distillate processing by-products. Sustainable Recovery and Reutilization of Cereal Processing. https://doi.org/10.1016/B978-0-08-102162-0.00007-1.
Lynch, K. M., Steffen, E. J., & Arendt, E. K. (2016). Brewers’ spent grain: a review with an emphasis on food and health. Journal of the Institute of Brewing. https://doi.org/10.1002/jib.363.
Abu-ghannam, N., & Balboa, E. (2018). 9 – Biotechnological, food, and health care applications. Sustainable Recovery and Reutilization of Cereal Processing. https://doi.org/10.1016/B978-0-08-102162-0.00009-5.
Steiner, J. (2016). Brewer’s spent grain—Waste material as potential raw material for a healthy diet. In World Brewing Congress, Denver Colorado, USA, August 13-17, 2016 (pp. 2–3).
Gomez, B., Miguez, B., Veiga, A., Parajó, J. C., & Alonso, J. L. (2015). Production, Purification, and in vitro evaluation of the prebiotic potential of arabinoxylooligosaccharides from brewer’s spent grain. Journal of Agricultural and Food Chemistry, 63(38), 8429–8438. https://doi.org/10.1021/acs.jafc.5b03132.
Gullón, P., González-Muñoz, M. J., & Parajó, J. C. (2011). Manufacture and prebiotic potential of oligosaccharides derived from industrial solid wastes. Bioresource Technology, 102(10), 6112–6119. https://doi.org/10.1016/j.biortech.2011.02.059.
Carvalheiro, F., Esteves, M. P., Parajo, J. C., Pereira, H., & Girio, F. M. (2004). Production of oligosaccharides by autohydrolysis of brewery’s spent grain. Bioresource Technology, 91(1), 93–100. https://doi.org/10.1016/S0960-8524(03)00148-2.
Moura, P., Carvalheiro, F., Esteves, M. P., & Gírio, F. M. (2008). Prebiotic Xylo-oligosaccharides as high-value co-products on an integrated biorefinery approach from lignocellulosic feedstock. In International Conference and Exhibition on Bioenergy April 6th – 9th 2008 Universidade do Minho, Guimarães, Portugal.
Galbe, M., & Zacchi, G. (2012). Pretreatment: the key to efficient utilization of lignocellulosic materials. Biomass and Bioenergy, 46, 70–78. https://doi.org/10.1016/j.biombioe.2012.03.026.
Yang, B., & Wyman, C. E. (2008). Pretreatment: the key to unlocking low-cost cellulosic ethanol. Biofuels, Bioproducts and Biorefining, 2(1), 26–40. https://doi.org/10.1002/bbb.49.
Cardenas-toro, F. P., Alcazar-alay, S. C., Forster-carneiro, T., & Meireles, M. A. A. (2014). Obtaining Oligo- and monosaccharides from agroindustrial and agricultural residues using hydrothermal treatments. Food and Public Health, 4(3), 123–139. https://doi.org/10.5923/j.fph.20140403.08.
Mosier, N. S., Ladisch, C. M., & Ladisch, M. R. (2002). Characterization of acid catalytic domains for cellulose hydrolysis and glucose degradation. Biotechnology and Bioengineering, 79(6), 610–618. https://doi.org/10.1002/bit.10316.
Negahdar, L., Delidovich, I., & Palkovits, R. (2016). Aqueous-phase hydrolysis of cellulose and hemicelluloses over molecular acidic catalysts : Insights into the kinetics and reaction mechanism. Applied Catalysis B: Environmental, 184, 285–298.
López-Linares, J. C., García-Cubero, M. T., Lucas, S., González-Benito, G., & Coca, M. (2019). Microwave assisted hydrothermal as greener pretreatment of brewer’s spent grains for biobutanol production. Chemical Engineering Journal, 368(December 2018), 1045–1055. https://doi.org/10.1016/j.cej.2019.03.032.
Modenbach, A. A., & Nokes, S. E. (2012). The use of high-solids loadings in biomass pretreatment-a review. Biotechnology and Bioengineering, 109(6), 1430–1442. https://doi.org/10.1002/bit.24464.
