Abstract
A probiotic biocatalyst was prepared through Lactobacillus plantarum ATCC 14917 immobilization on a prebiotic carrier (delignified wheat bran) and was used for fermentations of pomegranate juice. Initially, pomegranate juice was fermented for 24 h and then was stored for 28 days at 4 °C. The obtained results regarding sugar and organic acid analysis revealed that the probiotic biocatalyst was effective. Ethanol was produced in small amounts (0.4–1% v/v). Total phenolic content and antioxidant activity was greater in the fermented pomegranate juice than in unfermented juice after 24 h of fermentation and over the time span of 28 days. Viability of probiotic cells was well maintained (above 8.65 log cfu/mL) after 24 h of fermentation and during 4 weeks of storage at 4 °C, and it is noteworthy that no pathogens were observed. The strength of viability of probiotic cells can be attributed to the immobilization carrier (delignified wheat bran) that exhibits prebiotic properties providing a protective effect to the cells. Finally, the proposed bioprocess of employing the proposed synbiotic biocatalyst for pomegranate juice fermentation shows great potential for commercialization while sensory evaluation highlights the degree of quality of the produced functional pomegranate beverages.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Industry has had to address many challenges over the past years as consumers currently seek more delightful and appealing foods which are simultaneously safer, nutritional, and healthier [1]. The innovations introduced into the food industry mainly focus on the production of novel food reinforced by functional ingredients which can exert beneficial effects to the consumers [2, 3]. A very significant category of functional foods is foods that contain bioactive compounds such as dietary fiber, oligosaccharides, phenolics, and flavonoids as well as probiotics which consist of “friendly” bacteria that promote equilibrium of intestinal flora [4, 5]. Most of the probiotic foods are produced from dairy products. However, lately, the food industry is searching for additional vehicles in order to produce probiotic foods. The main reasons are that up to 70% of the human population of the world is affected by lactose-intolerance and that more and more consumers suffer from dairy product allergies and cholesterol diseases [6].
The dietary habits of consumers have progressively turned to more healthy options over the past few years while the increasing awareness of health benefits of fruit-based products has led to the consumption of these food items to be on the rise [1]. Fruit juice–based beverages can be proposed as appropriate novel substrates to produce non-dairy probiotic foods [5, 7]. Particularly, several research studies have reported fermentation of various fruit juices with probiotic bacteria such as oranges, apples, lemons, passion fruit, and pomegranates, for the production of probiotic beverage [5, 8, 9]. Among fruit juices, pomegranate possesses many functional characteristics since it contains several beneficial nutrients as well as bioactive compounds which have anti-atherosclerotic, anti-aging, anti-oxidative, anti-inflammatory, antimicrobial, and possibly anti-carcinogenic effects [10]. In addition, pomegranate juice has been used as a substrate of fermentation by various probiotic lactic acid bacteria targeting the production of pomegranate beverages with enhanced health benefits [11,12,13,14,15].
On the other hand, pomegranate juice as well as other juices cannot be considered as the best substrate to ensure probiotic cell viability because of acidic conditions that predominate in the juice. It has been established that the minimum concentration of viable bacterial cells for the production of probiotic food should be approximately 106–107 cfu/mL at the time of consumption [4]. Given this finding, the acidic environment of pomegranate juice can be characterized as a drawback for the growth of probiotic lactic acid bacteria. Many methods have been proposed as entrapment or immobilization on various food-grade carriers [16, 17], such as addition of prebiotic substances [17,18,19,20], spray drying and spray chilling [21, 22], and others, targeting enhanced viability of probiotic lactic acid bacteria within food matrices. The scientific approach is to combine probiotic lactic bacteria with a prebiotic material in order to protect or enhance cell viability against the acidic environment of pomegranate juice. Prebiotics are non-digestible food ingredients that have a specific stimulatory effect upon selected populations of gut bacteria such as probiotics [23].
