Abstract
Marine microalgae such as Isochrysis sp. and Pavlova sp. are the predominant source of polyunsaturated fatty acids (PUFAs) such as eicosapentaenoic acid (EPA, 20:5n–3) and docosahexaenoic acid (DHA, 22:6n–3). EPA biosynthesis pathway is predominant in lower eukaryotes, and its biosynthetic gene expressions are not well established. Till date, the C18 elongation enzymes for EPA biosynthesis have not been identified from lower eukaryote. In the present study, we describe the identification of two microalgal genes Δ6-elongase and Δ5-desaturase involved for EPA biosynthesis. By PCR-based technique, a novel elongase gene (Δ6Elo-Iso) was isolated from Isochrysis sp., and 654 bp of full-length sequence was identified, which catalysed the conversion of SDA into ETr in E. coli. The identified gene displayed unique substrate specificity for both n-3 and n-6 C18-substrates for Δ6-elongation, with no activity towards Δ9-elongase. In addition, a novel Δ5-desaturase gene (Δ5Des-Pav) was isolated from Pavlova sp. and found an ORF of 1149 bp in length, which was capable of converting ETr into EPA in omega-3 pathway. For the first time, the heterologous expressions of two novel microalgal genes were successfully expressed in Escherichia coli. EPA production from E. coli is being considered as an alternative and economic source for industrial and pharmaceutical sectors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Microalgae are unicellular photosynthetic organisms in an aquatic ecosystem, which are optimistic lipid producers and adopted to grow wide range of environmental conditions with short doubling times, and they have a higher growth rate than the conventional crops [1, 2]. Microalgae are used in several commercial applications such as protein, pigments, feed in animal and aquaculture, biofuel, and polyunsaturated fatty acids (PUFAs). Among microalgae like Pavlova, Isochrysis, and Nannochloropsis are the primary producers of PUFAs in the aquatic ecosystem. The major PUFAs are arachidonic acid (ARA), eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA) and are significantly produced from microalgae by de novo pathway using different desaturase and elongase enzymes. These fatty acids are highly significant for human growth and metabolisms. DHAs are found abundantly in grey matter of the brain and retina of the eyes and are essential for cognitive development, especially in infants. ARA and EPA are potent molecules involved in the production of eicosanoids, prostaglandin, leukotriene, and thromboxane, which are responsible for inflammatory response, reproduction, regulation of blood pressure, blood clotting, and cell signaling [3, 4]. EPA and DHA synthesize intermediate lipid mediators like resolvins (E and D) and protectins that are involved in anti-inflammatory and immune-regulatory processes and also in the prevention and treatment of cancer [5].
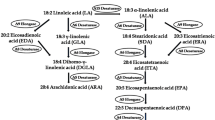
Fish and marine organisms are not synthesizing EPA and DHA, they are obtained from microalgae in marine food chain, and they are enriched with EPA and DHA [6]. The global demand for PUFA has increased from recent years, due to lack of present dietary sources such as decrease of fish population and ocean contamination of heavy metals (lead, cadmium, mercury, etc.); modern food habitat looks for the alternative source of PUFA for human use. Microalgae are considered as a potential alternative source for PUFA. Pavlova sp. is a unicellular brown/golden mircoalga, which is highly enriched with both EPA (18.0 mg g/DW) and DHA (13.2 mg g/DW). They found nearly 80% of PUFA in their total lipid contents [7, 8]. And also marine microalga Isochrysis sp. is enriched with high amounts of EPA and DHA [9]. In recent years, both Isochrysis sp. and Pavlova sp. gained more attention in the aquaculture field as food supplements. They have been used as a potential source for the isolation and identification of PUFA biosynthetic genes such as desaturases and elongases. Among them, Δ6-elongase gene converts the γ-linolenic acid (GLA) into dihomo-gamma-linolenic acid (DGLA) in omega-6 pathway and stearidonic acid (SDA) into eicosatetraenoic acid (ETr) in omega-3 pathway, respectively. And, Δ5-desaturase gene is most important for EPA and ARA biosynthesis, which helps to convert DGLA into arachidonic acid (ARA) in omega-6 pathway and ETr into eicosapentaenoic acid (EPA) in omega-3 pathway, respectively (Fig. 1).
Polyunsaturated fatty acid (PUFA) biosynthesis from lower eukaryotes by two different pathways (ω-3 and ω-6). Different enzymes like desaturases and elongases are involved in the biosynthesis of ARA, EPA, and DHA by two different ways such as classical method mediated by Δ6-desaturase enzyme and alternative method mediated by Δ9-elongase enzyme, respectively
From the past few decades, several attempts have been made from different research groups for the identification and characterization of desaturases and elongases from different organisms such as algae [10, 11], fungi [12], moss [13], higher plants [14], and mammals [15]. But, the commercial outcomes of PUFA from heterologous systems such as plants, yeast, fungi, and microalgae are not achieved yet. There are several recombinant products synthesized and commercialized from Escherichia coli for human use. And the present study also revealed about the identification and functional characterization of fatty acid Δ6-elongase and Δ5-desaturase genes from marine microalgae and its heterologous expression analysed in E. coli. This work is preliminary investigation about algal desaturase and elongase gene expression and functional characterization in E. coli, which may be useful in the PUFA demand for human consumption.