Leibbrandt, N. H., Knoetze, J. H., & Görgens, J. F. (2011). Comparing biological and thermochemical processing of sugarcane bagasse: an energy balance perspective. Biomass and Bioenergy, 35(5), 2117–2126. https://doi.org/10.1016/j.biombioe.2011.02.017.
Yang, B., & Tucker, M. (2013). Laboratory pretreatment systems to understand biomass deconstruction. In C. E. Wyman (Ed.), Aqueous Pretreatment of Plant Biomass for Biological and Chemical Conversion to Fuels and Chemicals (pp. 489–521). John Wiley & Sons Inc.. https://doi.org/10.1002/9780470975831.ch23.
Sui, W., & Chen, H. (2015). Study on loading coefficient in steam explosion process of corn stalk. Bioresource Technology, 179, 534–542. https://doi.org/10.1016/j.biortech.2014.12.045.
Cullis, I. F., Saddler, J. N., & Mansfield, S. D. (2004). Effect of initial moisture content and chip size on the bioconversion efficiency of softwood lignocellulosics. Biotechnology and Bioengineering, 85(4), 413–421. https://doi.org/10.1002/bit.10905.
Brownell, H. H., Yu, E. K. C., & Saddler, J. N. (1986). Steam-explosion pretreatment of wood: effect of chip size, acid, moisture content and pressure drop. Biotechnology and Bioengineering, 28(6), 792–801. https://doi.org/10.1002/bit.260280604.
Jönsson, L. J., & Martín, C. (2016). Pretreatment of lignocellulose: formation of inhibitory by-products and strategies for minimizing their effects. Bioresource Technology, 199, 103–112. https://doi.org/10.1016/j.biortech.2015.10.009.
Vallejos, M. E., Zambon, M. D., Area, M. C., & da Silva Curvelo, A. A. (2012). Low liquid–solid ratio (LSR) hot water pretreatment of sugarcane bagasse. Green Chemistry, 14(7), 1982. https://doi.org/10.1039/c2gc35397k.
Swart, L. J., Bedzo, O. K. K., Van Rensburg, E., & Görgens, J. (2020). Pilot-scale xylooligosaccharide production through steam explosion of screw press – dried brewers ’ spent grains. Biomass Conversion and Biorefinery, 23–25.
Gurgel, L. V. A., Marabezi, K., Zanbom, M. D., & Curvelo, A. A. D. S. (2012). Dilute acid hydrolysis of sugar cane bagasse at high temperatures: a kinetic study of cellulose saccharification and glucose decomposition. Part I: Sulfuric acid as the catalyst. Industrial and Engineering Chemistry Research, 51(3), 1173–1185. https://doi.org/10.1021/ie2025739.
Shen, J., & Wyman, C. E. (2011). A novel mechanism and kinetic model to explain enhanced xylose yields from dilute sulfuric acid compared to hydrothermal pretreatment of corn stover. Bioresource Technology, 102(19), 9111–9120. https://doi.org/10.1016/j.biortech.2011.04.001.
Kapoor, M., Soam, S., Agrawal, R., Gupta, R. P., Tuli, D. K., & Kumar, R. (2017). Pilot scale dilute acid pretreatment of rice straw and fermentable sugar recovery at high solid loadings. Bioresource Technology, 224, 688–693. https://doi.org/10.1016/j.biortech.2016.11.032.
Huige, N. J. (1994). Handbook of brewing: In Food Science and Technology. In W. A. Hardwick (Ed.), Marcel Dekker Inc (Vol. 15, pp. 501–550). CRC Press. https://doi.org/10.1002/9780470290118.
Weger, A., Jung, R., Stenzel, F., & Hornung, A. (2017). Optimized energetic usage of brewers’ spent grains. Chemical Engineering & Technology, 40(2), 306–312. https://doi.org/10.1002/ceat.201600186.
Finley, J. W., Walker, C. E., & Hautala, E. (1976). Utilization of press water from brewers spent grains. Journal of the Science of Food and Agriculture, 27(7), 655–660.
Yan, Q., Miazek, K., Grande, P. M., Domínguez de María, P., Leitner, W., & Modigell, M. (2014). Mechanical pretreatment in a screw press affecting chemical pulping of lignocellulosic biomass. Energy & Fuels, 28(11), 6981–6987. https://doi.org/10.1021/ef501706w.
