Abstract
Glucose phosphorylation by glucokinase exhibits a sigmoidal dependency on substrate concentration regardless of its simple structure. Dimorph mechanism suggested the existence of two enzymatic states with different catalytic properties, which has been shown to be plausible by structural analysis. However, the dimorph mechanism gives rise to a complicated or non-explicit non-closed mathematical form. It is neither feasible to apply the dimorph mechanism in effector characterizations. To improve the area of glucokinase study with stronger theoretical support and less complication in computation, we proposed the investigation of the enzyme from a pseudo-dimeric angle. The proposed mechanism started from the idealization of two monomeric glucokinase as a dimeric complex, which significantly simplified the glucose phosphorylation kinetics, while the differences in enzyme reconfiguration caused by variable substrates and effectors have been successfully characterized. The study presented a simpler and more reliable way in studying the properties of glucokinase and its effectors, providing guidelines of effector developments for hyperglycemia and hypoglycemia treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glucokinase (hexokinase IV, D, EC 2.7.1.2) is a key enzyme for substrate level phosphorylation. Glucokinase catalyzes the phosphorylation of glucose to glucose-6-phosphate with phosphate groups provided by adenosine triphosphate (ATP) [1, 2]. This reaction happens in most organisms and animal bodies under both aerobic and anaerobic conditions. Glucokinase in the animal body is majorly presented in hepatic cells, pancreatic cells, and brain cells where glucokinase is functioning as glucose sensor [3, 4]. It has been found that the interaction between glucose and pancreatic β cells glucokinase will stimulate insulin secretion, while glycogen synthesis is accelerated by glucose, hepatic glucokinase interaction [5]. A recent study proposed that the secretion of pancreatic glucagon is also regulated by glucokinase [6]. All these effects as well as the glucose phosphorylation help in reducing plasma glucose concentration to a desired level.
Glucokinase plays a critical role in regulating the glucose homeostasis that its malfunction can result in diseases like hyperglycemia or hypoglycemia [7,8,9]. The inhibitory of glucokinase variation results in a non-insulin-dependent diabetes mellitus named MODY-2 as well as a more severe diabetic disease known as permanent neonatal diabetes mellitus (PNDM) [10]. Patients with glucokinase inactivating malfunction exhibit low responsiveness on glucose, giving rise to the defection of insulin secretion and glycogen synthesis, which indicates the reduction of enzyme reactivity or affinity towards substrates [11,12,13]. On the other hand, glucokinase active variation induced hyperinsulinemic hypoglycemia. Glucokinase is endogenously inhibited by glucokinase regulatory protein (GKRP) through allosteric binding, which prevents an over-phosphorylation of glucose under hypoglycemic conditions [14,15,16,17]. Such inhibition is relieved at high glucose concentration or by fructose-1-phosphate. In vivo studies have revealed that glucokinase-GKRP complex is localized in the nucleus at low glucose concentration and glucokinase itself is released to cytosol at raised glucose level [18, 19]. However, this process is imbalanced in hypoglycemia patients. The excessive glucokinase activity leads to the over-phosphorylation of glucose and stimulates insulin secretion even at low glucose level, resulting in diseases such as hyperinsulinemic hypoglycemia of infancy (PHHI) [7,8,9]. A recent investigation also revealed the potential relation between glucokinase variations and tumorigeneses. It was reported that the energy metabolism reprograming in a cancer cell is associated with the active variation and/or stabilization effect of glucokinase [20]. In order to regulate the enzyme activity to the desired level, substantial amount of studies have been directed to glucokinase effector development including the early trials on human patients [21,22,23]. Effectors such as glucose analogs, carbohydrates, phosphorylated carbohydrates, lipids, phenolic, and amino compounds have been found effective in regulating the glucokinase activity [24,25,26,27,28]. On the other hand, methods in comparing and characterizing the regulative effectors have yet been unified, due to its unclosed kinetics study.
