Abstract
Selective ethanolysis of fish oil was catalyzed by immobilized lipases and their derivatives in organic media. Lipases from Candida antarctica B (CALB), Thermomyces lanuginosa (TLL) and Rhizomucor miehei (RML) were studied. The three lipases were immobilized by anion exchange and hydrophobic adsorption. The discrimination between the ethyl ester of eicosapentaenoic acid (EE-EPA) and the ethyl ester of docosahexaenoic acid (EE-DHA) depends on the lipase, the immobilization support, the physico-chemical modifications of the immobilized lipase derivatives and on the solvents used. TLL and RML were much more selective than CALB. EE-EPA is released 20-fold faster than EE-DHA when ethanolysis was catalyzed, in cyclohexane, by TLL hydrophobically adsorbed on Sepabeads C18. The selectivity and stability of the different derivatives in these polar organic solvents were further improved after physico-chemical modification. The best results for activity-selectivity-stability were obtained in cyclohexane for TLL adsorbed on Sepabeads C18 and further modified via solid-phase physical modification with a polyethylenimine polymer. In this case, the initial selectivity was higher than 20, and a 80 % of EPA was released as ethyl ester after 3 h at 25 °C. At this conversion, mixtures of ethyl esters highly enriched in the ethyl ester of EPA with less than 5 % of the EE-DHA were obtained. TLL derivatives remained fully active after incubation for 24 h in anhydrous solvents.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The synthesis of valued functional ingredients such as ethyl esters or triglycerides that are highly enriched in omega-3 fatty acids [containing 70 to 100 % of one or both polyunsaturated fatty acids, (PUFA)] is quite interesting [1]. The beneficial effects of omega-3 fatty acids derived from fish oils (mainly consisting of DHA and EPA) have prompted increasing interest among health professionals. DHA is a physiologically essential nutrient required at high levels by the brain and the retina and also to improve learning ability, mental development and visual acuity in the early stages of life [2]. In addition, EPA is considered to be beneficial in the prevention of cardiovascular diseases in adults [3, 4]. Therefore, the preparation of ethyl esters or triglycerides enriched in either or both DHA and EPA could be useful for promoting different health benefits for end consumers with varying ages, geographic locations, or health conditions [5, 6].
The enzymatic transformations of fish oils containing omega-3 fatty acids represents an attractive alternative to conventional chemical approaches because enzymatic processes can be performed under very mild conditions without the formation of undesirable byproducts [7–9].
Ethanolysis of fish oil promotes the synthesis of ethyl esters containing omega-3 fatty acids. Ethyl esters of omega-3 fatty acids are currently used as health/dietary and they are also good precursors for the production of triacylglycerol esters of omega-3 fatty acids [10]. Triacylglycerol esters could prove to be the ideal food ingredients based on omega-3 fatty acids.
The omega-3 fatty acids EPA and DHA have different the length of the aliphatic chain between the carboxylic acid and the first double bound. The enzymatic release of EPA could be favored because its saturated aliphatic chain is a bit larger (4 C–C bonds) than the saturated aliphatic chain of DHA (3 C–C bonds). In enzymatic hydrolysis methods previously developed by our group, EPA/DHA ratios from 1 to 20 were obtained using different lipases, different lipase derivatives and different experimental conditions [11]. Enzymatic separation of EPA and DHA by selective hydrolysis or by selective ethanolysis may be very interesting because both molecules are very similar and difficult to be separated by using physico-chemical protocols.
Ethanolysis of oils is performed better in organic solvents [12, 13]. On the one hand, organic solvents may modulate and improve the activity-selectivity properties of the different enzyme derivatives, on the other hand, polar organic solvents are deleterious to enzyme stability [14]. For this reason, particular attention should be paid to the stability and stabilization of different enzyme derivatives when using polar organic solvents [15].