Stiles, R. S., & Herbert, H. S. (1977). Centrifugal separation, isolation and characteristics of brewery spent grains and press liquor. MBAA Technical Quarterly, 14(1), 21–34.
Liao, W., Liu, Y., Liu, C., & Chen, S. (2004). Optimizing dilute acid hydrolysis of hemicellulose in a nitrogen-rich cellulosic material - Dairy manure. Bioresource Technology, 94(1), 33–41. https://doi.org/10.1016/j.biortech.2003.11.011.
Rommi, K., Niemi, P., Kemppainen, K., & Kruus, K. (2018). Impact of thermochemical pre-treatment and carbohydrate and protein hydrolyzing enzyme treatment on fractionation of protein and lignin from brewer’s spent grain. Journal of Cereal Science, 79, 168–173. https://doi.org/10.1016/j.jcs.2017.10.005.
Carvalheiro, F., Garrote, G., Parajo, J. C., Pereira, H., & Gı, F. M. (2005). Kinetic Modeling of brewery’ s spent grain autohydrolysis. Biotechnol.Prog., 21(1), 233–243.
Duarte, L. C., Carvalheiro, F., Lopes, S., Marques, S., Pajaró, J. C., & Gírio, F. (2004). Comparison of two posthydrolysis processes of brewery’s spent grain autohydrolysis liquor to produce a pentose-containing culture medium †. Applied Biochemistry and Biotechnology, 113–116, 1041–1058.
Mussatto, S. I., & Roberto, I. C. (2005). Acid hydrolysis and fermentation of brewer’s spent grain to produce xylitol. Journal of the Science of Food and Agriculture, 85(14), 2453–2460. https://doi.org/10.1002/jsfa.2276.
Chum, H. L., Johnson, D. K., Black, S. K., & Overend, R. P. (1990). Pretreatment-catalyst effects and the combined severity parameter. Applied Biochemistry and Biotechnology, 24–25(1), 1–14. https://doi.org/10.1007/BF02920229.
Sluiter, J. B., Ruiz, R. O., Scarlata, C. J., Sluiter, A. D., & Templeton, D. W. (2010). Compositional analysis of lignocellulosic feedstocks. 1. Review and description of methods. Journal of Agricultural and Food Chemistry, 58(16), 9043–9053. https://doi.org/10.1021/jf1008023.
Robertson, J. A., I’Anson, K. J. A., Treimo, J., Faulds, C. B., Brocklehurst, T. F., Eijsink, V. G. H., & Waldron, K. W. (2010). Profiling brewers’ spent grain for composition and microbial ecology at the site of production. LWT - Food Science and Technology, 43(6), 890–896. https://doi.org/10.1016/j.lwt.2010.01.019.
Pires, E. J., Ruiz, H. A., Teixeira, J. A., & Vicente, A. A. (2012). A new approach on brewer’s spent grains treatment and potential use as lignocellulosic yeast cells carriers. Journal of Agricultural and Food Chemistry, 60(23), 5994–5999. https://doi.org/10.1021/jf300299m.
García-Aparicio, M., Parawira, W., Van Rensburg, E., Diedericks, D., Galbe, M., Rosslander, C., et al. (2011). Evaluation of steam-treated giant bamboo for production of fermentable sugars. Biotechnology Progress, 27(3), 641–649. https://doi.org/10.1002/btpr.580.
Weger, A., Binder, S., Franke, M., & Hornung, A. (2014). Solid Biofuel production by mechanical pre-treatment of brewers ’ spent grain. Chemical Engineering Transactions, 37, 661–666. https://doi.org/10.3303/CET1437111.
Weber, B., & Stadlbauer, E. A. (2017). Sustainable paths for managing solid and liquid waste from distilleries and breweries. Journal of Cleaner Production, 149, 38–48. https://doi.org/10.1016/j.jclepro.2017.02.054.
Jay, A. J., Parker, M. L., Faulks, R., Husband, F., Wilde, P., Smith, A. C., Faulds, C. B., & Waldron, K. W. (2008). A systematic micro-dissection of brewers’ spent grain. Journal of Cereal Science, 47(2), 357–364. https://doi.org/10.1016/j.jcs.2007.05.006.