Mechanistic-based kinetics studies of glucokinase have been started since the last century. Structural investigation has revealed that glucokinase is a smaller molecule than other hexokinases with a molecular mass of 50 kDa [26, 27]. A human glucokinase is a monomeric enzyme, containing two domains that distinct in sizes. A single catalytic site is located between the two domains, composed of six amino acid residues (Glu256, Glu290, Thr168, Lys169, Asn204, and Asp205). Crystal structural analysis has revealed the interaction between the catalytic site and the anomeric carbon of glucose, resulting in the substrate binding. The ligand-free glucokinase presents an open form that the catalytic site is well exposed. However, the catalytic site of the open-formed glucokinase is disordered with incomplete amino acid residues, resulting in the low affinity to the substrate. With substrate binding, the small domain of glucokinase rotates about 99° leading to the “closure” of the enzyme with intact active site structure and enhanced activity [29]. In this case, unlike other single-sited enzymes, glucokinase activity displays a sigmoidal dependency on its substrates showing the stimulation effect of glucose [30,31,32]. A mathematical modeling of the glucokinase catalytic process can be achieved based on the dimorph catalytic mechanism. On the other hand, such mechanism yields tedious mathematical calculations, which lays obstacles to the investigation of the enzyme’s characteristics. Meanwhile, an independent allosteric site that interacts only with effectors was observed in human glucokinase characterization. Such finding manifests the incompleteness of the dimorph theory, as the activation/inhibition mechanisms caused by substrates and effectors are different. In this case, a new theory in characterizing the enzyme and its effectors is needed with less mathematical effort and stronger mechanistic support.
In this study, we proposed a pseudo-dimeric model by treating two known individual glucokinase molecule states as a dimeric community. The new model explained the catalytic mechanisms of glucokinase from a new angle, while simplifying the bimorph mechanistic model by reducing the number of parameters. It provides an easier way in understanding the kinetics properties of the enzyme as well as the impacts from effectors. Both single ligand and multiple ligands binding and reaction behaviors on glucokinase are modeled kinetically. The theory proposed in this study is potentially beneficial for the treatment of hyperglycemia and hypoglycemia.
Methods
Dimorph Catalysis Mechanism
Petit et al. revealed based on X-ray crystallography that glucokinase has two unique folding states: the stable or steady GK bound with glucose is distinctly different from that when no glucose is bound [33]. The glucose phosphorylation on glucokinase is catalyzed by two states of the enzyme with different binding affinities and catalytic activities. The bimorph catalytic mechanisms can be generalized as Fig. 1, where ER stands for the “relaxed” (inactive) state of the enzyme and EC stands for the “closed” (active) state. Such catalytic behavior was further proved from an atomic level with NMR characterization. Larion et al. reported that the glucose-free enzyme displays exchange behaviors in the small domain with an exchange rate constant of 509 ± 51 s−1 from the closed state to the relaxed state, while the exchange rate is significantly slower at the opposite direction with a rate constant of 81 s−1. The equilibrium between the two exchanging states results in the dominance of glucokinase in the relaxed-state (≥ 87%) with limited activity. On the other hand, the exchange behaviors were quenched in the glucose-bond enzyme, suggesting that glucose favors the stabilization of the enzyme to remain at the active state [31].
Based on the bimorph mechanism, catalytic rate expression can be derived, but it is very tedious because of the fact that pseudosteady-state hypothesis (PSSH) needs to be used as the active state or 2E only exists if glucose is bound or during turning over time. Shortly after the enzyme is mixed with glucose, there is a pseudosteady-state beginning to emerge. Pseudosteady-state hypothesis for the enzyme states leads to the following:
and enzyme balance leads to the following:
So, the concentrations of enzyme-glucose complexes can be solved by the following:
where,
Therefore, combining Eqs (5–10), the glucose phosphorylation rate is given by the following:
One can see from Eqs. (5–11), the biomorph catalytic model contains an overall ten reaction rate constants with only glucose as substrate. When effectors are involved, the bimorph mechanism becomes more complicated with three more equilibrium steps (Fig. 2). Together with the complexity of the equations, it gives difficulties in modeling and understanding the kinetics properties of glucokinase and its effectors, not mentioning the catalysis of both glucose and effector (fructose for example). Meanwhile, it is neither convincible to characterize the reconfigurations of the enzyme-glucose complex (ER × G) and enzyme-ignitor/inhibitor complex (ER × I) as the same.