Materials and Method
Organism and Culture Condition
Isochrysis sp. and Pavlova sp. were collected from the Central Marine Fisheries Research Institute (CMFRI), Tuticorin, Tamil Nadu, India, and cultured in Walne medium [16] with light intensity of 18.75 ± 2.5 μmol/m/s at 16:8 h of light and dark cycle at 22 ± 2 °C. Escherichia coli strain DH5α was used for gene cloning, and BL21 (DE3) was used for gene expression and grown in Luria–Bertani medium containing 100 μg/mL ampicillin at 37 °C.
Effects of Nitrogen Stress on Fatty Acid Gene Expression
Nitrate is one of the major nutrient sources in culture medium, and three different nitrate concentrations such as control (100 mg/L), nitrogen-depleted medium (10 mg/L), and nitrogen-repleted medium (500 mg/L) were used in this study. Primary culture of both Isochrysis sp. and Pavlova sp. was grown for 7 days, and the biomass was harvested by centrifugation at 4,000 × g for 5 min and inoculated in the stress-induced medium. At different time intervals like 3rd, 5th, and 7th day, the biomass was harvested from all the treatment cultures. Using this, total RNA was extracted by Tri-RNA Reagent (Favorgen Biotech, Taiwan), and simultaneously first-strand cDNA was synthesized using RevertAid Reverse Transcriptase enzyme (Thermo Fisher Scientific, USA). The fatty acid genes like Δ6-elongase from Isochrysis sp. and Δ5-desaturase from Pavlova sp. genes expression were analysed by qRT-PCR (Table 1).
Isolation and Cloning of Fatty Acid Genes
The algal biomass was harvested by centrifugation at 12, 000 × g for 10 min and frozen with liquid N2. Total RNA was isolated by Tri-RNA Reagent (Favorgen Biotech, Taiwan) as per manufacture’s instruction. The RNA quantity and quality were estimated by nanodrop and 1% MOPS agarose gel electrophoresis, respectively. Using 1 μg RNA, the first-strand cDNA was synthesized using RevertAid Reverse Transcriptase enzyme (Thermo Fisher Scientific, USA). Using Δ6-elongase and Δ5-desaturase gene-specific primers (Table 1), PCR was performed using cDNA as template with initial denaturation at 94 °C for 4 min followed by 35 cycles of denaturation at 94 °C for 30 sec, annealing at 58 °C for 30 sec, and extension at 72 °C for 1 min followed by a final extension at 72 °C for 10 min. The PCR products were analysed in 0.8% agarose gel electrophoresis, and the amplified products were named as Δ6Elo-Iso for Δ6-elongase gene and Δ5Des-Pav for Δ5-desaturase gene, respectively. Further, it was cloned into pXCM vector for Δ6Elo-Iso and pGEMT-easy vector for Δ5Des-Pav; the transformed colonies were screened by blue/white selection followed by colony PCR and restriction digestion, respectively. The positive clones were subjected for sequencing analysis.
Computational Analysis of Δ6Elo-Iso and Δ5Des-Pav
The sequencing service was performed by Shrimpex Biotech, Chennai, India, and the full-length sequence result was acquired. Bioinformatics analysis was performed by different tools such as NCBI-BLAST (www.ncbi.nlm.nih.gov/BLAST), multiple sequence alignment (www.ebi.ac.uk/Tools/msa/clustal), and ORF finder (www.ncbi.nlm.nih.gov/gorf/gorf.html), and the transmembrane domain was analysed by TMpred (http://www.ch.embnet.org/cgi-bin/TMPRED) and was carried out simultaneously. Phylogenetic tree was constructed by Mega (6.1), and protein 3D structure was predicted by protein-homology/analogy recognition engine 2 (PHYRE2—http://www.sbg.bio.ic.ac.uk/phyre2).
Functional Characterization of Δ6Elo-Iso and Δ5Des-Pav
The Δ6Elo-Iso and Δ5Des-Pav gene expression and functional characterization were performed in pGEX-4T2, which has isopropyl β-D-1-thiogalactopyranoside (IPTG)-inducible tac promoter for heterologous expression. The cDNA of Δ6Elo-Iso was amplified with primers having BamH1/EcoR1 restriction sites overhang, and simultaneously Δ5Des-Pav was amplified with primers having EcoR1 and Sal1 restriction sites overhangs. For gene expression, E. coli BL21 (DE3) was used for transformation, and the positive clones were screened by colony PCR and restriction digestion. Protein expression analysis was performed by 1 mM of IPTG, after 4 h cells were harvested and the protein profile was investigated in SDS-PAGE. Meanwhile with the same conditions, the functional characterization of Δ6Elo-Iso was analysed in the presence of external fatty acid substrates like 50 μM of linoleic acid (LA), alpha-linolenic acid (ALA), gamma-linolenic acid (GLA), and stearidonic acid (SDA). And simultaneously, Δ5Des-Pav activity was performed in the presence of external fatty acid substrates like 25 μM of DGLA and ETr separately. Control samples contain empty pGEX-4T2 plasmid in E. coli BL21 (DE3). The recombinant E. coli was inoculated as a primary culture overnight, and 1% inoculum was added to the secondary culture and allowed to grow up to the OD600 and reaches 0.4. And the respective fatty acid substrates were added separately in the culture medium and allowed to grow for 12 h. The biomass was harvested and the fatty acid profile was analysed in GC-MS.