Enzymatic protocols for the ethanolysis of fish oil are reported in this paper. The reaction was conducted at room temperature, and the mild conditions plus the absence of acids prevents any modification to the omega-3 fatty acids. Several lipases and several immobilization supports for each lipase were studied. The behavior of the lipases in two solvents (cyclohexane and tert-amyl alcohol) was tested. The immobilized lipases with a better performance were modified by physico-chemical protocols. The best results were sought for three main parameters: activity, stability and mainly discrimination between EPA and DHA.
Materials and Methods
Materials
Buffering salts, Triton® X-100 (TX), p-nitrophenyl butyrate (p-NPB), ethanol, tert-amyl alcohol, cyclohexane, polyethyleneimine (PEI) (MW 25,000), ethanolamine hydrochloride, 1-ethyl-3-(dimethylaminopropyl) carbodiimide (EDC), dextran sulfate sodium salt (MW 40,000) and succinic anhydride were obtained from Sigma Chemical Co. (St. Louis, Mo). Sardine oil was obtained from Biotec BTSA. Octyl Sepharose™ CL-4B was purchased from GE Healthcare (Uppsala, Sweden). Duolite A568, a weak base ion-exchange resin, was provided by Rohm and Haas (USA). Lewatit VP OC1600 was purchased from Bayer (Leverkusen, Germany). Sepabeads-C18 were kindly donated by Resindion S.R.L. (Milan, Italy). Lipases from Rhizomucor miehei (RML), Thermomyces lanuginose (TLL), Candida antarctica lipase B (CALB) were generously donated by Novo Nordisk (Denmark). All reagents and solvents used were of analytical or HPLC grade.
Methods
Determination of the Activity of Different Soluble and Immobilized Lipases
The activity assay used a spectrophotometer with a thermostated cell and continuous magnetic stirring (500 rpm) for 2 min. The increase in absorbance at 348 nm (∈ = 5.150 M−1cm−1) produced by p-nitrophenol (pNP) released in the hydrolysis of 0.4 mM p-nitrophenyl butyrate (pNPB) in 25 mM sodium phosphate at pH 7 and 25 °C was measured [14]. To initialize the reaction, 0.05–0.2 ml of the free lipases (blank or supernatant) and their immobilized preparations (suspension) was added to 2.5 ml of the substrate solution. Enzymatic activity was calculated as μmol of hydrolyzed pNPB per minute per mg of enzyme (IU) under the conditions described.
Purification of Lipases
Adsorption on Octyl-Sepharose To purify the lipases from any contaminant protein (e.g., esterases), the different lipase preparations were immobilized on octyl–sepharose by interfacial activation at a low ionic strength (5 mM) in sodium phosphate buffer at 25 °C and pH 7 [16]. Periodically, the activity of the suspension and supernatant was measured using the pNPB assay. In all cases, the enzyme load of the immobilized preparations was approximately 20 mg of lipase per mL of support.
Desorption of lipases from Octyl-Sepharose To desorb the purified lipase, 10 g of the octyl-sepharose lipase derivative was suspended in 100 ml of 1 % Triton X-100 (TX) (v/v) solution or 1 % lauryl sucrose solution in 10 mM sodium phosphate buffer at pH 7.0 and 25 °C. Lauryl sucrose was used to desorb TLL derivatives, as TX causes a strong inactivation of the enzyme. After 30 min, more than 90 % of the lipase was desorbed away from the octyl-sepharose derivatives of all the lipases.
Immobilization of Lipases on Duolite A568, Lewatit VP OC1600 or Sepabeads-C18 resins
The purified lipase was diluted ten-fold using a 10 mM phosphate buffer to dilute the detergent Triton X-100. The pH of the solution was then adjusted to 7, and 1 g of support Duolite A568 (derivatives named as Duo), Lewatit VPOC 1600 (derivatives named as Lew) or Sepabeads-C18 (derivatives named as Sepab) was added. The immobilization mixture was maintained at pH 7 for 3 h at 25 °C. Periodically, the activity of the suspension and supernatant was measured by the pNPB assay. In all cases, the yield was greater than 95 % as compared to the initial enzyme activity.