Pseudo-Dimeric Modeling
The stimulation effect of glucose can be regarded as the enhanced catalytic activity (kcG>>kGc), as well as the binding affinity (\( \frac{k_{G2}}{k_{-G2}} \)>>\( \frac{k_{G1}}{k_{-G1}} \)) of the 2E form, and the 2E form is accumulated with the enhanced glucose concentration. In this case, to model the two stages of the enzyme as ease, we proposed a pseudo-dimeric model as shown in Fig. 3. Two glucokinase molecules are regarded as a dimeric complex (E2) with two homosteric sites in the mechanism. The interaction of the dimeric complex with glucose results in three different enzyme states: relaxed-relaxed (E2), relaxed-closed (E2G), and closed-closed (E2G2). Instead of considering the activation of a single glucokinase molecule by glucose, the pseudo-dimeric mechanism considers the changes occurring on the dimeric complex. The interconversion from each states is controlled by content of glucose in the system, giving the expression of the stimulation effect of glucose. In the pseudo-dimeric mechanism, KG represents the equilibrium constant of glucose binding on the enzyme, and kG stands for the catalytic rate constant of glucose phosphorylation. αG and βG stands for the effect of glucose on the dimeric complex regarding to the binding affinity and catalytic activity, respectively.
The pseudo-dimeric also simplified the process with multiple substrates while capable in characterizing the properties of effectors. Figure 4 a shows the simplified reaction mechanism with effector when treating the enzyme as dimeric complexes. The impact of an effector on the binding and catalysis are characterized as αI and βI, respectively. When effectors can be phosphorylated under the catalysis of glucokinase, fructose for example, the system involves two types of phosphorylation reactions as shown in Fig. 4b, which can also be characterized with the pseudo-dimeric mechanism at ease.
Results and Discussion
Single Species Catalysis
Based on the pseudo-dimeric mechanism (Fig. 3), binding and catalysis on a dimeric glucokinase complex is impacted by the presence of glucose, indicated as Eqs. (12–14).
In equations, the initial binding affinity and catalytic activity of the enzyme were represented as KG and kG, respectively, which were further characterized by αG and βG as the stimulation/inhibition effect of the substrate on the pseudo-dimeric complex. Combining Eqs. (12–14), the glucose phosphorylation rate, rG6P, can be rewritten as follows:
A pseudo-dimeric glucokinase complex during the reaction has two other forms that are bound with glucose. The initial enzyme concentration, in this case, is two times the summation of three complexes forms. That is,
Combining Eqs. (12), (13), and (16), the unbound enzyme complex can be represented by the initial enzyme loading.
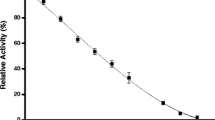
Based on Eqs. (15) and (17), the initial phosphorylation rate can be modeled with the pseudo-dimeric mechanism by monitoring the glucose concentration. Figure 5 showed the catalytic activity of glucokinase with glucose as the only substrate. The curve in Fig. 5 is fitted based on Eqs. (15) and (17).
Initial phosphorylation rate of glucose on hepatic glucokinase with a single substrate. The symbols are data obtained from Kamata et al. [29]. Experiments measured the initial glucose phosphorylation rate with different substrate loading. The curve was fitted with the pseudo-dimeric model with Eqs. (15) and (17). Parameters were shown in Table 1
Multiple Species Catalysis
The effects of different chemicals on glucokinase have been studied in order to study their impacts on the enzyme. Studies have found that most glucose analogs like fructose, mannoheptulose, N-acetylglucosamine, and 5-thioglucose work as inhibitors on glucose phosphorylation. Ignitors were also discovered like N-thiazol-2-yl-2-amino-4-fluoro-5-(1-methylimidazol-2-yl)thiobenzamide, which can substantially enhance the enzyme activity with a hyperbolic glucose dependence. However, a mechanistic characterization and a quantitative comparison of their impacts on the enzyme has yet been achieved due to the complexity of the dimorph mechanism (Fig. 2). On the other hand, the pseudo-dimeric mechanism with multiple substrates (Fig. 4) provided an alternative way in characterizing glucokinase effectors with less effort.