Fatty Acid Analysis
The total lipid from E. coli was extracted by chloroform/methanol (2/1), and fatty acid methyl ester (FAME) was prepared using methanol/acetyl chloride (9.5:0.5) by a standard method [17]. The samples were analysed in GC-MS (MS-QP2020; Shimadzu, Japan) with a non-polar capillary column RX-5SIL MS (30 m length, 0.25 mm inner diameter, and 0.25 μm thickness). GC-MS programme was run as started 120 °C and maintained for 5 min followed by an increase of 5 °C/min and finally peaking at 280 °C for 10 min.
Results
Identification and Sequence Analysis of Δ6Elo-Iso
The full length of Δ6-elongase gene was amplified from cDNA of Isochrysis sp. that gave amplification at 0.65 kb in size. The PCR amplified product was cloned into pXCM vector, the recombinant colony was screened by colony PCR, and restriction digestion analysis showed the gene of interest at 0.65 kb in size (Supplementary Fig. S1). The positive colony was subjected for sequence analysis, and the full-length sequence was retrieved (NCBI accession number MK579398). The isolated gene was confirmed as elongase using different bioinformatic tools. Protein BLAST analysis of Δ6Elo-Iso showed 99% high similarity to elongase of Isochrysis galbana (ADD51571.1) and 37% identity to Δ6-elongase of Pyramimonas cordata (ACR53359.1). Simultaneously, the multiple sequence alignment of Δ6Elo-Iso protein sequence with other available sequences showed that the major conserved domains for elongase are KxxExxDT, SFLxxxHH, MYxYY, and QxxQF (Fig. 2). The single ORF was detected as 654 bp in length having a polypeptide of 217 amino acids, which is the functional region of Δ6-elongase. Generally, elongase gene is a membrane-bound protein and Δ6Elo-Iso sequence results also found in the transmembrane domain regions. Phylogenetic tree analysis of Δ6Elo-Iso has close association with Δ6-elongase of Rebecca salina (AAY15135.1) followed by Phaeodactylum tricornutum (XP_002179048.1) and Pythium sp. (AIG53905.1) (Supplementary Fig. S2a). These bioinformatic analyses revealed that the isolated gene belongs to Δ6-elongase from Isochrysis sp.
Multiple sequence alignment of deduced amino acid sequences of Δ6-elongase (6Elo) from Isochrysis sp. (QEP54295.1) with other fatty acid elongase genes like Rebecca salina (AAY15135.1), Physcomitrella patens (AFU35740.1), Pyramimonas cordata (ACR53359.1), Ostreococcus tauri (AAV67797.1), Phaeodactylum tricornutum (XP_002179048.1), and Pythium sp. BCC53698 (AIG53905.1). The functional and identical amino acids of Δ6-elongase showed four histidine-rich domains KxxExxDT, SFLxxxHH, MYxYY, and QxxQF which are mentioned as H1, H2, H3, and H4 boxes
Functional Characterization of Δ6Elo-Iso
The single ORF of Δ6Elo-Iso was mobilized into pGEX-4T2 vector, and the recombinant colonies were screened by colony PCR which showed the amplification at 0.65 kb in size, and further it was confirmed by restriction digestion with BamHI/EcoRI that released the gene of interest at 0. 65 kb. The Δ6Elo-Iso protein was induced by 1 mM IPTG, and the protein profile in SDS-PAGE showed the overexpressed protein nearly at 50 kDa which is not observed in the wild pGEX-4T2 (Supplementary Fig. S3). The different fatty acid substrates such as LA, ALA, GLA, and SDA were added externally to the control and recombinant E. coli. The GC-MS analysis showed that GLA-supplemented culture produced a new peak in the recombinant E. coli that corresponds to dihomo-gamma-linolenic acid (DGLA) in omega-6 pathway. Even though the functional characterization of Δ6Elo-Iso was studied by different fatty acids (specific for Δ6-elongase and Δ9-elongase), the isolated gene utilized GLA more favourably and produced the product, DGLA. This confirms that the isolated gene has Δ6-elongation activity. Simultaneously, in the presence of SDA, the Δ6Elo-Iso was functionally active and produced eicosatetraenoic acid (ETr) in the recombinant E. coli which is not observed in the control and that confirms the presence of elongation activity (Fig. 3a, b). The isolated gene Δ6Elo-Iso from Isochrysis sp. was found in the long-chain fatty acid production in both omega-6 and omega-3 pathway such as GLA and ETr in recombinant E. coli.
