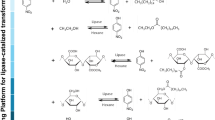
Abstract
Fungal enzymes are widely used in technological processes and have some interesting features to be applied in a variety of biosynthetic courses. Here, free and substrate-immobilised lipases from Fusarium verticillioides P24 were obtained by solid-state fermentation using wheat bran as substrate and fungal carrier. Based on their hydrolytic and transesterification activities, the lipases were characterised as pH-dependent in both reactions, with higher substrate conversion in an alkaline environment. Thermally, the lipases performed well from 30 to 45 °C, being more stable in mild conditions. Organic solvents significantly influenced the lipase selectivity using different vegetable oils as fatty acid source. Omega(ω)-3 production in n-hexane achieved 45% using canola oil, against ≈ 18% in cyclohexane. However, ω-6 production was preferably produced for both solvents using linseed oil with significant alterations in the yield (≈ 79% and 49% for n-hexane and cyclohexane, respectively). Moreover, the greatest enzyme selectivity for ω-6 led us to suppose a lipase preference for the Sn1 position of the triacylglycerol. Lastly, a transesterification reaction was performed, achieving 90% of ester conversion in 72 h. This study reports the characterisation and use of free and substrate-immobilised lipases from Fusarium verticillioides P24 as an economic and efficient method for the first time.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Filamentous fungi are widely used in industrial processes due to their metabolic versatility, well-known for producing a wide variety of chemicals, such as organic acids, polysaccharides, vegetable growth factors, pigments, antibiotics, and enzymes [1]. Fungal lipases from different microorganisms are extensively characterised by their high activity and stability, and, due to their flexibility as catalysts, these enzymes have been commonly used in different chemical reactions [2, 3]. Such extracellular lipases have been immobilised on inert supports, increasing their catalytic properties, such as enantioselectivity [4], hydrolytic [5], and transesterifying activities [6]. Lipases can act in both aqueous and organic solvents, promoting the hydrolysis of ester bonds of triacylglycerol or esterification/transesterification reactions [7, 8]. There are two main ways to use them: by recovering the extracellular lipases from the medium during the fungi cultivation or by using the intracellular lipases present in the cell-producing walls [9]. The use of intracellular lipases has the advantage of being simple, since steps such as separation, purification, and enzyme characterisation are directly linked to the fermentation process [10].
Different fungal enzymes have been produced by solid-state fermentation (SSF) [11, 12], showing operational advantages when compared with submerse fermentation (SmF). SSF can be carried out with low-cost materials without any pre-treatment, using less energy than SmF [13]. Also, in the SSF systems, the cell grows on the surface of solid organic materials with the absence of water between the particles. Once the SSF is over, the solid content can be recovered and used as an immobilised whole-cell (IWC) biocatalyst. Fungi are considered to be one of the organisms best adapted to SSF, as their hyphae can grow on the surface of the solid and penetrate the inter-particle spaces, thereby colonising the solid medium [14]. The fungal mycelium is able to retain several types of enzymes, which makes it a natural catalyst. Its use as an enzyme carrier provides additional advantages to immobilised enzymes on a synthetic support, due to their ease of management, low production costs, and no need for cell disruption [15]. Besides the lipases, numerous enzymes have been reported as also being produced by SSF, making different applications possible, but all research is looking at the same thing: the facility and efficiency of the SSF [14,15,16,17,18].
Dietary, polyunsaturated fatty acids (PUFAs) are essential in the prevention of numerous human illnesses, like cardiovascular diseases, atherosclerosis, high blood pressure, and gastrointestinal infections [19]. Omega-3 (ω-3) and omega-6 (ω-6) are vital cell growth constituents in eukaryotic cells [20], and, because of that, their presence in the human diet must be controlled [21]. Their production has been reported by several authors [2, 20, 22]. Lipase-catalysed enzymatic processes for transforming these acids are relevant from the industrial point of view because these reactions are usually performed under lower-energy conditions, as well as there being no sub-product generation [23]. The transesterification reaction is a chemical process of oil conversion into alkyl esters, which may be methyl or ethyl products depending on the alcohol used, as well as producing glycerol [24]. Lipases with relevant transesterifying activity are interesting from an industrial viewpoint, being used for different purposes. Here, the properties of lipases from the fungus Fusarium verticillioides P24 (LFv) were studied, using the soluble extracellular lipases and the intracellular lipases on immobilised whole cells. Both biocatalysts had their transesterifying potential analysed, based on their catalytic properties and application in the hydrolysis and transesterification reactions. Both the essential fatty acids obtained by hydrolysis (ω-3 and ω-6) and the alkyl esters obtained by transesterification were identified and quantified.
Materials and Methods
Chemical Reagents
p-Nitrophenyl palmitate (pNPP), isopropyl alcohol (isopropanol), fatty acids (palmitic, oleic, linoleic, and linolenic), and ethyl and methyl esters (palmitate, oleate, linoleate, and linolenate) were purchased from Sigma-Aldrich (St. Louis, USA). All other reagents were of analytical grade. Plant oils from olive, canola, linseed, and soybean were purchased from the local market in Sao Jose do Rio Preto, SP, Brazil.
Biological Section
Microorganism
The fungus Fusarium verticillioides P24 was isolated from a sugar cane field (sugar cane mill, Virgolino de Oliveira S/A) in the town of Jose Bonifácio, SP, Brazil (latitude: − 21° 03′ 10″ S and longitude: 49° 41′ 16″ W), and its culture was kept in a potato dextrose agar (PDA) medium under water and mineral oil at 25 °C. This strain belongs to the microbial work collection of the Laboratory of Biochemistry and Applied Microbiology, at the São Paulo State University (UNESP), São José do Rio Preto, SP, Brazil, headed by Prof Eleni Gomes.
Free Lipase Production and Whole-Cell Immobilisation by Solid-State Fermentation
The SSF was carried out using polypropylene bags (26.5 × 15.0 cm) containing 5 g of wheat bran plus 25 mL of nutrient solution (g L−1: 20 peptone, 2 K2HPO4, 0.5 MgSO4, and 20 olive oil). A spore suspension of 5 × 105 mL−1 in water (enough to achieve 70% humidity) was used to inoculate the substrate, and then, the mix was incubated at 28 °C for 72 h. To obtain the free enzyme, the fermented material was mixed with 6 mL of distilled water per gramme of wheat bran, stirred for 30 min, filtered, and centrifuged at 10,000×g at 4 °C for 5 min. The supernatant was used as a free enzyme solution (crude enzymatic extract). To obtain the immobilised whole-cell (IWC) lipases, the fermentation substrate (wheat bran plus mycelia) was filtered and washed with distilled water, acetone 1%, and sodium phosphate buffer 50 mM pH 7.0. Lastly, this natural biocatalyst was dried at 40 °C until constant weight (approximately 24 h) and lyophilised before storage and use.