Hence, the present study focused on the application of a synbiotic biocatalyst prepared through the immobilization of the probiotic Lactobacillus plantarum ATCC 14917 on delignified wheat bran, for pomegranate juice fermentation. The viability of the strain, the concentration of residual sugars, organic acids, and ethanol as well as the antioxidant activity, and total phenolics content were measured after 24 h of fermentation and monitored for a total of 28 days during storage at 4 °C.
Materials and Methods
Microorganism and Media
Probiotic Lactobacillus plantarum ATCC 14917 [24] was maintained at the Democritus University of Thrace in de Man-Rogosa-Sharpe (MRS) medium (LabM, UK) supplemented with glycerol (20%, v/v; Sigma–Aldrich, G7893, USA) at − 80 °C. It was grown under anaerobic conditions at 37 °C for 48 h in MRS broth. Wet biomass was harvested by centrifugation (Sigma 3K12, Bioblock Scientific, France) at 5000 rpm for 10 min at 25 °C [20]. All media were autoclaved at 120 °C and at 1–1.5 atm for 15 min prior to use.
Preparation of the Immobilized Synbiotic Biocatalyst
Wheat bran (Triticum aestivum L.), a by-product of flour mills, was supplied by a local industry (Orestiada, Greece) and was used as an immobilization carrier of the probiotic strain Lactobacillus plantarum ATCC 14917. Wheat bran consisted of approximately 50% dietary fiber, 20% protein, 7% ash, and 4% lipids. Prior to use, wheat bran was delignified by boiling in 1% NaOH solution targeting to increase carrier porosity by lignin removal [25]. The delignified wheat bran (DWB) was sterilized by autoclaving at 120 °C, 1–1.5 atm for 15 min. Immobilization (entrapment by absorption) was performed by mixing 5 g of DWB with 1.0 g of harvested Lactobacillus plantarum ATCC 14917 cell biomass (dry weight) in 500 mL MRS broth and incubating at 37 °C for 48 h [25]. The initial dry weight of the harvested cell biomass was determined by taking an aliquot of 5 mL, placing it in previously weighted tubes (priory sterilized and dried for 48 h at 105 °C) and centrifuged for 15 min at 1500g. Then, the supernatant was discarded, and the pellet was washed with distilled water and centrifuged again as described above. Finally, the biomass was dried until constant weight at 90 °C and used for cell immobilization. The produced synbiotic immobilized biocatalyst was washed with sterile 1/4 strength Ringer’s solution (Sigma–Aldrich) for the removal of free cells and was used as a starter culture for pomegranate juice fermentation.
Scanning Electron Microscopy
The immobilized Lactobacillus plantarum ATCC 14917 cells were observed on DWB by scanning electron microscopy (SEM) prior fermentation to ensure cell immobilization. For comparison, samples of plain DWB were also observed. All samples were freeze-dried prior to testing as described previously [17]. Parts of the immobilized synbiotic biocatalyst as well as parts of DWB were coated with gold in a Balzers SCD 004 Sputter coater (Bal-Tec, Schalksmühle, Germany) for 2 min, and then the samples were examined on a JSM-6300 microscope (JEOL, Tokyo, Japan), operated at an accelerating voltage of 20 kV.
Pomegranate Juice Fermentation
Fresh pomegranates (Punica granatum L.) were obtained from a local market (Orestiada, Greece). The fresh fruit was carefully washed, peeled, and processed into juice by blending the seeds for 10 min in a common household blender. Initial sugar concentration was adjusted to 80 g/L with the addition of sterilized, deionized water, and the prepared juice was pasteurized (5 min/80 °C) and cooled to room temperature (18–20 °C) [12]. Approximately 1 g (dry weight) of the immobilized biocatalyst was suspended in 100 mL of fermentation substrate. The pH of the substrate was adjusted to 3.5 with the appropriate addition of NaOH 4 N. The substrate was submitted to fermentation at 30 °C for 24 h and was kept at 4 °C for 28 days.