Solid-Phase Chemical Modification of Sepabeads-C18 Derivatives of Different Lipases
Amination of Immobilized Lipases Chemical amination was performed as previously described [17]. One gram of lipase immobilized on octyl-sepharose beads was added to 10 ml of 1 M ethylenediamine (EDA), pH 4.75, under continuous stirring. Solid EDC was added to the suspension to yield a final concentration of 10 mM. After a 90 min incubation at 25 °C with gentle stirring, the immobilized-aminated lipase derivatives were vacuum filtered using a sintered glass funnel and incubated for 4 h in 0.1 M hydroxylamine, pH 7, at 4 °C [18]. The immobilized lipases were filtered and washed with 25 mM sodium phosphate, pH 7.5, and then with an excess of distilled water.
Adsorption of Polymers on the Immobilized Lipases Immobilized lipase derivatives on Sepabeads-C18 resin were incubated in a solution of 1 % polymer (polyethylenimine or dextran sulfate sodium salt) in 25 mM phosphate buffer, pH 8 [19]. The suspension was gently stirred for 1.5 h and then washed with 5 mM phosphate buffer. Derivatives were filtered under vacuum to remove all buffer except that filling the pores of the derivatives.
Succinylation of Immobilized Lipases One gram of immobilized lipase derivative was added to 10 ml of 50 mM sodium phosphate at pH 8. Then, 0.1 g of solid succinic anhydride was added to the suspension under rapid stirring at 20 °C (final concentration was 100 mM) [20]. The pH was maintained at 8. The mixture was allowed to react for 2 h (100 % modification of the exposed groups). After the reaction, the succinylated derivative was filtered and washed with an excess of distilled water.
Drying of Immobilized Lipases and their Derivatives
Organic solvents were dried by overnight incubation in an excess of dry molecular sieves (500 g of sieves per liter of solvent). Then, 5 g of the lipase derivative was suspended in 50 ml of dry solvents in the presence of 20 g of dried molecular sieves for 3 h. In this way, the water filling the pores of derivatives was dissolved and diluted in the organic solvent and then adsorbed onto the sieves.
Enzymatic Synthesis of Ethyl Esters of Omega-3 Fatty Acids
First, 0.1 g of dried immobilized lipase was added to the substrate solution containing 0.623 mmol of sardine oil and 6.2 mmol of ethanol dissolved in 4.11 ml of organic solvent (tert-amyl alcohol or cyclohexane). Second, 100 mg of dry molecular sieves was also added to the reaction mixture to obtain activity values in the absence of water. The final concentration of sardine oil in the solution was 125 mM. The reaction was carried out at 25 °C and it was followed by HPLC. Initial enzymatic discrimination between EPA and DHA was measured when the transesterification degree is lower than 10 % (2.2 mM of ethyl ester of EPA). Experiments were conducted in triplicate, and the standard error never exceeded 5 %.
HPLC Analysis
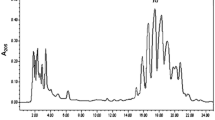
Reactants and products were analyzed by RP-HPLC (Spectra Physic SP 100 coupled with a UV detector Spectra Physic SP 8450) using a reversed-phase column (Ultrabase-C8, 150 × 4.6 mm, 5 μm). Products were eluted at a flow rate of 1.5 ml/min with acetonitrile/water/CH3COOH (80:20:0.1 v:v) and pH 3. The UV detection was performed at 215 nm. At this wavelength, synthetic yields could be easily calculated from the pure peak areas corresponding to eicosapentaenoic acid (EPA) ethyl ester [retention time (RT) of 9 min] and docosahexaenoic acid (DHA) ethyl ester (RT of 12 min).
Thermal Inactivation of Different Immobilized Lipases
Enzyme derivatives were equilibrated with the solvent (tert-amyl alcohol or cyclohexane) at the desired temperature (45 °C). Subsequently, at different times, 0.1 g of catalyst incubated in the inactivation conditions was added to the synthesis reaction solution, and the activity was checked using the enzymatic synthesis assay described in the previous section. Relative activity is expressed as the ratio of activity at derivatives incubated at different times and the initial enzyme activity of each derivative before incubation in the solvent. Experiments were conducted in triplicate, and the standard error was always under 5 %.