Scanning Electronic Microscopy
The biomaterial (free fungal cells and the final material after the SSF) was fixed in 2.5% glutaraldehyde in 0.1 M phosphate buffer (pH 7.3) for 36 h at room temperature. Next, the material was washed with distilled water and post-fixed in 1% osmium tetroxide diluted in distilled water for 30 min at room temperature. Following fixation, the bagasse was dehydrated in an ethanol series, critical point-dried with CO2, and coated with gold using a Bal-Tec SCD 050 sputter coater (Oerlikon Balzers, Pfäffikon, Switzerland). The samples were examined using a Quanta 200 scanning electron microscope (SEM, FEI Company, Eindhoven, Netherlands) with an accelerating voltage of 12.5 kV at the Electron Microscopy Center of the Biosciences Institute (UNESP, Botucatu, Brazil).
Enzyme Characterisation
Enzymatic Extract Obtaining
To check the all possibilities of lipase expression by the fungus Fusarium verticillioides P24, different crude enzymatic extracts were obtained aiming to identify the extracellular, associated membrane, and cytoplasmic lipases. For that, the method proposed by Hama and co-authors [25] was used and the total protein quantification was based on the Bradford method [26].
Effect of the pH and Temperature on the Lipase Activity
For the free enzyme, the activities were determined using the hydrolytic spectrophotometric assay and transesterification assay for IWC. Optimum pH conditions were determined with different buffers (sodium citrate at pH 3.0–4.0, sodium acetate at pH 4.0–6.0, sodium phosphate at pH 6.0–8.0, and glycine-NaOH at pH 8.0–9.0) at 35 °C. The optimum temperatures for the assays were determined by incubating the respective reaction mixtures at temperatures from 25 to 45 °C.
Effect of the pH and Temperature on the Lipase Stability
The stability of pH was only tested with free enzymes. The crude enzyme was incubated at 28 °C for up to 5 h in buffers of different pH values (sodium acetate at pH 5.0–6.0 and sodium phosphate at pH 7.0–8.0). In each assay, at the end of the prearranged time, aliquots of 0.05 mL were withdrawn and analysed in a hydrolytic spectrophotometric assay. Stability of temperature assays were done by incubating crude enzyme at 30 to 45 °C, in the absence of substrate, for up to 5 h. In each assay, at the end of the allotted time, aliquots of 0.05 mL were withdrawn and assayed in a hydrolytic spectrophotometric assay as previously described.
Influence of Carbon Chain Lengths on the Hydrolytic Activity of Lipases
Aiming to check the lipase preference for different substrate lengths, this assay was performed with the free extracellular enzymes, measuring the amount of p-nitrophenol released by the hydrolysis of different synthetic substrates: pNPB, pNPD, pNPDD, and pNPP. The total p-nitrophenol released by the hydrolytic activity was measured at 410 nm according to the method described by Winkler and Stuckmann [27] and described in hydrolytic activity of extracellular lipases by spectrophotometric assay.
Zymogram SDS-PAGE Analysis
Zymogram assay was performed according to Fuciños et al. [28] using a polyacrylamide gel at 13% with 0.1% of SDS (sodium dodecyl sulphate). Lipase detection was made with a solution containing α- and β-naphthyl acetate in the presence of Fast Blue. A standard kit (SIGMA) of low molecular weight proteins was used: phosphorylase b (97 kDa), albumin (66.2 kDa), ovalbumin (45 kDa), carbonic anhydrase (31 kDa), trypsin inhibitor (21.5 kDa), lactalbumin (14.4 kDa). To detect other proteins, a silver stain solution was used.
Enzymatic Activity Assays
Hydrolytic Activity of Extracellular Lipases by Spectrophotometric Assay
This assay was performed with the free extracellular enzymes by measuring the amount of p-nitrophenol released by the hydrolysis of the pNPP at 410 nm, according to the method described by Winkler and Stuckmann [27]. Solution A contains 3.0 mg pNPP mL−1 isopropanol. Solution B contains 2.0 g of Triton X-100 plus 0.5 g Arabic gum, in 450 mL 0.05 M phosphate buffer pH 7.0. Solution A (1 mL) was mixed with solution B (9 mL) slowly under agitation, and then, 0.45 mL of the resulting solution was added to 0.05 mL of the crude enzyme extract. After incubation for 1 min at 35 °C, the reaction was ended by adding 0.5 mL sodium tetraborate. Absorbance was determined in a Lambda 25 spectrophotometer (Perkin-Elmer, Norwalk, USA), by using a 1-cm path length quartz cuvette. One unit (U) of activity was defined as the amount of enzyme able to release one micromole of p-nitrophenol per minute, under the assay conditions, based on a calibration curve. The analyses were done three times with standard deviation less than 2%.
Hydrolytic Activity of Intracellular Lipases by Titrimetric Assay
A reaction mixture was composed of 0.09 g Arabic gum, 3 mL of distilled water, 3 mL of 0.05 M phosphate buffer pH 7.0, and 1 mL of olive oil in 50-mL Erlenmeyer flasks. 0.25 g of IWC was used to start the reaction. After incubation for 30 min at 37 °C, the reaction was ended by adding 10 mL of acetone/ethyl alcohol (1:1), and the reaction solutions were titrated with 0.05 M KOH in a Metrohm Titrando 902 pH-STAT automatic titratror (Herisau, Appenzell, Switzerland). One unit (U) of enzyme activity was defined as the amount of enzyme able to release one micromole of fatty acids per minute, under the assay conditions, based on the volume of KOH consumed [29]. The analyses were done three times with standard deviation less than 5%.
Transesterification Reaction
For the determination of the transesterifying activity, the reaction mixture was composed of 0.25 g IWC, 3.58 g soybean oil, 0.56 g ethanol, and 5.81 mL hexane and maintained under agitation (150 rpm) at 35 °C. The reaction products were determined by gas chromatography with flame ionisation detection (GC-FID) in an HP-5890 series II gas chromatograph (Hewlett-Packard, Palo Alto, USA) using a SUPELCO SPB-35 (30 m × 0.32 mm × 0.25 μm) column. The temperature programme was 160–235 °C, with a heating rate of 4 °C/min (160–180 °C) and 6 °C/min (180–235 °C). Injector and detector temperatures were both 250 °C, and nitrogen was used as the carrier gas (1 mL min−1). Calibration was performed with methyl esters (palmitate, oleate, linoleate, and linolenate) and the yield calculated according to Urioste et al. [30]. The analyses were done three times with standard deviation less than 2%.
Chemical Section
Determination of Fatty Acids in Composition of Oils
Prior to the study of the substrate selectivity, the concentration of each fatty acid present in the canola, linseed, and soybean oils was obtained from acid catalysis reactions, using the method with modifications [31]. The reactions were performed with 0.5 g of sample, 10 mL of methanol, and 0.5 mL of sulphuric acid for 2 h in reflux systems. Then, a mixture of water/ether was added to the solution to effect phase separation (the glycerol remains in the water phase) and the organic phase was concentrated using a Heidolph rotary evaporator (Heidolph Instruments, Schwabach, Germany). The fatty acid methyl esters (FAMEs) were determined by the GC-FID and the yield calculated according to Urioste et al. [30].