Microbiological Analysis
Aliquots of 10 mL were removed from pomegranate juice (after homogenization by shaking thoroughly) at various time intervals during fermentation and storage. The samples were blended with 90 mL of sterile 1/4 strength Ringer’s solution (Sigma–Aldrich) and mixed in a stomacher blender. The suspension was then subjected to serial decimal dilutions in 1/4 strength Ringer’s solution. Microbial counts were performed by pour plating 0.1 mL or 1 mL of appropriate dilutions on the selective media for each species according to the instructions provided by the manufacturer (LabM, UK) as described previously [19].
Specifically, viable counts of Lactobacillus plantarum ATCC 14917 were performed on acidified MRS agar at 37 °C for 48 h. Yeasts and fungi were determined by plating on potato dextrose agar (PDA) after incubation at 30 °C for 72 h. Coliforms were enumerated on Violet Red Bile Agar (VRBA) after incubation at 30 °C for 24 h. Enterobacteria were enumerated on Violet Red Bile Glucose Agar (VRBGA) after incubation at 37 °C for 24 h. Staphylococci were counted on Baird Parker agar with added egg yolk tellurite medium (BP) after incubation at 37 °C for 48 h. All media were sterilized by autoclaving at 120 °C and at 1–1.5 atm for 15 min and then cooled to 45–50 °C before use. The original count in each sample was expressed as log CFU per milliliters of pomegranate juice. All results are presented as the mean plus standard deviation of three replications.
Ethanol and Residual Sugar Analysis
Samples were collected at various time intervals (days 1, 7, 14, 21, and 28) after storage at 4 °C and were analyzed for concentrations of residual sugar and ethanol by high-performance liquid chromatography on a Shimadzu HPLC system (Shimadzu, Kyoto, Japan) consisting of a SCR-101N stainless steel column, a LC-9A pump, a CTO-10A oven set at 60 °C, and a RID-6A refractive index detector. Ultra-pure water obtained by a Milli-Q water purifying system (resistivity 18.2 MΩ cm−1) was used as the mobile phase with a flow rate of 0.8 mL/min, and 1-butanol (0.1% v/v) was used as the internal standard [12]. Samples were filtered through 0.2-μm microfilters, before injection. Ethanol (% v/v) and residual sugar (g/L) concentrations were calculated using standard curves. All results are presented as mean plus standard deviation of at least three replicates.
Organic Acid Analysis
Organic acids (lactic and acetic) were determined by ion-exchange liquid chromatography. The analysis was carried out on an ion-exchange HPLC Shimadzu system consisting of a Shim-pack ICA1 column, an LC-10AD pump, a CTO-10A oven, and a CDD-6A conductivity detector. A solution of 2.5 mM phthalic acid and 2.4 mM tris (hydroxymethyl) aminomethane (pH 4.0) was used as the mobile phase (1.2 mL/min). The column temperature was 40 °C. The sample dilution was 5% v/v, and the injection volume was 60 μL (28). Determinations were carried out by means of standard curves.
Total Phenolics
Total phenolic content was determined using the Folin-Ciocalteu reagent based on colorimetric reduction [26]. Phenolic compounds were oxidized to phenolates by treatment in a saturated solution of sodium carbonate in alkaline pH resulting in a blue complex. About 1 mL of Folin-Ciocalteau (10%, w/v) was added to 0.2 mL of prepared pomegranate juice, followed by the addition of 1.2 mL of aqueous Na2CO3 (7.5%, w/v). The mixture was left in the dark for 90 min. The absorbance of the blue color solution was monitored by UV-visible spectrophotometer at 760 nm, and all samples were compared with blank (distilled water). The TPC was assessed by plotting the gallic acid calibration curve and expressed as milligrams of gallic acid equivalents (GAE)/100 mL of juice.