Results
Ethanolysis of Fish Oil by Different Immobilized Lipases
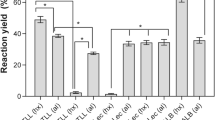
Lipase derivatives with the active center stabilized by adsorption on hydrophobic supports (Sepabeads C18, C18Sep) [16] were utilized were utilized for enzymatic ethanolysis of sardine oil. Enzymatic ethanolysis in two different solvents (cyclohexane and tert-amyl alcohol) was carried out at 25 °C (Tables 1, 2). In spite of the stabilization of the active center, the functional properties (e.g., activity and EPA/DHA selectivity) of the derivatives were different in each solvent. CALB discriminated poorly between EPA and DHA in both solvents; its activity greatly increased in tert-amyl alcohol, but its selectivity decreased. TLL was very active and selective in cyclohexane (EE-EPA/EE-DHA = 29) and showed similar activity but decreased selectivity in tert-amyl alcohol. RML was more active in tert-amyl alcohol than in cyclohexane, and its selectivity was similar and quite good in both solvents (EPA/DHA = 13). The selectivity is defined as the relationship between the initial rate of release EE-EPA and EE-DHA.
Ethanolysis of Fish Oil by Different Lipases Immobilized on Different Supports
The same lipase immobilized on different supports exhibited different functional properties (Tables 3, 4) [21]. For example, derivatives obtained by ionic exchange exhibited very low activity and selectivity. In these derivatives, the active center of the lipases directly interacts with the solvent. In the other two derivatives, the active center is stabilized on hydrophobic supports (C18-Sep and Lewatit), the use of hydrophobic supports allows adsorption of lipase through the lid, so stabilizing the active center of lipase [21].
Derivatives of lipases adsorbed on Lewatit were less active and less selective than derivatives on C18-Sep. One possible explanation for this difference is the varying morphology or surface structure between the two supports, with C18Sep being more purely hydrophobic. The best TLL derivative working in cyclohexane is the best alternative in terms of activity-selectivity.
Physico-Chemical Modification of Lipase Derivatives
Activity and Selectivity
Modification of the TLL derivatives showed very good activity and selectivity properties. As an exception, amination (conversion of Asp and Glu into amino groups) promoted a decrease in activity and selectivity, which was recovered after coating the derivative with dextran sulfate (Table 5). Modifications of RML improved selectivity (from 13 up to 23) but in some cases caused a decrease in activity (Table 6).
Stability
In general, stability of derivatives decreased after modification. For example, amination or succinylation of both enzymes strongly decreased stability; however, an improvement in stability was observed after coating both derivatives with PEI. Coated TLL derivatives preserved 100 % of activity after 24 h in cyclohexane, and the activity of the least stable derivatives decreased to 50 % (Fig. 1). Coated derivatives of RML preserved 100 % of their activity after 24 h in tert-amyl alcohol, and the least stable derivatives exhibited a half-life of 1 h under the same conditions (Fig. 2).
Time-course of inactivation of TLL derivatives in dried cyclohexane. All derivatives were obtained by adsorption of TLL on Sepabeads C18. Physico chemical modifications: squares coating with polyethyleneimine, rhombus unmodified derivatives, crosses aminated derivative, triangles succinylated derivatives. Derivatives were incubated in dried cyclohexane in the presence of molecular sieves. At different times aliquots were withdrawn and assay for ethanolysis as described in “Methods”
Time-course of inactivation of RML derivatives in dried tert-amyl alcohol. All derivatives were obtained by adsorption of RML on Sepabeads C18. Physico chemical modifications: squares coating with polyethyleneimine, rhombus unmodified derivatives, crosses aminated derivative, triangles succinylated derivatives. Derivatives were incubated in dried tert-amyl alcohol in the presence of molecular sieves. At different times aliquots were withdrawn and assay for ethanolysis as described in “Methods”
The TLL derivatives in cyclohexane and coated with PEI exhibited the best properties after a simultaneous consideration of activity-selectivity-stability parameters. The same derivatives of RML have also an interesting selectivity in tert-amyl alcohol, but the catalytic activity is sixfold lower.