Functional characterization of PUFA biosynthesis genes in E. coli. GC-MS results for control and recombinant E. coli cultured supplemented with precursor fatty acid substrates such as GLA (a), SDA (b), DGLA (c), and ETr (d), respectively. E. coli expression was induced by 1 mM of IPTG. The recombinant E. coli harbouring Δ6Elo-Iso plasmids showed Δ6-elongase activity and produced corresponding fatty acids like DGLA (a) and Etr (b). Meanwhile, the recombinant E. coli having Δ5Des-Pav plasmid showed desaturation activity towards the production of new fatty acids like ARA (c) and EPA (d), respectively. New fatty acids were marked as asterisk, and its mass fragmentation was analysed by GC-MS
Identification and Sequence Analysis of Δ5Des-Pav
The full-length Δ5-desaturase gene was amplified from microalga Pavlova sp. using respective gene-specific primers, which gave nearly 1.2 kb amplified product. Further, it was mobilized into pGEMT-easy vector, and the recombinant clones were identified by colony PCR, and restriction analysis showed the presence of 1.2 kb gene of interest (Supplementary Fig. S4). The sequence analysis of Δ5Des-Pav revealed 1169 bp (NCBI GenBank accession no KR062003) containing an ORF of 1149 bp that encodes 382 amino acids. The different bioinformatic analyses such as protein BLAST of Δ5Des-Pav showed 85.2% similarity with Δ5-desaturase sequence of Isochrysis galbana (AFD22890), and the multiple sequence alignment detected the functional regions of Δ5-desaturase conserved histidine motifs (HEGGH, HNKHH, and QIEHH) and cytochrome b5 region (HPGG) (Fig. 4). The phylogenetic analysis of Δ5Des-Pav has close association with microalgae Isochrysis galbana (Supplementary Fig. S2b), and the transmembrane domain analysis of Δ5Des-Pav sequences was located in the inner, outer, and in-between the cell membrane regions. Even the protein 3D structure also found that Δ5Des-Pav has 70% sequence similarity and 99.9% confidence with human strearoyl coA-desaturase2 (Supplementary Fig. S5a, b). These bioinformatic analyses confirmed that the isolated gene Δ5Des-Pav belongs to front-end desaturase family.
Multiple sequence alignment of deduced amino acid sequence of Δ5-desaturase from Pavlova sp. (5DPav: ALE15225) with the other available known desaturase sequences from different organisms like Thraustochytrium sp. FJN-10 (FAD5: ACD03117), Thraustochytrium sp. ATCC21685 (FAD52: AAM09687), Pavlova salina (FAD53: ABL96295), Ostreococcus tauri (FAD6: AAW70159), and M. squamata (FAD62: CAQ30479). Δ5-desaturase functional and conserved histidine domains (HEGGH, HNKHH, and QIEHH) and cytochrome b5 (HPGG) domain are marked as boxes
Functional Characterization of Δ5Des-Pav
The ORF of Δ5Des-Pav was amplified from pGEMT vector and further cloned into expression vector pGEX-4T2. The recombinant clones were identified by colony PCR and restriction digestion that showed the gene of interest at 1.2 kb in size (Supplementary Fig. S4). Protein expression of Δ5Des-Pav was induced by 1 mM IPTG, and the result showed the overexpressed protein found at 70 kDa in size (Δ5Des-Pav is 44 kDa and N-terminal GST protein is 26 kDa in size) (Supplementary Fig. S6). For the functional characterization of Δ5Des-Pav, external fatty acid substrates like DGLA and ETr were supplied to the E. coli culture medium. The fatty acid profile of recombinant E. coli showed the new fatty acid peaks corresponding to arachidonic acid (ARA) and eicosapentaenoic acid (EPA), respectively, which is not found in the control (Fig. 3c, d). The Δ5Des-Pav functional activity was involved in both omega-3 and omega-6 pathways and produced significant levels of EPA and ARA in the heterologous system E. coli.
Effects of Nitrogen Stress on Δ6Elo-Iso and Δ5Des-Pav Gene Expressions
In the different nitrogen treatments, microalgae Isochrysis sp. and Pavlova sp., were cultured at 100 mg/L as control (nitrogen concentration in the Walne medium). The qRT-PCR results for Δ6Elo-Iso described that maximum gene expression was found in both nitrogen-repleted and -depleted culture. The results showed that nearly 1.7-fold higher gene expression was quantified under nitrogen treatment cultures at the 3rd day. As well as, the highest Δ5Des-Pav expression was detected as 3.3-fold after 500 mg/L nitrate treatment for 3 days (Fig. 5). However, both the fatty acid genes’ expression levels were gradually decreased with increasing the treatment days. For example, Δ6Elo-Iso gene expression was showed gradual decreases from 3rd to 7th days under both nitrogen-repleted and -depleted treatments. But, in case of Δ5Des-Pav, nitrogen-repleted condition was initially decreased at 0.4-fold from 3rd day culture, and it was gradually increased as 1.2-fold at 7th day treatment. The maximum fatty acid genes expression was found at early logarithmic phase of culture conditions in both Isochrysis sp. and Pavlova sp., respectively.
Discussion
The role of PUFAs in human growth and development has been well described, and especially DHA, EPA, and ARA are having many significant roles in several metabolic processes in major systems including cardiovascular, immune, nervous, reproductive system, etc. For humans, the present available dietary sources of PUFAs are from marine organisms. But due to pollution and toxicity nature of marine environment, there is a need of alternative sources. Recombinant strategies such as heterologous production of PUFA in plants, microalgae, fungi, and yeast have also been attempted in the recent past, and here we are establishing the heterologous production of PUFAs in E. coli. We had already collected and identified the marine microalgae species for isolation and functional characterization of different PUFA genes. We have also described the isolation of Δ6-desaturase gene from Isochrysis sp. functionally characterized and successfully expressed in E. coli for recombinant production of GLA and SDA [10]. For successful recombinant production of PUFAs from E. coli, several other PUFA biosynthetic genes are needed which are mainly available in marine microalgae that can be explored for the heterologous production in E. coli.