Antioxidant Activity
The antioxidant activity (AA) of pomegranate juices was evaluated by applying the ABTS radical cation decolorization assay [27]. ABTS·+ was prepared by reacting ABTS with potassium persulfate. Samples were analyzed at five different dilutions, within the linearity range of the assay, as described previously [28]. AA was expressed as milligrams of Trolox equivalent (TE)/100 mL juice. All measurements were repeated three times.
Sensory Evaluation
The evaluation of fermented pomegranate juice was carried out by a panel of 30 non-trained laboratory members after the end of juice fermentation and during storage in terms of aroma, taste, and overall quality of produced juices. For comparison, non-fermented pomegranate juice was also examined. The samples were coded by a different 3-digital number and were served in a randomized order, and the panel was asked to evaluate them based on a 0–10 preference scale [16]. The results are presented as average scores plus standard deviations.
Statistical Analysis
The data obtained from microbiological analysis, physicochemical characteristics, antioxidant activity, total phenolics content, and sensory evaluation of the initial and the fermented pomegranate juice were analyzed for their mean differences with the analysis of variance (ANOVA) procedure followed by Duncan’s post hoc multiple range test to extract the specific differences among the various treatments. Analysis was performed by using IMB SPSS v20 (IBM Corp.) at an alpha level of 5%. Differences noted as significant in the results and discussion are statistically significant at this level.
Results and Discussion
Pomegranate (Punica granatum), which belongs to the Punicaceae family, is a plant that contains beneficial compounds, many of which characterized as both bioactive components and as important phytochemicals. As a result, the value of pomegranate juice can be attributed to many therapeutic purposes [10]. As an addition to the beneficial nutrients of pomegranate juice, the present study was conducted to evaluate a novel pomegranate beverage having enhanced functional (probiotic and prebiotic) characteristics. A novel immobilized biocatalyst of probiotic Lactobacillus plantarum ATCC 14917 [24, 29], delignified wheat bran, was prepared and applied for pomegranate juice fermentation. The biocatalyst was examined by electron microscopy, showing the immobilized cells attached on the surface of DWB (Fig. 1b) and compared with DWB prior to immobilization (Fig. 1a). Results verified the immobilization of the probiotic cells which was determined by pomegranate juice successful fermentations.
Cell Survival
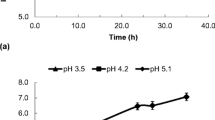
The viability of the probiotic strain Lactobacillus plantarum ATCC 14917 within the fermented pomegranate juice during 28 days of storage at 4 °C was determined (Fig. 2a). During fermentation and storage, the viable L. plantarum cell counts remained within the international guidelines (> 7 log cfu/mL) for probiotic products [4]. This result is similar to those of previous studies that described the suitability of wheat grains and wheat bran as cell immobilization carriers for deliver probiotics to the human gut [20, 30, 31]. Considering that the pH of the initial pomegranate juice was approximately 2.8~3.0, a slight increase of the substrate pH (value of 3.5) was sufficient to enhance the viability of L. plantarum ATCC 14917 (Fig. 2b). Specifically, the survival of L. plantarum ATCC 14917 was enhanced by 30% of the initial concentration (8.88 cfu/mL) during the 14th day of storage at 4 °C and viability was maintained over 90% during the entire storage period.