Time-Course of Enzymatic Ethanolysis
The time-course of ethanolysis of EPA was studied with the TLL derivatives (Fig. 3). The discrimination between EPA and DHA was very high, up to 60–75 % of ethanolysis, but the reaction rate decreased drastically, and the ethanolysis of DHA increased from these conversions. At conversions around 75 % (16.5 mM of ethyl ester of EPA), reached after 3 h at 25 °C by using a ratio of reaction volume/catalyst volume of 50. Mixtures of the esters of omega-3 fatty acids were obtained with 95 % purity for the ethyl ester of EPA versus the ethyl ester of DHA. Similar results were obtained with RML in tert-amyl alcohol with lower reaction rates.
Time course of ethanolysis of omega-3 fatty acids in cyclohexane catalyzed by TLL adsorbed on Sepabeads C18 and coated with PEI. Symbols: rhombus release of ethyl ester of EPA, squares release of ethyl ester of DHA. Arrow indicates an optimal reaction mixture with more than 90 % of purity in ethyl ester of EPA
Conclusions
The three lipases studied exhibited different functional properties for the ethanolysis of sardine oil. Interestingly, different derivatives of each lipase also exhibit quite different catalytic properties. Furthermore, two derivatives obtained with different hydrophobic supports were also different. The selectivity EPA/DHA of TLL varied from 4 to 29.
The ethanolysis could be performed enzymatically at 25 °C; therefore, the omega-3 fatty acids were minimally modified, and selectivity of enzymatic processes could be obtained (up to 20–29 molar ratios).
Physico-chemical modification of the lipase derivatives mainly affected selectivity and stability. The PEI coating of the derivatives adsorbed on hydrophobic supports (C18-Sep) greatly improved both selectivity and stability for RML in tert-amyl alcohol and stability only for TLL in cyclohexane.
TLL adsorbed on hydrophobic supports and coated with PEI exhibited the best activity-selectivity-stability properties.
References
Fernandez L, Banuelos O, Zafra A, Ronchel C, Perez-Victoria I, Morales JC, Velasco J, Adrio JL (2008) Alteration of substrate specificity of Galactomyces geotrichum BT107 lipase I on eicosapentaenoic acid-rich triglycerides. Biocatal Biotransform 26:296–305
Heird WC (2001) The role of polyunsaturated fatty acids in term and preterm infants and breastfeeding mothers. Pediatr Clin North Am 48:173–188
Demaison L, Moreau D (2002) Dietary n-3 polyunsaturated fatty acids and coronary heart disease-related mortality: a possible mechanism of action. Cell Mol Life Sci 59:463–477
Saremi A, Arora R (2009) The utility of omega-3 fatty acids in cardiovascular disease. Am J Ther 16:421–436
Antypa N, Van Der Does AJW, Smelt AHM, Rogers RD (2009) Omega-3 fatty acids (fish-oil) and depression-related cognition in healthy volunteers. J Psychopharmacol 23:831–840
Bougnoux P, Hajjaji N, Maheo K, Couet C, Chevalier S (2010) Fatty acids and breast cancer: sensitization to treatments and prevention of metastatic re-growth. Prog Lipid Res 49:76–86
Kralovec JA, Zhang S, Zhang W, Barrow CJ (2012) A review of the progress in enzymatic concentration and microencapsulation of omega-3 rich oil from fish and microbial sources. Food Chem 131:639–644
Yan J, Liu S, Hu J, Gui X, Wang G, Yan Y (2011) Enzymatic enrichment of polyunsaturated fatty acids using novel lipase preparations modified by combination of immobilization and fish oil treatment. Bioresour Technol 102:7154–7158
Treichel H, Oliveira D, Mazutti MA, Luccio MD, Oliveira JV (2010) A review on microbial lipases production. Food Bioprocess Technol 3:182–196