Fructose was found that can be co-phosphorylated together with glucose. With the presence of both sugars as substrates, a competitive catalysis of glucose phosphorylation was observed that the glucose phosphorylation rate was reduced with increased fructose content. Interestingly, such competitively on fructose phosphorylation was not observed that fructose phosphorylation was actually stimulated by glucose. Based on the dimorph mechanism (Fig. 2), this phenomenon was explained by considering an easier activation of the enzyme by glucose (ER × G to EC×G) than fructose (ER × I to EC × I). However, due to the complexity of the dimorph catalysis model, such hypothesis is difficult to be proven by experimental data, neither is it plausible to consider the changes of the enzyme configuration caused by glucose and fructose as the same.
In the pseudo-dimeric modeling of the glucose-fructose co-phosphorylation process as shown in Fig. 4a, the different impacts of the enzyme-binding affinity and catalysis activity by glucose and fructose was characterized αG, βG, and αF, βF, respectively, distinguishing the differences of glucose and fructose on the enzyme reconfiguration. According to the mechanism, the phosphorylation rate of glucose (rG6P) and fructose (rF6P) can be written as follows:
Enzyme balance gives the substrate free glucokinase dimeric complex as follows:
Equations (18–20) can be applied to calculate the phosphorylation rates of glucose and fructose on glucokinase, based on the substrates concentrations. Figure 6 shows the glucose phosphorylation rate with the existence of fructose. Fructose loading ranged from 0 to 200 mM. Curves were fitted by Eqs. (18) and (20) with kinetic parameters shown in Table 2. On the other hand, glucose is an ignitor for fructose phosphorylation as shown in Fig. 7.
On the basis of the co-phosphorylation process, the impact of effectors can be characterized similarly with the pseudo-dimeric mechanism shown as in Fig. 4b. The phosphorylation rate of glucose and fructose with impact from effectors can be written as Eqs. (21) and (22), respectively.
where (I) stands for the effector concentration; KI is the equilibrium constant of the effector; αI and βI characterize the impact of an effector on the binding and catalysis of the dimeric enzyme complex. We applied Eq. (22) on a fructose phosphorylation process with mannoheptulose and N-acetylglucosamine as effectors as shown in Fig. 8. Some parameters were inherited from Table 2 due to the same enzyme origin and operational conditions, with characteristics of the effector given in Table 3.
Fructose phosphorylation process with mannoheptulose and N-acetylglucosamine as effectors interpreted by the pseudo-dimeric model. Symbols are experimental data obtained from Moukil and Van-Schaftingen [25]. Initial fructose concentration was at 100 mM
Discussions
It is previously known that the glucose phosphorylation process is self-stimulating that glucokinase is stabilized in the closed form with the presence of glucose. However, the bimorph model is overcomplicated in the mathematical form. We considered that instead of characterizing the variations of a single enzyme molecule, the changed enzyme properties represented in an idealized enzyme complex or the whole-enzyme environment can actually be easier to characterize. In this case, we proposed the pseudo-dimeric model for an easier glucokinase characterization, as well as explaining some previously uninterpreted data.
According to the simulation result given in Fig. 5, one can see that the pseudo-dimeric model is capable of characterizing the sigmoidal kinetic behaviors of glucokinase. In the simulation results, KG and kG represents the binding and catalytic properties of the glucose-free enzyme complex. The low KG and kG value suggests the poor binding and catalysis of glucose on the original enzyme. On the other hand, αG larger than 1 indicates that the binding affinity can be improved with the presence of a substrate, which is consistent with the previous knowledges. Besides, the catalytic activity of glucokinase can also be improved by glucose according to the βG value. Such cooperativity explains the sigmoidal phosphorylation rate with increased glucose concentration. Since the complex form E2G2 possesses a higher reactivity to be converted to E2G with high binding affinity, one can tell the cooperativity of the glucose stimulation effect between the binding and reaction steps. Such characteristics can be translated to the dimorph mechanism that the reconfigured glucokinase is improved by its affinity and reactivity.