Enzyme Hydrolysis and Fatty Acid Production
Enzyme fatty acids were produced separately using canola, linseed, and soybean oils as substrates as already described in the literature [32] with some modifications. Five millilitres of n-hexane or cyclohexane, 5 mL of TRIS–HCL buffer (0.1 M) pH 7, and 0.5 mL of oil were placed in a reactor and pre-incubated at 25 °C for 30 min. The reaction was then initiated by adding 0.3 g of IWC, and the reaction suspension was stirred at 150 rpm for 12 h. The concentration of free fatty acids was determined using a Flexar high-performance liquid chromatograph with ultraviolet detection (HPLC–UV) method (Perkin-Elmer, Waltham, USA). Column: Dionex C8 reversed phase (4.6 mm × 250 mm) was used with 70% acetonitrile, 29.9% water, and 0.1% acetic acid solution as the mobile phase with a flow rate of 1.0 mL/min. The elution was monitored by recording the absorbance at 215 nm. The analyses were done three times with standard deviation less than 2%.
Enzyme Transesterification and Ethyl Ester Production
A reaction mixture totalling 12 mL, composed of 1.5 mL ethanol, 4 mL soybean oil, and 6.5 mL n-hexane, was incubated at 35 °C under agitation at 150 rpm in 50-mL flasks. The enzymatic transesterification was started by the addition of 1 U (according to the hydrolytic titrimetric method) of IWC for up to 144 h. At fixed intervals, 0.5-mL samples of the mixture were collected. The synthesis products were concentrated by evaporation, centrifuged for glycerol removal, and the fatty acid ethyl esters (FAEE) were analysed by GC-FID according to the methodology used in the transesterification assay, except that the calibration was performed with ethyl esters (palmitate, oleate, linoleate, and linolenate). Since the transesterification using lipases should be performed in an aqua-restricted environment, the effect of the amount of water present in the reaction mixture was also evaluated.
Results and Discussion
Lipases are enzymes expressed by different living systems, such as animals, vegetables, and microorganisms, among which the microbial lipases have often been exploited in manufacturing processes [33]. These organisms may be found in distinct environmental conditions, and, due to their ease of cultivation, fungi, yeast, and bacteria are the most common microbial lipase producers which are of industrial interest [34]. The Fusarium verticillioides P24 fungus was isolated from a sugar cane field (data not published yet) and kept in a PDA medium with mineral oil at 25 °C. Theoretically, all currently characterised enzymes can be produced by SSF [13]. Firstly, the main features of the fungus cultivation were characterised. The best thermal conditions for the microorganism growth and lipase production were 25 and 35 °C, respectively (Supplementary material–S1). Also, distinct carbon and nitrogen sources for the fungus development were checked considering the hydrolytic activity of the enzymatic extract from the fungus fermentation. Based on that, corn oil and yeast were the best carbon and nitrogen sources, used singly. All the following work was done under these conditions (Supplementary material–S1). Then, the SSF was performed as described in the “Material and Methods” section, aiming at the hydrolytic enzyme production, followed by the extraction of the extracellular lipases and recovery of the immobilised whole-cell lipases on the substrate surface (Scheme 1). The lipases produced by the fungi were firstly evaluated for their physicochemical properties to determine the ideal conditions for the hydrolysis and transesterification reactions. Once optimised, the enzymes were applied to ω-3 production using linseed, canola, and soy oils as fatty acyl acid source. Lastly, the transesterification reaction was carried out with the IWC to check the ethyl ester production from soy oil, the most common vegetable source for biodiesel production.
The fungus Fusarium verticillioides P24 was isolated and used in the SSF with wheat bran as both substrate and support for the cell growth. After the SSF, the extracellular lipases were separated and the solid content was recovered and washed with distilled water, lyophilised and characterised according to their enzymatic activity.
Enzyme Characterisation: Optimal Conditions for LFv Performance
Determination of the Lipolytic Activity of Distinct Lipases
Knowing the physicochemical properties of enzymes is crucial in achieving optimal activity during a biotechnological process. Each enzyme presents specific reaction conditions under which it performs best. Also, two type of lipases are known: extracellular, which are secreted by the microorganism to the medium environment, and the intracellular, which may be found in the cytoplasm or linked to the cell membrane [35]. Aiming to identify the possible presence of both enzymes, the fungus cells were treated according to a description in the literature [25] and crude intra- and extracellular extracts were obtained. Compared with the extracellular lipase extract, the hydrolytic activity of the membrane-bound enzyme extract was 14 times greater (Table 1). Besides that, the specific activity of each enzymatic extract was calculated using the amount of proteins (mg mL−1) present in the medium. The enzyme extract present in the membrane showed specific activity 9 times higher than the extracellular enzymes and 6 times greater than the extract of the cytoplasm enzyme. These results were enough to prove the existence of both hydrolytic enzymes (intra and extracellular), allowing the future fungal immobilisation and its application as a biocatalyst.
Lipase Size and Selectivity
Afterwards, a zymogram assay was performed based on the a-b-naphtylin hydrolysis, in which the bands in the gel identified the presence of two enzymes with hydrolytic activity and molecular weight between 45 and 66 kDa (Fig. 1a). Since their discovery, lipases have been identified and characterised by many authors, showing particular sizes, depending on their producer microorganism [25, 36,37,38]. The fungus Fusarium verticillioides P24 proved to be efficient in producing two enzymes with lipase catalytic characteristics and distinct molecular weight, as already reported [39]. A stronger band with lower molecular weight was detected, which indicates that, probably, there is a preference for smaller lipases in the extracellular extract. However, the production of lipases with different sizes from one microorganism source is commonly reported and well-discussed in the literature. The crude extract of Staphylococcus warneri has been characterised by the presence of three different lipases with distinct properties and molecular weights between 28 and 30 kDa [36]. Lipases with higher molecular weight (from 45 to 57 kDa) have been also identified [2, 40, 41], and here, in the present study, there are two lipases approximated to the latter size range. Furthermore, small lipases with molecular weight around 30 kDa may interact with each other or even with different chemical molecules present in the medium, promoting the establishment of aggregates [42] and, consequently, combinations with higher molecular mass. However, this hypothesis was not considered here, since no band for a small lipase was identified, suggesting the expression of only two distinct lipases with approximately 45 and 60 kDa.
Biochemical characterisation of the soluble LFv from the crude protein extract. a Lipase detection by zymogram (polyacrylamide 15%) stained with specific dye α-β-naphtylin in the presence of Fast Blue and molecular weight by SDS-PAGE submitted to silver staining and b lipase preference in the face of distinct lengths of synthetic substrate chains (white circle) based on the hydrolytic activity (black circle). Influence of c pH and d temperature on the (white circle ) hydrolytic and (black circle) transesterification LFv activity
The enzyme was also characterised based on its selectivity by the chromogenic substrate, using different substrate saturated chain lengths: p-NPB (4 carbons), p-NPD (10 carbons), p-NPDD (12 carbons), and p-NPP (16 carbons). The greatest p-NP release was using p-NPB as substrate (Fig. 1b). However, this substrate is also used to quantify the esterase activity, which may be present in the medium. Thus, it is not the most appropriate substrate for lipase activity quantification when a crude extract is used. Among substrates of larger carbon chains, p-NPD was greatly converted to p-NP, with an enzyme activity of about 41 U g−1, followed by p-NPDD and p-NPP with activities of 27 and 22 U g−1, respectively. For the lipase activity, the substrate p-NPP is the most used [15, 41] because it has 16 carbons in its saturated chain. According to that, it is possible to verify the lipolytic activity only, as esterases do not have affinity to structures with more than 6 carbons atoms as substrate. The following experiments were carried out using the p-NPP as lipase substrate.