The present study discusses about the isolation of novel Δ6Elo-Iso from Isochrysis sp. and Δ5Des-Pav from Pavlova sp. which are crucial for EPA biosynthesis. Δ6-elongase gene is specific for the conventional PUFA pathway that acts as an intermediate in the EPA and DHA biosynthesis. Only few C18-elognase genes were identified and functionally characterized from Ostreococcus lucimarinus [18], Myrmecia incisa [19], Physcomitrella patens [20], and Pythium sp. [21]. But their expression levels and product conversion in heterologous system are not fulfilled due to the bottleneck in the gene [22]. And still isolation and characterization of elongase gene from different organisms are being attempted in the recent years for PUFA pathway construction and heterologous production. Microalgae are the major producers of PUFA in marine ecosystem, and especially Isochrysis sp. is highly enriched with EPA and DHA due to the presence of major PUFA biosynthetic genes. Using Δ6-elongase gene-specific primers, the full-length sequence of 0.65 kb was retrieved that possessed major conserved domains like KxxExxDT, SFLxxxHH, MYxYY, and QxxQF and has been categorized for typical C18-elongase gene family [23, 24]. These conserved regions were found in the transmembrane helices that are similar to elongases from microalga Ostreococus tauri [25] and the oomycetes Pythium sp. [21]. The fatty acids like LA and ALA (substrate for Δ9-elongase) and GLA and SDA (substrate for Δ6-elongase) were provided separately, and the functions of isolated gene were evaluated. However, Δ6Elo-Iso showed activity towards Δ6-elongase specific substrates that produced new fatty acids such as dihomo-gamma-linolenic acid (DGLA) and eicosatetraenoic acid (ETr), which is not present in control. There were no Δ9-elongase responsible products found in GC-MS analysis. Similarly, Jeennor et al. [21] also described the isolation and functional characterization of C18-elongase gene from Pythium sp., which is more specific for Δ6-elongase functional activity. Likewise, Jiang et al. [11] have identified Δ5-elongase gene from Phaeodactylum, and its functional characterization was studied in Pichia pastoris. Identification of Δ6 and Δ5-elongase genes from different organisms showed consistent expression in heterologous system for both ω-3 and ω-6 fatty acids production. From our knowledge, this is first reported that Δ6-elongase gene has been functionally characterized in E. coli, whereas the previous studies were limited to Saccharomyces cerevisiae [25, 26].
Eicosapentaenoic acid (EPA) and arachidonic acid (ARA) production in E. coli required an additional enzyme to convert DGLA into ARA in omega-6 pathway and ETr into EPA in omega-3 pathway. The respective Δ5-desaturase gene was isolated from microalga Pavlova sp. and characterized in E. coli. The full length 1186 bp of Δ5-desaturase gene was isolated using respective gene-specific primers, which encodes 382 amino acid sequences, and having three conserved histidine domains such as HEGGH, HNKHH, and QIEHH and cytochrome b5 domain fused to the N-terminal extremity [27]. These results illustrated that the isolated gene has front-end desaturase features with high similarity to other Δ5-desaturase genes from Phaeodactylum tricornutum [28] and Mortierella alpina [29]. Heterologous expression of Δ5Des-Pav in E. coli confirmed its functional activity by producing the corresponding fatty acids like EPA and ARA (Fig. 3c, d) in the presence of external fatty acid substrates. However, the low desaturation activity was found in the E. coli, due to heterologous expression modulating factors like formation of inclusion bodies, protein folding, mistargeting, etc. A similar low activity was found in yeast for the expression of Δ12 and Δ5-desaturase genes from O. tauri and Chlorella vulgaris [30, 31]. The efficiency of Δ5Des-Pav expression is similar in both ω-3 and ω-6 fatty acid pathway, which does not show any substrate preference in this study. Domergue et al. [28] also described the identification and functional characterization of Δ6 and Δ5-desaturase gene from Phaeodactylum tricornutum, which produced both n-3 and n-6 fatty acid in equal efficiency. Guihéneuf et al. [8] examined about the biosynthesis of EPA in Pavlova sp. through Δ17-desaturation pathway by radiolabeled substrates, and the Δ5-desaturase gene effectively converted the radiolabeled eicosatetraenoic acid (ETr) into EPA at higher rate, and further it extends to produce DHA (see Fig. 1).
Nutrient is one of the major stress factor influences on microalgal growth and metabolism. As nitrogen plays a significant role in biomass and lipid production, previous reports suggested that the concentrations of nitrogen affect the proportion of saturated and unsaturated fatty acid production [32]. This was also confirmed in the present study that Pavlova sp. was cultured at higher nitrogen concentration (500 mg/L) and enhanced the Δ5Des-Pav expression of 3.3-fold higher than control. Simultaneously, Δ6Elo-Iso expression also found to be 1.7-fold higher in both nitrogen-repleted and -depleted conditions. The maximum gene expression and PUFA production were observed at 3rd day culture, which showed that the early logarithmic phase is more suitable for PUFA production, and even microalga Tetraselmis sp. also exhibits the similar gene expression pattern at early logarithmic phase [33]. Generally, low nitrogen concentration improves the high lipid production, and high nitrogen concentration helps to enhance the PUFA production in the microalgae culturing condition [34]. This also proved from the previous studies that high EPA production in Phaeodactylum tricornutum was achieved in nitrogen-rich condition [35].