The unique advantage of the pseudo-dimeric model is its capability in characterizing multiple substrates and effector involved glucokinase catalysis with substantially less efforts. Meanwhile, the pseudo-dimeric model provides a more reliable mechanism support, as it describes the enzyme variations resulted from glucose and other species differently. From the glucose-fructose co-phosphorylation results shown in Table 2, one can tell that glucose again showed ignition effect on both binding and phosphorylation processes. However, the parameters in Table 2, for example, KG, kG, αG, and βGare not directly comparable with those in Table 1 due to the differences in enzyme origins and ATP content. The binding equilibrium constants KG and KF suggest the binding of fructose to glucokinase is even weaker that of glucose. Meanwhile, the low αF, and βF values indicate a strong inhibition effect of fructose. Previously, we discussed the deficiency of the bimorph mechanism in treating the reconfiguration of glucokinase by different substrates as the same. Based on the different values of βG and βF, one can further tell the presence of three catalytic activities to a single substrate (kG, βGkG, and βFkG towards glucose), indicating the presence of more than two enzyme states.
When employing the pseudo-dimeric model in effector involved catalysis, we obtained high KI values as shown in Table 3, suggesting strong bindings between mannoheptulose and N-acetylglucosamine to the enzyme. Together with their impediment on the substrate binding (αI<1), the two species result in inhibitory effect. With a comparison between the two effectors, N-acetylglucosamine exhibits a stronger inhibition effect due to its higher affinity to the enzyme. At this point, one can tell the pseudo-dimeric mechanism is capable to provide a quantitative characterization of effectors, which is desired for effector developments in treating hyperglycemia and hypoglycemia.
Conclusion
For the kinetics studies of complex enzymes, instead of investigating the elementary steps of a single-enzyme molecule, the characterizations from an idealized enzyme complex or the whole catalytic environment level can potentially reduce the modeling effort to a great extent. The pseudo-dimeric model proposed the characterization of glucokinase reconfiguration and its changes in binding affinity and catalytic activity by treating two glucokinase molecules as a dimeric complex. The validity of the proposed model has been proved with glucose and fructose phosphorylation data with and without effectors. Glucokinase was found to be substrate-stimulated by glucose on both its affinity and reactivity, which is consistent with previous studies. Dimorph mechanism suggests the existence of only two states of glucokinase, which is insufficient with the presence of effectors, as effectors cause different enzyme reconfigurations from each other. Such differences were successfully characterized by the pseudo-dimeric model with detailed effectors’ properties, which provided guidelines for the development and characterization of glucokinase effectors for hyperglycemia and hypoglycemia treatment.
References
Vinuela, E., Salas, M., & Sols, A. (1963). Glucokinase and hexokinase in liver in relation to glycogen synthesis. The Journal of Biological Chemistry, 238, 1175–1177.
González, C., Ureta, T., Sánchez, R., & Niemeyer, H. (1964). Multiple molecular forms of atp: hexose 6-phosphotransferase from rat liver. Biophysical Research Communications, 16, 347–352.
McFarland, J., Seckinger, K., & Rizzo, M. (2017). Glucokinase mediated glucosensing in hypothalamic neurons. Biophysical Journal, 112, 443.
Matschinsky, F. M., Glaser, B., & Magnuson, M. A. (1998). Pancreatic beta-cell glucokinase: closing the gap between theoretical concepts and experimental realities. Diabetes, 47, 307–315.
Agius, L. (2016). Hormonal & metabolite regulation of hepatic glucokinase. Annual Review of Nutrition, 36, 389–415.
Basco, D., Zhang, Q., Salehi, A., Tarasov, A., Dolci, W., Herrera, P., Spiliotis, I., Berney, X., Tarussio, D., Rorsman, P., & Thorens, B. (2018). α-cell glucokinase suppresses glucose-regulated glucagon secretion. Nature Communications, 9, 546.