Optimal Conditions for Lipolytic Activity
A pH range from 3 to 9 was used to evaluate the influence of H+ ion content on the LFv hydrolytic activity assayed by the spectrophotometric method (Fig. 1c). Due to self-hydrolysis of the synthetic substrate p-NPP, the hydrolysis reaction was not carried out above pH 9. Transesterification activity was also evaluated in the same pH range using the soy oil as substrate. A prior assay was carried out to determine a minimum amount of water to be used in the reaction system to modify the pH with no yield alteration (Supplementary material–S2). Both optimum enzymatic activities (hydrolytic and transesterification) were observed at pH 8, characterising the enzyme as an alkaline lipase, a common characteristic reported for most lipases from animal and microbial origin [34]. Thus, the LFv showed similar behaviour to that observed for different fungi of the genus Fusarium [43,44,45], with minor differences from F. graminearum, which showed better activity at a neutral pH (pH 7) [45]. On the other hand, a subtle peak was observed in the LFv hydrolytic activity in acid conditions (pH 4), suggesting the occurrence of isozymes, which has also been reported for lipases from other Fusarium species [39, 44]. Alkaline lipases are extremely important in the food and pharmaceutical industry due to the conditions used in these industrial processes. In addition, this type of lipase is also widely used in the detergent industry, since washing processes are usually carried out in an alkaline environment [46,47,48].
Since the optimum pH was 8, the next experiments were carried out at this pH. The thermal characteristic is a crucial enzyme property from the industrial point of view. Here, the hydrolytic activity here was subtly dependent on the temperature, with a wide temperature working range. At 35 °C, the best LFv action was observed, with at least 70% of its activity retained across the temperature range from 25 to 45 °C (Fig. 1d). Studies about the enzymatic properties of five extracellular lipases secreted by the fungus Fusarium graminearum showed that all these enzymes revealed optimum activity at 35 to 38 °C [45]. However, three of them had a narrow temperature range of action, since a significant decrease was observed for their activity above 40 °C, with the other two lipases showing an extensive temperature range with activities of about 60% at 50 °C. Two optimum temperature peaks were observed for lipases from Fusarium solani FS1 (25 °C and 45 °C), suggesting the possible presence of isozymes [44]; result was similar to that found here for the pH influence. Optimum activity at 35 °C was also seen for the lipases expressed by the fungus Fusarium oxysporum AM3, but their activity was only kept up to 40 °C [43]. Analysing the transesterification activity, it presented delicate temperature dependence, with a well-defined peak at 30 °C, defining the highest LFv activity. However, the enzyme retained more than 70% of its transesterification activity in a range from 35 to 45 °C, proving its better thermal behaviour for hydrolytic activity. A different performance was found for LFv reported by another author, suggesting the best thermal condition at 40 °C [39]. The difference between these studies may be directly due to the fungus source and cultivation, which could modify its enzyme expression. In this sense, both LFv reports show the largest activity temperature ranges among lipases from this fungus genus. Also, this profile may suggest the presence of more than one lipase enzyme extract with the same molecular weight, but different catalytic properties.
Thermal and pH Influence on the LFv Stability
The effect of pH on enzyme stability was evaluated by keeping LFv incubated in a respective buffer for 5 h, checking their hydrolytic activity by the spectrophotometric assay. For that, the reaction system was maintained at the previously established optimum conditions of pH and temperature. Commonly, due the electrostatic repulsion between the protonated substrate and the negative characteristic of the asparagine residue in the enzyme active site at alkaline conditions, lipases from different sources have a constant behaviour at neutral or alkaline conditions [49]. Despite the alkaline characteristic, LFv had their hydrolytic activity reduced by about 50% after one hour of incubation at pH 8 (Fig. 2a). In contrast, they proved to be stable at acid pH, with a 40% activation activity in the first hour at pH 5 and that remained for the next 5 h of incubation. It was a curious and relevant result, which matches some reports in the literature, which show loss of activity by the lipases from Fusarium solani FS1 in alkaline conditions, with more stability in acidic conditions [44]. It is extremely important to remember that, even though lipases are categorised by their catalytic properties and active site construction, the rest of the enzyme structure may show some differences in terms of amino acid composition. This is enough to promote different secondary structure rearrangement, which is crucial to the enzyme-substrate interaction and may be affected differently by external factors, such as pH and temperature. For thermo stability, the enzymatic activity remained stable for 3 h for all temperatures tested, with a decrease of 40% in activity after 5 h at 40 and 45 °C (Fig. 2b). A slight LFv activity decrease (≈ 10%) was observed at 30 and 35 °C after 5 h of incubation. Usually, enzymes from mesophilic sources are active in mild temperature conditions, from 25 to 40 °C [39]. Lipases from Fusarium solani FS1 strains showed a loss of about 90% of activity when kept at 25 °C for 1 h [44]. Independently, LFv showed here a resistant stability in all conditions studied, keeping at least about 55–60% of their activity.
Stability assays performed with LFv. a pH influence in the lipase stability at different conditions: pH (black square) 5, (black circle) 6, (black up-pointing triangle) 7, and (black down-pointing triangle) 8. Enzymes were kept for 5 h in different buffer solutions. b Thermal stability of lipase activity at (black square) 30 °C; (black circle) 35 °C; (black up-pointing triangle) 40 °C; and (black down-pointing triangle) 45 °C. Previously, lipases were diluted in a buffer solution pH 7 followed by their incubation at different temperatures for up to 5 h. After the incubation period, LFv had their hydrolytic activity measured by the spectrophotometric assay using the synthetic substrate p-NPP for both assays. Standard deviation was lower than 5% for all assays
Lipases Application in Biocatalysis
Production of ω-3 and ω-6
From enzymatic processes, it is possible to produce functional and “clean” dietary components interesting for the food industry, such as ω-3 and ω-6, due to the non-production of undesirable product traces or toxic components [22]. In addition, lipases have been proved as a great biocatalyst to produce different types of triacylglycerols with medium and long carbonic chains [50]. Here, initially, the fatty acid composition of canola, soybean, and linseed oils was determined by acid catalysis according to the literature [51] in order to prove that all oils are composed of ω-3 and 6 (Supplementary material–S3). Linseed oil was the richest in ω-3 (55.5 mM) while soybean and canola oils showed about 6.8 mM. Soybean oil was the richest in ω-6 (53.4 mM) while linseed and canola oils showed 15.1 mM and 20.9 mM, respectively, results which are in accordance with those already reported [52]. From these data, it was possible to calculate the yields of enzymatic hydrolysis of all the oils tested. Although the presence of organic solvents is an important emulsification issue during the reaction, some of them may induce biophysical and biochemical alterations, leading to significant changes in the enzymatic activity [53]. Bearing this in mind, hydrolysis reactions were evaluated using n- or cyclohexane as solvents. Both hexane isomers are not reactive, and they are often used as inert solvents in the oil industry (extraction of essential oils). The production of polyunsaturated fatty acids was carried out for 12 h using IWC as biocatalyst. The highest relative conversion rate of triglyceride to linolenic acid (ω-3) was achieved by using canola oil and n-hexane, with a yield of 45.6% (Fig. 3). However, for cyclohexane, the greatest conversion rate occurred in soybean oil (≈ 31%). These results make clear the direct influence of solvents on the hydrolysis efficiency, since the content of fatty acid was similar in the two oils. Considering absolute values, ω-3 production was greater from linseed oil (9.6 mM in n-hexane and 7.6 mM in cyclohexane), which was expected, since this oil is mainly composed of this acid. However, its relative conversion rate was proportionally lower than those obtained from the canola and soybean oils (17.3% in n-hexane and 13.7% in cyclohexane). Regarding linoleic acid (ω-6), both concentration and conversion rates were higher in linseed oil, even though it is found in lesser concentrations in this oil. Probably, this occurs because the ω-6 position exerts a favourable influence in facilitating its hydrolysis in this oil. It is known that some lipases may exhibit specificity according to the position of the fatty acid in the triglyceride, for instance, the 1,3-regiospecific lipase from the fungus Mucor miehei [54]. Furthermore, many vegetable oils were characterised regarding the position in which the fatty acid is linked to the glycerol [55, 56]. Linoleic acid is found mainly in the Sn1,3 position in linseed oil, whereas, in soybean and canola oils, this position was occupied by linolenic acid. Hence, our results suggest that the LFv has a preference for hydrolysis in the Sn1,3 position. This is an essential characteristic to know about lipases of industrial interest because they may be used to specifically produce some molecules, decreasing costs and purification methods.