Haraldsson GG, Kristinsson B, Sigurdardottir R, Gudmundsson GG, Breivik H (1997) The preparation of concentrates of eicosapentaenoic acid and docosahexaenoic acid by lipase catalyzed transesterification of fish oil with ethanol. J Am Oil Chem Soc 74:1419–1424
Fernández-Lorente G, Pizarro C, López-Vela D, Betancor L, Carrascosa AC, Pessela B, Guisan JM (2011) Hydrolysis of fish oil by lipases immobilized inside porous supports. J Am Oil Chem Soc 88:819–826
Medina AR, Cerdán LE, Giménez AG, Páez BC, Ibáñez González MJ, Grima EM (1999) Lipase-catalyzed esterification of glycerol and polyunsaturated fatty acids from fish and microalgae oils. J Biotechnol 70:379–391
Rozieres MS, Deyris V, Comeau LC (2000) Enrichment of polyunsaturated fatty acids from sardine cannery effluents by enzymatic selective esterification. J Am Oil Chem Soc 77:329–332
Muñío MDM, Esteban L, Robles A, Hita E, Jiménez MJ, González PA, Camacho B, Molina E (2008) Synthesis of 2-monoacylglycerols rich in polyunsaturated fatty acids by ethanolysis of fish oil catalyzed by 1,3 specific lipases. Process Biochem 43:1033–1039
Fernandez-Lorente G, Filice M, Lopez-Vela D, Pizarro C, Wilson L, Betancor L, Avila Y, Guisan JM (2011) Cross-linking of lipases adsorbed on hydrophobic supports: highly selective hydrolysis of fish oil catalyzed by RML. J Am Oil Chem Soc 88:801–807
Bastida A, Sabuquillo P, Armisen P, Fernández-Lafuente R, Huguet J, Guisan Jose M (1998) A single step purification, immobilization, and hyperactivation of lipases via interfacial adsorption on strongly hydrophobic supports. Biotechnol Bioeng 58:486–493
Fernández-Lorente G, Godoy CA, Mendes AA, Lopez-Gallego F, Grazu V, de las Rivas B, Guisan Jose M (2008) Solid-phase chemical amination of a lipase from Bacillus thermocatenulatus to improve its stabilization via covalent immobilization on highly activated glyoxyl-agarose. Biomacromolecules 9:2553–2561
Carraway KL, Koshland DE Jr (1968) Reaction of tyrosine residues in proteins with carbodiimide reagents. Biochim Biophys Acta 160:272–274
Montes T, Grazú V, Manso I, Galán B, López-Gallego F, González R, Hermoso JA, García JL, Guisán JM, Fernández-Lafuente R (2007) Improved stabilization of genetically modified penicillin G acylase in the presence of organic cosolvents by co-immobilization of the enzyme with polyethyleneimine. Adv Synth Catal 349:459–464
Montes T, Grazu V, López-Gallego F, Hermoso JA, Guisán JM, Fernández-Lafuente R (2006) Chemical modification of protein surfaces to improve their reversible enzyme immobilization on ionic exchangers. Biomacromolecules 7:3052–3058
Mateo C, Palomo JM, Fernandez-Lorente G, Guisan JM, Fernandez-Lafuente R (2007) Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzyme Microb Technol 40:1451–1463
Acknowledgments
This work was sponsored by the Spanish Ministry of Science and Innovation (project AGL-2009-07526) We gratefully recognize the Spanish Ministry of Science and Innovation for the “Ramón y Cajal” contract for Dr. Fernandez-Lorente and for the FPI contract granted to Sonia Moreno-Perez. We would like to thank Novozymes and Ramiro Martinez for the generous gift of commercial lipases.
Author information
Authors and Affiliations
Corresponding authors
About this article
Cite this article
Moreno-Pérez, S., Guisan, J.M. & Fernandez-Lorente, G. Selective Ethanolysis of Fish Oil Catalyzed by Immobilized Lipases. J Am Oil Chem Soc 91, 63–69 (2014). https://doi.org/10.1007/s11746-013-2348-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11746-013-2348-3