Glaser, B., Kesavan, P., Heyman, M., Davis, E., Cuesta, A., Buchs, A., Stanley, C. A., Thornton, P. S., Permutt, M. A., Matschinsky, F. M., & Herold, K. C. (1998). Familial hyperinsulinism caused by an activating glucokinase mutation. The New England Journal of Medicine, 338, 226–230.
Christesen, H. B., Jacobsen, B. B., Odili, S., Buettger, C., Cuesta-Munoz, A., Hansen, T., Brusgaard, K., Massa, O., Magnuson, M. A., Shiota, C., & Matschinsky, F. M. (2002). The second activating glucokinase mutation (A456V) implications for glucose homeostasis and diabetes therapy. Diabetes, 51, 1240–1246.
Rudland, V. L., Hinchcliffe, M., Pinner, J., Cole, S., Mercorella, B., Molyneaux, L., Constantino, M., Yue, D. K., Ross, G. P., & Wong, J. (2016). Identifying glucokinase monogenic diabetes in a multiethnic gestational diabetes mellitus cohort: new pregnancy screening criteria and utility of HbA1c. Diabetes Care, 39, 50–52.
Larion, M., & Miller, B. G. (2012). Homotropic allosteric regulation in monomeric mammalian glucokinase. Archives of Biochemistry and Biophysics, 519, 103–111.
Gutierrez-Nogués, A., García-Herrero, C. M., Oriola, J., Vincent, O., & Navas, M. A. (2018). Functional characterization of MODY2 mutations in the nuclear export signal of glucokinase. Biochimica et Biophysica Acta, 1864, 2385–2394.
Clement, K., Pueyo, M. E., Vaxillaire, M., Rakotoambinina, B., Thuillier, F., Passa, P. H., Froguel, P. H., Robert, J. J., & Velho, G. (1996). Assessment of insulin sensitivity in glucokinase-deficient subjects. Diabetologia, 39, 82–90.
Grimsby, J., Sarabu, R., Corbett, W. L., Haynes, N. E., Bizzarro, F. T., Coffey, J. W., Guertin, K. R., Hilliard, D. W., Kester, R. F., Mahaney, P. E., & Marcus, L. (2003). Allosteric activators of glucokinase: potential role in diabetes therapy. Science, 301, 370–373.
Agius, L. (2008). Glucokinase and molecular aspects of liver glycogen metabolism. The Biochemical Journal, 414, 1–18.
Casey, A. K., & Miller, B. G. (2016). Kinetic basis of carbohydrate-mediated inhibition of human glucokinase by the glucokinase regulatory protein. Biochem., 55, 2899–2902.
Honardoost, M., Sarookhani, M. R., Arefian, E., & Soleimani, M. (2014). Insulin resistance associated genes and mirnas. Applied Biochemistry and Biotechnology, 174, 63–80.
Brouwers, M. C., Jacobs, C., Bast, A., Stehouwer, C. D., & Schaper, N. C. (2015). Modulation of glucokinase regulatory protein: a double-edged sword. Trends in Molecular Medicine, 21, 583–594.
Farrelly, D., Brown, K. S., Tieman, A., Ren, J., Lira, S. A., Hagan, D., Gregg, R., Mookhtiar, K. A., & Hariharan, N. (1999). Mice mutant for glucokinase regulatory protein exhibit decreased liver glucokinase: a sequestration mechanism in metabolic regulation. Proceedings of the National Academy of Sciences of the United States of America, 96, 14511–14516.
Kaushik, A., & Kaushik, M. (2018). Recent updates on glucokinase activators and glucokinase regulatory protein disrupters for the treatment of type 2 diabetes mellitus. Current Diabetes Reviews, 14, 1–8.
Těšínský, M., Šimčíková, D., & Heneberg, P. (2019). First evidence of changes in enzyme kinetics and stability of glucokinase affected by somatic cancer-associated variations. Biochimica et Biophysica Acta, Proteins Proteomics, 1867, 213–218.