Production (%) of ω-3 and ω-6 (bars) and concentration (mM) achieved (dot-line) through the enzymatic hydrolysis of vegetable oils catalysed by immobilised whole-cell lipases. Reactions were carried out in n-hexane or cyclohexane, as presented on the X-axis. The bars correspond to the production in percentage (left Y-axis) for each acid, while the dot lines show the concentration obtained of each acid during the reaction (right Y-axis). Standard deviation was not more than 5% for all reactions. For the relative production (%), statistical analysis was performed by the Dunnett test considering P values < 0.05, with significant difference to the samples indicated with an asterisk (*)
Production of Ethyl Esters from Soybean Oil by Enzymatic Transesterification
The transesterification reactions for the ethyl ester production were carried out using the IWC as biocatalyst for 144 h of reaction. Besides enzymes, microorganisms with relevant metabolic rate are interestingly applied as biocatalysts in some chemical processes [57]. The use of non-immobilised whole cells in biocatalysis presents some limitations, similar to those arising from the use of soluble enzymes, such as their poor reuse and stability during the course of the reactions [58]. In order to overcome this experimental issue, fungus whole cells were immobilised on wheat bran during their cultivation by SSF, followed by lyophilisation. Once immobilised, these cells can be used as a biocatalyst, using both intracellular and any extracellular lipases which may be anchored on the cell membrane. Before their use in ethyl ester production, the initial activity of IWC was standardised by the hydrolysis activity by titration. Based on this assay, 1 U g−1 of IWC for the transesterification reactions was calculated as presented in the literature [59]. Alcohol esters are commonly produced by the transesterification between triglycerides of vegetable oils and linear monohydroxy alcohols, in the presence of some catalyst. In general, methanol is the predominant alcohol used due to its high reactivity and low cost. However, since ethanol is obtained from a renewable source, a final product from 100% renewable sources can be obtained when ethanol is used, making the process an environmentally friendly one. The transesterification reaction was carried out using soybean oil and ethanol incubated with the standard amount of IWC. For transesterification reactions, there should be total absence or a very low amount of water, since water facilitates the hydrolysis instead of the synthesis reaction [60,61,62]. Thus, the ethyl ester synthesis in the presence of water was also evaluated and no negative effect was observed by the addition of up to 1% of water (Fig. S1). However, with 5% of water in the reaction mixture, the yield fell substantially, stopping the production of esters. These results are in accordance with those in Atadashi et al. [62] but differ from the ones reported by Foresti et al. [61], in which the authors found that the addition of up 20% of water did not affect the ester production. Anyway, a maximum water amount of 1% was used for performing the experiments in this study.
The use of immobilised whole-cell catalysts with different biotechnological applications is a method already reported by some authors, and they show many advantages compared with the use of free cells or enzymes [39, 57, 58]. In addition to the different types of cells which can be immobilised, many low-cost materials have been exploited as carriers for the cell growth and immobilisation. Cells of the fungus Thermomucor indicae seudaticae were immobilised during its fermentation on loofah sponges, showing a lipolytic activity higher than 100 U g−1 of the biocatalyst [63]. Besides that, when immobilised, Rhizopus oryzae IFO4697 cells maintained their transesterification activity higher than 75%, even after six batches of reaction [64]. In this study, substantial production of ethyl esters catalysed by IWC occurred after 48 h, reaching a maximum of 90% at 72 h (Figs. 3 and 4). The yield was calculated from the main ethyl ester production characterised by gas chromatography and identified as palmitate, oleate, linoleate, and linolenate ethyl esters, the most common in the biodiesel blend [30]. Compared with chemical catalysis, a huge yield was observed using IWC as biocatalyst, proving the potential of this fungus as a lipase producer. Lipases have considerable flexibility as catalysts in different environments, making many reactions happen. This makes them a very interesting biotechnological tool for many different processes [65]. This study has identified a fungus which produces lipases with hydrolytic and transesterifying potential. Since biocatalysis presents many advantages over the conventional catalytic process [66], the combination of whole-cell immobilisation and its application in ethyl ester synthesis is a promising alternative that can be applied on a large scale for production of these esters. No significant difference in the ester production was observed for reactions carried out for 72 h or longer periods. This period seems to be enough for the use of all available acids by the enzymes, resulting in greater production of linoleate ester, which is present in a higher concentration in soybean oil (53.4 mM).
Fusarium verticillioides P24 cells: free fungus hyphae (a) and immobilised whole cells (b) by screening electron microscopy and its application in ethyl ester production for different periods (c). The ethyl ester (EE) production was quantified by gas chromatography based on the most common esters in the biodiesel blend: ethyl palmitate, ethyl oleate, ethyl linoleate, and ethyl linolenate. All analyses were performed in triplicate and the standard deviations are shown. Statistical analysis was performed by the Dunnett test considering P values < 0.05, indicating no significant difference between the samples indicated with an asterisk (*)
Conclusion
The fungus Fusarium verticillioides P24 produced two distinct lipases with hydrolytic and transesterifying potential. As is common among lipases, the optimum pH was characterised in alkaline conditions, but with better stability at lower pH values. This finding is a relevant point in biotechnological terms because the reaction environment with soybean shows a pH of around 5, facilitating the reaction with no addition of buffers and also decreasing unwanted products. Optimum thermal condition was found at 35 °C which was expected since these lipases are expressed by a mesophilic fungus. Besides that, the lipases had high thermostability between 25 and 40 °C, enabling their use in processes with different thermal requirements. Among the lipase properties, the enzyme selectivity was modified by the use of organic solvents, with a preference for ω-6 in n-hexane. Also, due to the position of the fatty acids, the lipases had a considerable preference for those acids which are commonly indicated in the S1,3 position. When immobilised, the fungus whole-cell biocatalyst was efficient in promoting the transesterification reaction between soybean oil and ethanol, achieving a yield of 90% in 72 h, confirming the industrial potential of these lipases.
References
Hesham, A., & E. E. (2007). Filamentous fungal cultures-process characteristics, products, and applications. In Bioprocessing for Value-Added Products from Renewable Resources. Elsevier B.V. https://doi.org/10.1016/B978-0-444-52114-9.50010-4.