In the past few decades, several PUFA biosynthetic genes were isolated from Mortierella alpina, Pythium irregulare, Thraustochytrium sp., Phaeodactylum tricornutum, Parietochloris incise, and Caenorhabditis elegans, and the PUFA biosynthesis was attempted in Pichia pastoris, Saccharomyces cerevisiae, and other fungi and plants. But, PUFA production from these organisms was limited and requires more time to achieve maximum production. Although, an attempt has been already made to achieve recombinant production of PUFAs in Yarrowia lipolytica [36], the high cost effects of PUFA production can be minimized if E. coli may be explored as an alternative source.
Previous studies strongly suggest that E. coli can produce EPA and DHA in their system. Amiri-Jami et al. [37] reported that a sequence of gene cluster was isolated from bacterium, Shewanella baltica, and functionally expressed in E. coli Nissle 1917 that produces high amount of EPA (31.36 mg/g cell dry weight) by polyketide synthase pathway. And also, Cahoon et al. [38] described that the plant Δ6-palmitoyl ACP desaturase gene helps to convert palmitic acid (C16:1) into stearic acid (C18:1) in E. coli by acetyl-CoA fatty acid synthesis. From these studies, it has been confirmed that E. coli can be used as an alternative organism for heterologous PUFA production. The previous report from our group successfully expressed Δ6-desaturase gene from Isochrysis sp. in E. coli and produced GLA and SDA. And the present study describes the isolation of two new novel genes like Δ6-elongase and Δ5-desaturase from Isochrysis sp. and Pavlova sp. to extend the PUFA biosynthesis pathway up to EPA and ARA synthesis in E. coli. In the future, PUFA biosynthetic pathway will be constructed in E. coli using these microalgal genes, and subsequent optimization is required to enhance the PUFA level for commercial outcomes and hope that it helps to overcome the demand of PUFA both in health sector and nutraceutical industries.
References
Mata, T. M., Martins, A. A., & Caetano, N. S. (2010). Microalgae for biodiesel production and other applications: a review. Renewable & Sustainable Energy Reviews, 14, 217–232.
Wu, L. F., Chen, P. C., & Lee, C. M. (2013). The effects of nitrogen sources and temperature on cell growth and lipid accumulation of microalgae. International Biodeterioration & Biodegradation, 85, 506–510.
Kinsella, J. E., Lokesh, B., Broughton, S., & Whelan, J. (1990). Dietary polyunsaturated fatty acids and eicosanoids: potential effects on the modulation of inflammatory and immune cells: an overview. Nutrition, 6, 24–44.
Greene, E. R., Huang, S., Serhan, C. N., & Panigrahy, D. (2011). Regulation of inflammation in cancer by eicosanoids. Prostaglandins & Other Lipid Mediators, 96(1-4), 27–36.
Lee, J. M., Lee, H., Kang, S., & Park, W. J. (2016). Fatty acid desaturases, polyunsaturated fatty acid regulation, and biotechnological advances. Nutrients, 8, 23.
Sargent, J. R., Tocher, D. R., & Bell, J. G. (2002). The lipids. Fish Nutrition, 3, 181–257.
Patil, V., Källqvist, T., Olsen, E., Vogt, G., & Gislerød, H. (2007). Fatty acid composition of 12 microalgae for possible use in aquaculture feed. Aquaculture International, 15, 1–9.
Guihéneuf, F., Ulmann, L., Mimouni, V., & Tremblin, G. (2013). Use of radiolabeled substrates to determine the desaturase and elongase activities involved in eicosapentaenoic acid and docosahexaenoic acid biosynthesis in the marine microalga Pavlova lutheri. Phytochemistry, 90, 43–49.
Mohy El-Din, S. (2019). Accumulation of lipids and triglycerides in Isochrysis galbana under nutrient stress. Applied Biochemistry and Biotechnology, 189(2), 359–373. https://doi.org/10.1007/s12010-019-02997-0.
Thiyagarajan, S., Arumugam, M., Senthil, N., Vellaikumar, S., & Kathiresan, S. (2018). Functional characterization and substrate specificity analysis of Δ6-desaturase from marine microalga Isochrysis sp. Biotechnology Letters, 40(3), 577–584.
Jiang, M., Guo, B., Wan, X., Gong, Y., Zhang, Y., & Hu, C. (2014). Isolation and characterization of the diatom Phaeodactylum Δ5-elongase gene for transgenic LC-PUFA production in Pichia pastoris. Marine Drugs, 12(3), 1317–1334.
Huang, J. Z., Jiang, X. Z., Xia, X. F., Yu, A. Q., Mao, R. Y., Chen, X. F., & Tian, B. Y. (2010). Cloning and functional identification of delta5 fatty acid desaturase gene and its 5′-upstream region from marine fungus Thraustochytrium sp. FJN-10. Marine Biotechnology, 13, 12–21.