The immobilization of Lactobacillus plantarum ATCC 14917 on DWB also appears to affect cell viability positively. In particular, the initial viability value of 8.88 log cfu/mL significantly increased to 11.74 log cfu/mL during the 14th day of storage (Fig. 2a) and remained elevated at the 28th day of storage (8.74 cfu/mL). These findings are important since in previous studies, the viability of other probiotic bacteria used for pomegranate juice fermentation frequently suffered a dramatic decrease during the last 2 weeks of storage and specifically this decrease was below the critical value of 107 cfu/mL [15, 32]. In a case where a similar set of fermentations was conducted using free cells of Lactobacillus paracasei K5, only a slight decrease of viability from 8.8 to 8.1 log cfu/mL was observed during 28 days of storage; however, there was no increase in cell viability [12]. Likewise, it seems that the immobilization carrier protected and even enhanced cell viability similar to the application of wheat bran or DWB as a carrier of lactic acid bacteria in dairy products [20, 25, 31]. In particular, wheat bran consists of elevated content of fructo-oligosaccharides, galacto-oligosaccharides, and xylo-oligosaccharides, compounds which are well known as prebiotics and are capable of enhancing the growth of specific beneficial microbial populations like Lactobacilli [33]. Likewise, in the current study, cell immobilization as well as the prebiotic ingredients of wheat bran could have provided a protective effect to Lactobacillus plantarum ATCC 14917 that was not reported during pomegranate juice fermentation by Lactobacillus paracasei K5 free cells [12].
A microbiological assessment evaluating the presence of possible pathogenic or spoilage microorganisms was carried out. It is noteworthy that the produced pomegranate beverages were not contaminated by yeasts, fungi, enterobacteria, coliforms, or staphylococci even after 28 days of storage at 4 °C (data not shown). Yeasts and fungi are mainly responsible for industrial juice spoilage and can cause severe economic losses [34] being present in parallel fruit natural habitats; as a result, they are most detrimental in fruit juices and beverages. In general, the absence of foodborne soilage or pathogenic microorganisms in the produced pomegranate beverages could be the result of successful pasteurization or from the inhibition of growth by pomegranate antimicrobial properties as well as the antagonist effect of Lactobacillus plantarum ATCC 14917 [35, 36].
Ethanol, Organic Acid, and Residual Sugar Concentrations
The results for residual sugar, lactic acid, and ethanol are presented in Table 1. The concentration of total sugars decreased and those of organic acids (mainly lactic acid) increased, illustrating the efficiency of the biocatalyst for lactic acid fermentation of pomegranate juice (Table 1). Specifically, the total sugar concentration was reduced significantly by approximately 19% (60.84 g/L) after 28 days of storage (statistically significant). The concentration of organic acids was increased, rising significantly from 1.48 g/L on the 1st day, to 3.94 g/L on the 28th day of storage (statistically significant). Ethanol concentration was significantly increased from 0.4 to 1.0% (v/v) by the end of storage period (28th day). In the specific fermentation conditions (low pH and storage temperature), the alcohol content was less than 1%, which is in accordance with the standards set for low or non-alcoholic beverages [37].
Total Phenolics Content
The total phenolics content (TPC) of the fermented pomegranate juice increased during the storage period. Specifically, initial total phenolic content of freshly prepared pomegranate juice was about 138 ± 10 mg GAE/100 mL. By the end of the 24-h fermentation, total phenolic content of the fermented pomegranate juice increased significantly to an average of 185 mg GAE/100 mL, compared to the respective value of the non-fermented juice (control) that decreased to 125 mg GAE/100 mL (Table 2). This significant increase of TPC in fermented pomegranate juice was observed also over the entire storage time, reaching its maximum value at the final storage day (234.59 mg GAE/100 mL), while TPC of the unfermented pomegranate juice decreased to 88.59 mg GAE/100 mL. This outcome also has been observed by other researchers who reported that in general, lactic acid fermentation enhances the TPC of pomegranate juice [38,39,40]. Improvements in the free radical scavenging effect can be related to the increase in the free form of phenolic compounds through the fermentation and the production of other by-products [14, 41]. Specifically, various enzymes are produced, from lactic acid bacteria during fermentation such as β-glycosidase leading to degradation of complex phenolic compounds usually initially conjugated to simpler types, thus increasing the total phenolic content [15].