Pérez, D., Martín, S., Fernández-Lorente, G., Filice, M., Guisán, J. M., Ventosa, A., et al. (2011). A novel halophilic lipase, LipBL, showing high efficiency in the production of eicosapentaenoic acid (EPA). PloS one, 6(8), e23325. https://doi.org/10.1371/journal.pone.0023325.
Hung, T.-C., Giridhar, R., Chiou, S.-H., & Wu, W.-T. (2003). Binary immobilization of Candida rugosa lipase on chitosan. Journal of Molecular Catalysis B: Enzymatic, 26(1–2), 69–78. https://doi.org/10.1016/S1381-1177(03)00167-X.
da Silva, C. C. F., Contesini, F. J., & Carvalho, P. d. O. (2009). Enantioselective behavior of lipases from Aspergillus niger immobilized in different supports. Journal of Industrial Microbiology and Biotechnology, 36(7), 949–954. https://doi.org/10.1007/s10295-009-0573-4.
Krishna, S. H., Prapulla, S. G., & Karanth, N. G. (2000). Enzymatic synthesis of isoamyl butyrate using immobilized Rhizomucor miehei lipase in non-aqueous media. Journal of Industrial Microbiology and Biotechnology, 25(3), 147–154.
Lima, L. N., Oliveira, G. C., Rojas, M. J., Castro, H. F., & Tardioli, P. W. (2015). Immobilization of Pseudomonas fluorescens lipase on hydrophobic supports and application in biodiesel synthesis by transesterification of vegetable oils in solvent-free systems. Journal of industrial microbiology & biotechnology, 42(4), 523–535. https://doi.org/10.1007/s10295-015-1586-9.
Fernandez-Lafuente, R., Armisén, P., Sabuquillo, P., Fernández-Lorente, G., & Guisán, J. M. (1998). Immobilization of lipases by selective adsorption on hydrophobic supports. Chemistry and physics of lipids, 93(1–2), 185–97. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/9720258
Ben Akacha, N., & Gargouri, M. (2015). Microbial and enzymatic technologies used for the production of natural aroma compounds: Synthesis, recovery modeling, and bioprocesses. Food and Bioproducts Processing, 94(May 2014), 675–706. https://doi.org/10.1016/j.fbp.2014.09.011.
Lokha, Y., Arana-Peña, S., Rios, N. S., Mendez-Sanchez, C., Gonçalves, L. R. B., Lopez-Gallego, F., & Fernandez-Lafuente, R. (2020). Modulating the properties of the lipase from Thermomyces lanuginosus immobilized on octyl agarose beads by altering the immobilization conditions. Enzyme and Microbial Technology, 133, 109461. https://doi.org/10.1016/j.enzmictec.2019.109461.
de Oliveira, B. H., Coradi, G. V., de Oliva-Neto, P., & do Nascimento, V. M. G. (2020). Biocatalytic benefits of immobilized Fusarium sp. (GFC) lipase from solid state fermentation on free lipase from submerged fermentation. Industrial Crops and Products, 147(February), 112235. https://doi.org/10.1016/j.indcrop.2020.112235.
de Oliveira Rodrigues, P., Gurgel, L. V. A., Pasquini, D., Badotti, F., Góes-Neto, A., & Baffi, M. A. (2020). Lignocellulose-degrading enzymes production by solid-state fermentation through fungal consortium among Ascomycetes and Basidiomycetes. Renewable Energy, 145, 2683–2693. https://doi.org/10.1016/j.renene.2019.08.041.
Singh, R. S., Chauhan, K., Kaur, K., & Pandey, A. (2020). Statistical optimization of solid-state fermentation for the production of fungal inulinase from apple pomace. Bioresource Technology Reports, 9(November 2019), 100364. https://doi.org/10.1016/j.biteb.2019.100364.
Wang, L., & Yang, S. T. (2007). Solid state fermentation and its applications. In Bioprocessing for Value-Added Products from Renewable Resources. Elsevier B.V. https://doi.org/10.1016/B978-044452114-9/50019-0.
Muller Dos Santos, M., Souza Da Rosa, A., Dal’Boit, S., Mitchell, D. A., & Krieger, N. (2004). Thermal denaturation: is solid-state fermentation really a good technology for the production of enzymes? Bioresource Technology, 93(3), 261–268. https://doi.org/10.1016/j.biortech.2003.11.007.
Fernandes, M. L. M., Saad, E. B., Meira, J. A., Ramos, L. P., Mitchell, D. A., & Krieger, N. (2007). Esterification and transesterification reactions catalysed by addition of fermented solids to organic reaction media. Journal of Molecular Catalysis B: Enzymatic, 44(1), 8–13. https://doi.org/10.1016/j.molcatb.2006.08.004.
Shinkawa, S., & Mitsuzawa, S. (2020). Feasibility study of on-site solid-state enzyme production by Aspergillus oryzae. Biotechnology for Biofuels, 3(1), 1–15. https://doi.org/10.1186/s13068-020-1669-3.
Perez, C. L., Casciatori, F. P., & Thoméo, J. C. (2019). Strategies for scaling-up packed-bed bioreactors for solid-state fermentation: the case of cellulolytic enzymes production by a thermophilic fungus. Chemical Engineering Journal, 361(December 2018), 1142–1151. https://doi.org/10.1016/j.cej.2018.12.169.
Rodrigues, I. d. S. V., Barreto, J. T., Moutinho, B. L., Oliveira, M. M. G., da Silva, R. S., Fernandes, M. F., & Fernandes, R. P. M. (2020). Production of xylanases by Bacillus sp. TC-DT13 in solid state fermentation using bran wheat. Preparative Biochemistry and Biotechnology, 50(1), 91–97. https://doi.org/10.1080/10826068.2019.1663536.
Brotas, M. S. C., Carvalho, G. A., & Pereira, P. A. P. (2020). Determination, through derivatization and GC-MS analysis, of omega-3 and omega-6 fatty acids in fish oil capsules sold in Salvador, Bahia. Journal of Brazilian Chemical Society, 31(3), 447–455.
Adarme-vega, T. C., Lim, D. K. Y., Timmins, M., Vernen, F., Li, Y., & Schenk, P. M. (2012). Microalgal biofactories: a promising approach towards sustainable omega-3 fatty acid production. Microbial Cell Factories, 11(96), 1–10.
Simopoulos, A. P. (2016). An increase in the omega-6/omega-3 fatty acid ratio increases the risk for obesity. Nutrients, 8(3), 1–17. https://doi.org/10.3390/nu8030128.
Moreno-Perez, S., Turati, D. F. M., Borges, J. P., Luna, P., Señorans, F. J., Guisan, J. M., & Fernandez-Lorente, G. (2017). Critical role of different immobilized biocatalysts of a given lipase in the selective ethanolysis of sardine oil. Journal of Agricultural and Food Chemistry, 65(1), 117–122. https://doi.org/10.1021/acs.jafc.6b05243.
Moreno-Pérez, S., Guisan, J. M., & Fernandez-Lorente, G. (2014). Selective ethanolysis of fish oil catalyzed by immobilized lipases. JAOCS, Journal of the American Oil Chemists’ Society, 91(1), 63–69. https://doi.org/10.1007/s11746-013-2348-3.