Vegas, R., Alonso, L., Domı, H., & Parajo, J. C. (2005). Manufacture and refining of oligosaccharides from industrial solid wastes, 614–620.
Morais, A. R. C., Mata, A. C., & Bogel-Lukasik, R. (2014). Integrated conversion of agroindustrial residue with high pressure CO 2 within the biorefinery concept. Green Chemistry, 16(9), 4312–4322. https://doi.org/10.1039/C4GC01093K.
Gurgel, L. V. A., Pimenta, M. T. B., & da S. Curvelo, A. A. (2014). Enhancing liquid hot water (LHW) pretreatment of sugarcane bagasse by high pressure carbon dioxide (HP-CO2). Industrial Crops and Products, 57, 141–149. https://doi.org/10.1016/j.indcrop.2014.03.034.
Luft, L., Confortin, T. C., Todero, I., Ugalde, G., Zabot, G. L., & Mazutti, M. A. (2018). Transformation of residual starch from brewer’ s spent grain into fermentable sugars using supercritical technology. The Journal of Supercritical Fluids, 140(April), 85–90. https://doi.org/10.1016/j.supflu.2018.06.006.
de Moura, F. A., Macagnan, F. T., & da Silva, L. P. (2015). Oligosaccharide production by hydrolysis of polysaccharides: a review. International Journal of Food Science and Technology, 50(2), 275–281. https://doi.org/10.1111/ijfs.12681.
Amorim, C., Silvério, S. C., & Rodrigues, L. R. (2019). One-step process for producing prebiotic arabino-xylooligosaccharides from brewer’s spent grain employing Trichoderma species. Food Chemistry, 270(March 2018), 86–94. https://doi.org/10.1016/j.foodchem.2018.07.080.
Lischeske, J. J., Crawford, N. C., Kuhn, E., Nagle, N. J., Schell, D. J., Tucker, M. P., McMillan, J. D., & Wolfrum, E. J. (2016). Assessing pretreatment reactor scaling through empirical analysis. Biotechnology for Biofuels, 9(1), 213. https://doi.org/10.1186/s13068-016-0620-0.
Funding
This work was supported by funding from the Council for Scientific and Industrial Research (CSIR) of South Africa.
Author information
Authors and Affiliations
Contributions
Conceptualisation: Johann F. Görgens and Eugéne van Rensburg; methodology: Lukas J. Swart; data analysis and investigation: Lukas J. Swart and Oscar K.K. Bedzo; writing-original draft preparation: Lukas J. Swart; writing-review and editing: Lukas J. Swart, Oscar K.K. Bedzo, Johann F. Görgens and Eugéne van Rensburg.
Corresponding author
Ethics declarations
Ethics Approval
This article followed the ethical standard of the institute.
Consent to Participate
All authors consented to participation in this research.
Consent for Publication
All authors consented to publish this research in this journal.
Competing Interest
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 994 kb)
Rights and permissions
About this article
Cite this article
Swart, L.J., Bedzo, O.K.K., van Rensburg, E. et al. Intensification of Xylo-oligosaccharides Production by Hydrothermal Treatment of Brewer’s Spent Grains: The Use of Extremely Low Acid Catalyst for Reduction of Degradation Products Associated with High Solid Loading. Appl Biochem Biotechnol 193, 1979–2003 (2021). https://doi.org/10.1007/s12010-021-03525-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-021-03525-9



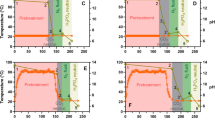



 ] hemicellulose yield, [purple rectangle] CSF (right axes)
] hemicellulose yield, [purple rectangle] CSF (right axes)



 ] xylobiose (X2) and [
] xylobiose (X2) and [  ] xylotriose (X3) from ELA HTT (left axis; yield in XOS, dashed [
] xylotriose (X3) from ELA HTT (left axis; yield in XOS, dashed [  ] for BSG-R; solid [
] for BSG-R; solid [  ] for BSG-SPD, right axis; [
] for BSG-SPD, right axis; [  ] CSF, [
] CSF, [  ] XOS yield, and [
] XOS yield, and [  ] xylan Eq yield) (* average for values for triplicate runs used)
] xylan Eq yield) (* average for values for triplicate runs used)