Antioxidant Activity
The same outcome as with TPC was observed regarding the antioxidant activity (AA) of fermented pomegranate juice. Particularly, initial TAA of freshly prepared pomegranate juice was about 87 ± 10 mg TE/100 mL. During the first 24-h fermentation, ΑΑ of the fermented pomegranate juice increased significantly to an average of 120.05 mg TE/100 mL, compared to the respective value of the non-fermented juice that decreased to 85 mg TE/100 mL (Table 2). This significant increase of AA concentration in fermented pomegranate juice also was observed over all the weeks of storage time, reaching its maximum value during the 21st days of storage (130.87 mg TE/100 mL), while TAA of non-fermented pomegranate juice decreases constantly to 58.47 mg TE/100 mL during the last day of storage. It appears that lactic acid fermentation enhanced the AA of pomegranate juice in the present study as other researchers have demonstrated in the past. Specifically, similar findings were observed in pomegranate juice fermented with probiotic lactic acid bacteria [13,14,15].
Sensory Evaluation
The preliminary sensory comparison of non-fermented and fermented juices that was performed by non-trained tasters (consumers) in terms of aroma, taste, and overall quality (preference) showed no significant differences until the end of storage (Table 3). It is noteworthy that consumers scored the fermented pomegranate juice significantly better in terms of aroma, taste, and overall quality. This is likely attributed to the lactic acid fermentation as other researchers have proposed. Specifically, during lactic acid fermentation concentration of desirable chemical groups such as alcohols, ketones and esters are increased while fewer undesirable aldehydes are produced. As a result, we can conclude that lactic acid fermentation can enhance the flavor profile of pomegranate juice and can ensure better control of flavor changes during juice processing [42].
Conclusions
The outcome of this study clearly highlighted that the probiotic strain Lactobacillus plantarum ATCC 14917 immobilized on DWB can be successfully applied for functional pomegranate beverage production. Enhanced viability of Lactobacillus plantarum ATCC 14917 above the critical level of 106–107 cfu/mL was observed during the entire period of storage. Total phenolic content and antioxidant activity of fermented pomegranate juice was greater compared to unfermented juice, and no contamination was observed by various foodborne pathogens during the entire storage period. Likewise, the proposed immobilized biocatalyst led to a novel functional food with elevated concentration of probiotic bacteria and enhanced nutritional value.
References
Oliveira, A., Amaro, A. L., & Pintado, M. (2018). Impact of food matrix components on nutritional and functional properties of fruit-based products. Current Opinion in Food Science, 22, 153–159.
Adefegha, S. A. (2018). Functional foods and nutraceuticals as dietary intervention in chronic diseases; novel perspectives for health promotion and disease prevention. Journal of Dietary Supplements, 15(6), 977–1009.
Santeramo, F. G., Carlucci, D., De Devitiis, B., Seccia, A., Stasi, A., Viscecchia, R., & Nardone, G. (2018). Emerging trends in European food, diets and food industry. Food Research International, 104, 39–47.
Plessas, S., Bosnea, L., Alexopoulos, A., & Bezirtzoglou, E. (2012). Potential effects of probiotics in cheese and yogurt production: a review. Engineering in Life Sciences, 12(4), 433–440.
Ephrem, E., Najjar, A., Charcosset, C., & Greige-Gerges, H. (2018). Encapsulation of natural active compounds, enzymes, and probiotics for fruit juice fortification, preservation, and processing: an overview. Journal of Functional Foods, 48, 65–84.
Perricone, M., Bevilacqua, A., Corbo, M. R., & Sinigaglia, M. (2014). Technological characterization and probiotic traits of yeasts isolated from Altamura sourdough to select promising microorganisms as functional starter cultures for cereal-based products. Food Microbiology, 38, 26–35.
Perricone, M., Bevilacqua, A., Altieri, C., Sinigaglia, M., & Corbo, R. M. (2015). Challenges for the production of probiotic fruit juices. Beverages, 1(2), 95–103.