Moazeni, F., Chen, Y. C., & Zhang, G. (2019). Enzymatic transesterification for biodiesel production from used cooking oil, a review. Journal of Cleaner Production, 216, 117–128. https://doi.org/10.1016/j.jclepro.2019.01.181.
Hama, S., Tamalampudi, S., Fukumizu, T., Miura, K., Yamaji, H., Kondo, A., & Fukuda, H. (2006). Lipase localization in Rhizopus oryzae cells immobilized within biomass support particles for use as whole-cell biocatalysts in biodiesel-fuel production. Journal of bioscience and bioengineering, 101(4), 328–333. https://doi.org/10.1263/jbb.101.328.
Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72(1–2), 248–254. https://doi.org/10.1016/0003-2697(76)90527-3.
Winkler, U. K., & Stuckmann, M. (1979). Glycogen, hyaluronate, and some other polysaccharides greatly enhance the formation of exolipase by Serratia marcescens. Journal of Bacteriology, 138(3), 663–670. https://doi.org/10.1128/jb.138.3.663-670.1979.
Fuciños, P., Abadín, C. M., Sanromán, A., Longo, M. A., Pastrana, L., & Rúa, M. L. (2005). Identification of extracellular lipases/esterases produced by Thermus thermophilus HB27: partial purification and preliminary biochemical characterisation. Journal of Biotechnology, 117(3), 233–241. https://doi.org/10.1016/j.jbiotec.2005.01.019.
Soares, C. M. F., De Castro, H. F., De Moraes, F. F., & Zanin, G. M. (1999). Characterization and utilization of Candida rugosa lipase immobilized on controlled pore silica. Applied Biochemistry and Biotechnology - Part A Enzyme Engineering and Biotechnology, 77–79, 745–757. https://doi.org/10.1385/abab:79:1-3:745, 1-3
Urioste, D., Castro, M., Biaggio, F., & Castro, H. (2008). Síntese de padroes cromatográficos e estabelecimento de método para dosagem da composiçao de ésteres de ácidos graxos presentes no biodiesel a partir do óleo de babaçu. Química Nova, 31(2), 407–412.
Ichihara, K., & Fukubayashi, Y. (2010). Preparation of fatty acid methyl esters for gas-liquid chromatography. Journal of Lipid Research, 51(3), 635–640. https://doi.org/10.1194/jlr.D001065.
Fernández-Lorente, G., Betancor, L., Carrascosa, A. V., & Guisán, J. M. (2011). Release of omega-3 fatty acids by the hydrolysis of fish oil catalyzed by lipases immobilized on hydrophobic supports. Journal of the American Oil Chemists’ Society, 88(8), 1173–1178. https://doi.org/10.1007/s11746-011-1776-1.
Lima, L. G. R., Gonçalves, M. M. M., Couri, S., Melo, V. F., Sant’Ana, G. C. F., & Costa, A. C. A. d. (2019). Lipase production by Aspergillus niger C by submerged fermentation. Brazilian Archives of Biology and Technology, 62, 1–14. https://doi.org/10.1590/1678-4324-2019180113.
Melani, N. B., Tambourgi, E. B., & Silveira, E. (2020). Lipases: from production to applications. Separation and Purification Reviews, 49(2), 143–158. https://doi.org/10.1080/15422119.2018.1564328.
Solarte, C., Yara-Varón, E., Eras, J., Torres, M., Balcells, M., & Canela-Garayoa, R. (2014). Lipase activity and enantioselectivity of whole cells from a wild-type Aspergillius flavus strain. Journal of Molecular Catalysis B: Enzymatic, 100, 78–83. https://doi.org/10.1016/j.molcatb.2013.12.005.
Volpato, G., Filice, M., Ayub, M. A. Z., Guisan, J. M., & Palomo, J. M. (2010). Single-step purification of different lipases from Staphylococcus warneri. Journal of chromatography. A, 1217(4), 473–478. https://doi.org/10.1016/j.chroma.2009.11.055.
Wang, Q., Hou, Y., Ding, Y., & Yan, P. (2012). Purification and biochemical characterization of a cold-active lipase from Antarctic sea ice bacteria Pseudoalteromonas sp. NJ 70. Molecular biology reports, 39(9), 9233–9238. https://doi.org/10.1007/s11033-012-1796-4.
Ilesanmi, O. I., Adekunle, A. E., Omolaiye, J. A., Olorode, E. M., & Ogunkanmi, A. L. (2020). Isolation, optimization and molecular characterization of lipase producing bacteria from contaminated soil. Scientific African, 8, e00279. https://doi.org/10.1016/j.sciaf.2020.e00279.
Facchini, F. D. A., Pereira, M. G., Vici, A. C., Filice, M., Pessela, B. C., Guisan, J. M., et al. (2018). Immobilization effects on the catalytic properties of two Fusarium verticillioides lipases: stability, hydrolysis, transesterification and enantioselectivity improvement. Catalysts, 8(2). https://doi.org/10.3390/catal8020084.
Salameh, M. a., & Wiegel, J. (2007). Purification and characterization of two highly thermophilic alkaline lipases from Thermosyntropha lipolytica. Applied and environmental microbiology, 73(23), 7725–7731. https://doi.org/10.1128/AEM.01509-07.
Ulker, S., & Karaoğlu, S. A. (2012). Purification and characterization of an extracellular lipase from Mucor hiemalis f. corticola isolated from soil. Journal of bioscience and bioengineering, 114(4), 385–390. https://doi.org/10.1016/j.jbiosc.2012.04.023.
Galvis, M., Barbosa, O., Ruiz, M., Cruz, J., Ortiz, C., Torres, R., & Fernandez-Lafuente, R. (2012). Chemical amination of lipase B from Candida antarctica is an efficient solution for the preparation of crosslinked enzyme aggregates. Process Biochemistry, 47(12), 2373–2378. https://doi.org/10.1016/j.procbio.2012.09.018.
Camargo-De-Morais, M. M., Maia, M. M. D., Borba, F. F. S., Melo, K. G., Santos, C. M. S. O., Reis, E. R. A., et al. (2003). Oil/mineral-salts medium designed for easy recovery of extracellular lipase from Fusarium oxysporum AM3. World Journal of Microbiology and Biotechnology, 19(1), 17–20. https://doi.org/10.1023/A:1022543125420.
Maia, M. D. M. D., Camargo De Morais, M. M., De Morais, M. A., Melo, E. H. M., & De Lima Filho, J. L. (1999). Production of extracellular lipase by the phytopathogenic fungus Fusarium solani Fs1. Revista de Microbiologia, 30(4), 304–309. https://doi.org/10.1590/S0001-37141999000400003
Nguyen, L. N., Dao, T. T., Živković, T., Fehrholz, M., Schäfer, W., & Salomon, S. (2010). Enzymatic properties and expression patterns of five extracellular lipases of Fusarium graminearum in vitro. Enzyme and Microbial Technology, 46(6), 479–486. https://doi.org/10.1016/j.enzmictec.2010.02.005.
Niyonzima, F. N., & More, S. (2014). Biochemical properties of the alkaline lipase of Bacillus flexus XJU-1 and its detergent compatibility. Biologia (Poland), 69(9), 1108–1117. https://doi.org/10.2478/s11756-014-0429-x.