Brocklehurst, K. J., Payne, V. A., Davies, R. A., Carroll, D., Vertigan, H. L., Wightman, H. J., Aiston, S., Waddell, I. D., Leighton, B., Coghlan, M. P., & Agius, L. (2004). Stimulation of hepatocyte glucose metabolism by novel small molecule glucokinase activators. Diabetes, 53, 535–541.
Cheruvallath, Z. S., Gwaltney, S. L., Sabat, M., Tang, M., Wang, H., Jennings, A., Hosfield, D., Lee, B., Wu, Y., Halkowycz, P., & Grimshaw, C. E. (2017). Discovery of potent and orally active 1, 4-disubstituted indazoles as novel allosteric glucokinase activators. Diabetes Obes. Metab., 27, 2678–2682.
Katz, L., Manamley, N., Snyder, W. J., Dodds, M., Agafonova, N., Sierra-Johnson, J., Cruz, M., Kaur, P., Mudaliar, S., Raskin, P., & Kewalramani, R. (2016). AMG 151 (ARRY-403), a novel glucokinase activator, decreases fasting and postprandial glycaemia in patients with type 2 diabetes. Diabetes, Obesity & Metabolism, 18, 191–195.
Detheux, M., Vandercammen, A., & Van Schaftingen, E. (1991). Effectors of the regulatory protein acting on liver glucokinase: a kinetic investigation. The FEBS Journal, 200, 553–561.
Moukil, M., & Van-Schaftingen, E. (2001). Analysis of the cooperativity of human beta-cell glucokinase through the stimulatory effect of glucose on fructose phosphorylation. The Journal of Biological Chemistry, 276, 3872–3878.
Lenzen, S. (2014). A fresh view of glycolysis and glucokinase regulation: history and current status. The Journal of Biological Chemistry, 289, 12189–12194.
Cárdenas, M. L., Rabajille, E., & Niemeyer, H. (1978). Maintenance of the monomeric structure of glucokinase under reacting conditions. Archives of Biochemistry and Biophysics, 190, 142–148.
Vella, A., Freeman, J. L., Dunn, I., Keller, K., Buse, J. B., & Valcarce, C. (2019). Targeting hepatic glucokinase to treat diabetes with TTP399, a hepatoselective glucokinase activator. Science Translational Medicine, 11, eaau3441.
Kamata, K., Mitsuya, M., Nishimura, T., Eiki, J. I., & Nagata, Y. (2004). Structural basis for allosteric regulation of the monomeric allosteric enzyme human glucokinase. Structure, 12, 429–438.
Niemeyer, H., De, I. L. C. M., Rabajille, E., Ureta, T., Clark-Turri, L., & Peñaranda, J. (1975). Sigmoidal kinetics of glucokinase. Enzyme, 20, 321–333.
Larion, M., Hansen, A. L., Zhang, F., Bruschweiler-Li, L., Tugarinov, V., Miller, B. G., & Brüschweiler, R. (2015). Kinetic cooperativity in human pancreatic glucokinase originates from millisecond dynamics of the small domain. Angewandte Chemie, 127, 8247–8250.
Matschinsky, F. M. (2002). Regulation of pancreatic β-cell glucokinase from basics to therapeutics. Diabetes, 51, 394–404.
Petit, P., Antoine, M., Ferry, G., Boutin, J. A., Lagarde, A., Gluais, L., Vincenteli, R., & Vuillard, L. (2011). The active conformation of human glucokinase is not altered by allosteric activators. Acta Crystallographica, 67, 929–935.3
Funding
This study is financially support from the Zhejiang Provincial Natural Science Foundation of China (No. Y19B060022) and National Natural Science Foundation of China (No. 31601390).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chen, H., Cai, X., Xu, A. et al. Characterization of Glucokinase Catalysis from a Pseudo-Dimeric View. Appl Biochem Biotechnol 189, 345–358 (2019). https://doi.org/10.1007/s12010-019-02998-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-019-02998-z