Kaewsuwan, S., Cahoon, E. B., Perroud, P. F., Wiwat, C., Panvisavas, N., Quatrano, R. S., Cove, D. J., & Bunyapraphatsara, N. (2006). Identification and functional characterization of the moss Physcomitrella patens delta(5)-desaturase gene involved in arachidonic and eicosapentaenoic acid biosynthesis. The Journal of Biological Chemistry, 281(31), 21988–21997.
Li, L., Wang, X., Gai, J., & Yu, D. (2007). Molecular cloning and characterization of a novel microsomal oleate desaturase gene from soybean. Journal of Plant Physiology, 164, 1516–1526.
Park, W. J., Kothapalli, K. S., Lawrence, P., Tyburczy, C., & Brenna, J. T. (2009). An alternate pathway to long-chain polyunsaturates: the FADS2 gene product Δ8-desaturates 20:2n-6 and 20:3n-3. Journal of Lipid Research, 50(6), 1195–1202.
Walne, P. R. (1970). Studies on the food value of nineteen genera of algae to juvenile bivalves of the genera Ostrea, Crassostrea, Mercenaria, and Mytilus. Fishery Investigations, 26, 1–62.
Christie, W. W. (1982). Lipid analysis (2nd ed.). New York: Pergamon Press.
Petrie, J. R., Liu, Q., Mackenzie, A. M., Shrestha, P., Mansour, M. P., Robert, S. S., Frampton, D. F., Blackburn, S. I., Nichols, P. D., & Singh, S. P. (2010). Isolation and characterisation of a high-efficiency desaturase and elongases from microalgae for transgenic LC-PUFA production. Marine Biotechnology, 12(4), 430–438.
Yu, S. Y., Li, H., Tong, H., Ouyang, L. L., & Zhou, Z. G. (2012). Identification of a Δ6 fatty acid elongase gene for arachidonic acid biosynthesis localized to the endoplasmic reticulum in the green microalga Myrmecia incisa Reisigl. Gene, 493, 219–227.
Eiamsa-ard, P., Kanjana-Opas, A., Cahoon, E. B., Chodok, P., & Kaewsuwan, S. (2013). Two novel Physcomitrella patens fatty acid elongases (ELOs): identification and functional characterization. Applied Microbiology and Biotechnology, 97(8), 3485–3497.
Jeennor, S., Cheawchanlertfa, P., Suttiwattanakul, S., Panchanawaporn, S., Chutrakul, C., & Laoteng, K. (2014). Novel elongase of Pythium sp. with high specificity on D6-18C desaturated fatty acids. Biochemical and Biophysical Research Communications, 450, 507–512.
Qi, B., Fraser, T., Mugford, S., et al. (2004). Production of very long chain polyunsaturated omega-3 and omega-6 fatty acids in plants. Nature Biotechnology, 22, 739–745. https://doi.org/10.1038/nbt972.
Parker-Barnes, J. M., Das, T., Bobik, E., Leonard, A. E., Thurmond, J. M., Chaung, L. T., Huang, Y. S., & Mukerji, P. (2000). Identification and characterization of an enzyme involved in the elongation of n-6 and n-3 polyunsaturated fatty acids. Proceedings of the National Academy of Sciences of the United States of America, 97, 8284–8289.
Leonard, A. E., Pereira, S. L., Sprecher, H., & Huang, Y. S. (2004). Elongation of long-chain fatty acid. Progress in Lipid Research, 43(1), 36–54.
Meyer, A., Kirsch, H., Domergue, F., Abbadi, A., Sperling, P., Bauer, J., Cirpus, P., Zank, T. K., Moreau, H., Roscoe, T. J., Zähringer, U., & Heinz, E. (2004). Novel fatty acid elongases and their use for the reconstitution of docosahexaenoic acid biosynthesis. Journal of Lipid Research, 45(10), 1899–1909.
Kajikawa, M., Matsui, K., Ochiai, M., et al. (2008). Production of arachidonic and eicosapentaenoic acids in plants using bryophyte fatty acid Δ6-desaturase, Δ6-elongase, and Δ5-desaturase genes. Bioscience, Biotechnology, and Biochemistry, 72, 435–444.
Hashimoto, K., Yoshizawa, A. C., Okuda, S., Kuma, K., Goto, S., & Kanehisa, M. (2008). The repertoire of desaturases and elongases reveals fatty acid variations in 56 eukaryotic genomes. Journal of Lipid Research, 49(1), 183–191.
Domergue, F., Lerchl, J., Zähringer, U., & Heinz, E. (2002). Cloning and functional characterization of Phaeodactylum tricornutum front-end desaturases involved in eicosapentaenoic acid biosynthesis. European Journal of Biochemistry, 269(16), 4105–4113.
Knutzon, D. S., Thurmond, J. M., Huang, Y. S., Chaudhary, S., Bobik Jr., E. G., Chan, G. M., Kirchner, S. J., & Mukerji, P. (1998). Identification of Delta5-desaturase from Mortierella alpina by heterologous expression in Bakers' yeast and canola. Journal of Biological Chemistry, 273(45), 29360–29366.
Hoffmann, M., Wagner, M., Abbadi, A., Fulda, M., & Feussner, I. (2008). Metabolic engineering of ω3-very long chain polyunsaturated fatty acid production by an exclusively acyl-CoA-dependent pathway. Journal of Biological Chemistry, 283(33), 22352–22362.