Bhosale, H., Shaheen, U., & Kadam, T. (2016). Characterization of a hyperthermostable alkaline lipase from Bacillus sonorensis 4R. Enzyme Research, 1, 1–11. https://doi.org/10.1155/2016/4170684.
Wang, H., Zhong, S., Ma, H., Zhang, J., & Qi, W. (2012). Screening and characterization of a novel alkaline lipase from. Brazilian Journal of Microbiology, 1, 148–156.
Reis, P., Holmberg, K., Watzke, H., Leser, M. E., & Miller, R. (2009). Lipases at interfaces: a review. Advances in Colloid and Interface Science, 147–148(C), 237–250. https://doi.org/10.1016/j.cis.2008.06.001.
Korma, S. A., Zou, X., Ali, A. H., Abed, S. M., Jin, Q., & Wang, X. (2018). Preparation of structured lipids enriched with medium- and long-chain triacylglycerols by enzymatic interesterification for infant formula. Food and Bioproducts Processing, 107, 121–130. https://doi.org/10.1016/j.fbp.2017.11.006.
Porto, B. L. S., Faria, I. D. L., de Oliveira Mendes, T., & de Oliveira, M. A. L. (2015). Fast screening method for the analysis of trans fatty acids in processed food by CZE-UV with direct detection. Food Control, 55, 230–235. https://doi.org/10.1016/j.foodcont.2015.02.027.
Damodaran, S., Parkin, K. L., & Fennema, O. R. (2007). Fennema’s Food Chemistry (4th ed.).
Kamal, Z., Yedavalli, P., Deshmukh, M. V., & Rao, N. M. (2013). Lipase in aqueous-polar organic solvents: activity, structure, and stability. Protein Science, 22(7), 904–915. https://doi.org/10.1002/pro.2271.
Muralidhar, R. V., Chirumamilla, R. R., Marchant, R., Ramachandran, V. N., Ward, O. P., & Nigam, P. (2002). Understanding lipase stereoselectivity. World Journal of Microbiology and Biotechnology, 18(2), 81–97. https://doi.org/10.1023/A:1014417223956.
Holčapek, M., Jandera, P., Zderadička, P., & Hrubá, L. (2003). Characterization of triacylglycerol and diacylglycerol composition of plant oils using high-performance liquid chromatography-atmospheric pressure chemical ionization mass spectrometry. Journal of Chromatography A, 1010(2), 195–215. https://doi.org/10.1016/S0021-9673(03)01030-6.
Neff, W. E., Mounts, T. L., & Rinsch, W. M. (1997). Oxidative stability as affected by triacylglycerol composition and structure of purified canola oil triacylglycerols from genetically modified normal and high stearic and lauric acid canola varieties. LWT - Food Science and Technology, 30(8), 793–799. https://doi.org/10.1006/fstl.1997.0274.
Żur, J., Piński, A., Michalska, J., Hupert-Kocurek, K., Nowak, A., Wojcieszyńska, D., & Guzik, U. (2020). A whole-cell immobilization system on bacterial cellulose for the paracetamol-degrading Pseudomonas moorei KB4 strain. International Biodeterioration and Biodegradation, 149(February), 104919. https://doi.org/10.1016/j.ibiod.2020.104919.
Yang, S. Y., Choi, T. R., Jung, H. R., Park, Y. L., Han, Y. H., Song, H. S., et al. (2019). Production of glutaric acid from 5-aminovaleric acid by robust whole-cell immobilized with polyvinyl alcohol and polyethylene glycol. Enzyme and Microbial Technology, 128(May), 72–78. https://doi.org/10.1016/j.enzmictec.2019.05.003.
Quilles Junior, J. C., Ferrarezi, A. L., Borges, J. P., Brito, R. R., Gomes, E., da Silva, R., … Boscolo, M. (2016). Hydrophobic adsorption in ionic medium improves the catalytic properties of lipases applied in the triacylglycerol hydrolysis by synergism. Bioprocess and Biosystems Engineering, 39(12), 1933–1943. https://doi.org/10.1007/s00449-016-1667-9
Lee, N. K., Oh, S. W., Kwon, D. Y., & Yoon, S. H. (2015). Production of 1, 3-dioleoyl-2-palmitoyl glycerol as a human milk fat substitute using enzymatic interesterification of natural fats and oils. Food Science and Biotechnology, 24(2), 433–437. https://doi.org/10.1007/s10068-015-0057-4.
Foresti, M. L., & Ferreira, M. L. (2007). Chitosan-immobilized lipases for the catalysis of fatty acid esterifications. Enzyme and Microbial Technology, 40(4), 769–777. https://doi.org/10.1016/j.enzmictec.2006.06.009.
Atadashi, I. M., Aroua, M. K., Abdul Aziz, A. R., & Sulaiman, N. M. N. (2012). The effects of water on biodiesel production and refining technologies: a review. Renewable and Sustainable Energy Reviews, 16(5), 3456–3470. https://doi.org/10.1016/j.rser.2012.03.004.
Ferrarezi, A. L., Hideyuki Kobe Ohe, T., Borges, J. P., Brito, R. R., Siqueira, M. R., Vendramini, P. H., et al. (2014). Production and characterization of lipases and immobilization of whole cell of the thermophilic Thermomucor indicae seudaticae N31 for transesterification reaction. Journal of Molecular Catalysis B: Enzymatic, 107(2014), 106–113. https://doi.org/10.1016/j.molcatb.2014.05.012.
Zhou, G. X., Chen, G. Y., & Yan, B. b. (2015). Two-step biocatalytic process using lipase and whole cell catalysts for biodiesel production from unrefined jatropha oil. Biotechnology Letters, 37(10), 1959–1963. https://doi.org/10.1007/s10529-015-1883-4.
Villeneuve, P., Muderhwa, J. M., Graille, J., & Haas, M. J. (2000). Customizing lipases for biocatalysis: a survey of chemical, physical and molecular biological approaches. Journal of Molecular Catalysis B: Enzymatic, 9(4–6), 113–148. https://doi.org/10.1016/S1381-1177(99)00107-1.
Zhang, B., Weng, Y., Xu, H., & Mao, Z. (2012). Enzyme immobilization for biodiesel production. Applied microbiology and biotechnology, 93(1), 61–70. https://doi.org/10.1007/s00253-011-3672-x.
Funding
This study received financial support from the São Paulo Research Foundation (FAPESP Grants 2010/03555-5 and 2008/58077-0) and the National Council for Scientific and Technological Development (CNPq).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing of Interests
The authors declare that they have no competing interests.
Research Involving Human Participants and/or Animals
The current research has no involved human participants and/or animal models.
Informed Consent
All authors declare that the current paper has not been under review by other journal, besides approving its submission on Applied Biochemistry and Biotechnology.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
ESM 1
(DOCX 129 kb)
Rights and permissions
About this article
Cite this article
Borges, J.P., Quilles Junior, J.C., Ohe, T.H.K. et al. Free and Substrate-Immobilised Lipases from Fusarium verticillioides P24 as a Biocatalyst for Hydrolysis and Transesterification Reactions. Appl Biochem Biotechnol 193, 33–51 (2021). https://doi.org/10.1007/s12010-020-03411-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-020-03411-w