Lu, Y., Chi, X., Yang, Q., Li, Z., Liu, S., Gan, Q., & Qin, S. (2009). Molecular cloning and stress-dependent expression of a gene encoding D12-fatty acid desaturase in the Antarctic microalga Chlorella vulgaris NJ-7. Extremophiles, 13(6), 875–884.
Liang, Y., Mai, K. S., & Sun, S. C. (2003). Effect of NaNO3 concentrations on the growth and fatty acid compositions of Nitzschia closterium and Chaetoceros gracilis. Bulletin of Marine Science, 5, 69–74.
Catalina Adarme-Vega, T., Thomas-Hall, S. R., Lim, D. K. Y., & Schenk, P. M. (2004). Effects of long chain fatty acid synthesis and associated gene expression in microalga Tetraselmis sp. Marine Drugs, 12, 3381–3398.
Yodsuwan, N., Sawayama, S., & Sirisansaneeyakul, S. (2017). Effect of nitrogen concentration on growth, lipid production and fatty acid profiles of the marine diatom Phaeodactylum tricornutum. Agriculture & Natural Resources, 51, 190–197.
Hamilton, M. L., Haslam, R. P., Napier, J. A., & Sayanova, O. (2014). Metabolic engineering of Phaeodactylum tricornutum for the enhanced accumulation of omega-3 long chain polyunsaturated fatty acids. Metabolic Engineering, 22, 3–9.
Zeng, S. Y., Liu, H. H., Shi, T. Q., Song, P., Ren, L. J., Huang, H., & Ji, X. J. (2018). Recent advances in metabolic engineering of Yarrowia lipolytica for lipid overproduction. European Journal of Lipid Science and Technology, 120, 1700352.
Amiri-Jami, M., Abdelhamid, A. G., Hazaa, M., Kakuda, Y., & Griffths, M. W. (2015). Recombinant production of omega-3 fatty acids by probiotic Escherichia coli Nissle 1917. FEMS Microbiology Letters, 362, fnv166.
Cahoon, E. B., Mills, L. B., & Shanklin, J. (1996). Modification of the fatty acid composition of Escherichia coli by coexpression of a plant acyl-acyl carrier protein desaturase and ferredoxin. Journal of Bacteriology, 178(3), 936–939.
Acknowledgements
This work was supported by grants from Science and Engineering Research Board (SERB), Government of India (ECR/2017/002914, SB/EMEQ-219/2014). The authors thank DBT-IPLS, NRCBS, CEGS, DST-PURSE, UGC-CAS Phase III, School of Biological Sciences, and Madurai Kamaraj University, Tamil Nadu, India, for the instrumentation facilities and the supports provided for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
All authors declare that they have no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Fig. S1
Cloning and expression of Δ6-elongase gene from Isochrysis sp. and the PCR amplification were found at 0.65 kb in size (a), TA cloning of Δ6Elo-Iso in pXCM vector was confirmed by BamH1 restriction digestion (b), and colony PCR (c) showed gene of interest at 0.65 kb. For gene expression, Δ6Elo-Iso cloned into pGEX-4T2, and recombinant clone was confirmed by BamH1/EcoR1 restriction digestion (d) and colony PCR (e) respectively (JPG 50 kb)
Fig. S2
Phylogenetic tree analysis of Δ6-elongase and Δ5-desaturase gene from microalgae Isochrysis sp. and Pavlova sp. The tree was constructed by Mega 6.1 and neighbour-joining method with the other available elongases and desaturases sequences from different organisms. The identified genes of Δ6Elo-Iso from Isochrysis sp. (a) and Δ5Des-Pav from Pavlova sp. (b) are represented as * (JPG 62 kb)
Fig. S3
Protein expression analysis of Δ6Elo-Iso in E. coli and the overexpressed protein were found at 50 kDa in size (Lane 2, 3) than wild type pGEX-4T2 (Lane 1) (JPG 33 kb)
Fig. S4
Cloning and expression of Δ5-desaturase gene from Pavlova sp. and the PCR amplification were found at 1.2 kb in size (a), TA cloning in pGEMT-easy vector was confirmed by EcoR1 restriction digestion (b), and colony PCR (c) showed gene of interest at 1.2 kb. For gene expression, Δ5Des-Pav cloned into pGEX-4T2, and recombinant clone was confirmed by EcoR1/Sal1 restriction digestion (d) and colony PCR (e) respectively (JPG 72 kb)
Fig. S5
Protein 3D homology model for Δ5Des-Pav was developed by Phyre tool. (a) The 3D view for human strearoyl coA-desaturase2 has shared 99.9% confidence, and (b) 3D view for cytochrome b5 has shared 99.7% confidence to the newly identified Δ5Des-Pav (JPG 64 kb)
Fig. S6
Protein expression analysis of Δ5Des-Pav in E. coli and the overexpressed protein were found at 70 kDa in size (Lane 4–6) than wild type pGEX-4T2 (Lane 1) (JPG 31 kb)
Rights and permissions
About this article
Cite this article
Thiyagarajan S, Arumugam M & Kathiresan S Identification and Functional Characterization of Two Novel Fatty Acid Genes from Marine Microalgae for Eicosapentaenoic Acid Production. Appl Biochem Biotechnol 190, 1371–1384 (2020). https://doi.org/10.1007/s12010-019-03176-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-019-03176-x