Dias, C. O., dos Santos Opuski de Almeida, J., Pinto, S. S., de Oliveira Santana, F. C., Verruck, S., Müller, C. M. O., Prudêncio, E. S., & de Mello Castanho Amboni, R. D. (2018). Development and physico-chemical characterization of microencapsulated bifidobacteria in passion fruit juice: a functional non-dairy product for probiotic delivery. Food Bioscience, 24, 26–36.
Nualkaekul, S., & Charalampopoulos, D. (2011). Survival of Lactobacillus plantarum in model solutions and fruit juices. International Journal of Food Microbiology, 146(2), 111–117.
Khwairakpam, A. D., Bordoloi, D., Thakur, K. K., Monisha, J., Arfuso, F., Sethi, G., Mishra, S., Kumar, A. P., & Kunnumakkara, A. B. (2018). Possible use of Punica granatum (pomegranate) in cancer therapy. Pharmacological Research, 133, 53–64.
Murthy, S. N., Patnaik, A., Srinivasan, N., Selvarajan, E., Nivetha, A. & Mohanasrinivasan, V. (2017). Fermentative preparation of functional drink from Punica granatum using lactic acid bacteria and exploring its anti-tumor potential. IOP Conference Series: Materials Science and Engineering.
Plessas, S., Nouska, C., Karapetsas, A., Kazakos, S., Alexopoulos, A., Mantzourani, I., Chondrou, P., Fournomiti, M., Galanis, A., & Bezirtzoglou, E. (2017). Isolation, characterization and evaluation of the probiotic potential of a novel Lactobacillus strain isolated from Feta-type cheese. Food Chemistry, 226, 102–108.
Filannino, P., Azzi, L., Cavoski, I., Vincentini, O., Rizzello, C. G., Gobbetti, M., & Di Cagno, R. (2013). Exploitation of the health-promoting and sensory properties of organic pomegranate (Punica granatum L.) juice through lactic acid fermentation. International Journal of Food Microbiology, 163(2-3), 184–192.
Mousavi, Z. E., Mousavi, S. M., Razavi, S. H., Hadinejad, M., Emam-Djomeh, Z., & Mirzapour, M. (2013). Effect of fermentation of pomegranate juice by Lactobacillus plantarum and Lactobacillus acidophilus on the antioxidant activity and metabolism of sugars, organic acids and phenolic compounds. Food Biotechnology, 27(1), 1–13.
Mousavi, Z. E., Mousavi, S. M., Razavi, S. H., Emam-Djomeh, Z., & Kiani, H. (2011). Fermentation of pomegranate juice by probiotic lactic acid bacteria. World Journal of Microbiology & Biotechnology, 27(1), 123–128.
Schoina, V., Terpou, A., Bosnea, L., Kanellaki, M., & Nigam, P. S. (2018). Entrapment of Lactobacillus casei ATCC393 in the viscus matrix of Pistacia terebinthus resin for functional myzithra cheese manufacture. LWT - Food Science and Technology, 89, 441–448.
Terpou, A., Gialleli, A. I., Bosnea, L., Kanellaki, M., Koutinas, A. A., & Castro, G. R. (2017). Novel cheese production by incorporation of sea buckthorn berries (Hippophae rhamnoides L.) supported probiotic cells. LWT - Food Science and Technology, 79, 616–624.
Fonteles, T. V., & Rodrigues, S. (2018). Prebiotic in fruit juice: processing challenges, advances, and perspectives. Current Opinion in Food Science, 22, 55–61.
Terpou, A., Bekatorou, A., Bosnea, L., Kanellaki, M., Ganatsios, V., & Koutinas, A. A. (2018). Wheat bran as prebiotic cell immobilisation carrier for industrial functional Feta-type cheese making: chemical, microbial and sensory evaluation. Biocatalysis and Agricultural Biotechnology, 13, 75–83.
Terpou, A., Bekatorou, A., Kanellaki, M., Koutinas, A. A., & Nigam, P. (2017). Enhanced probiotic viability and aromatic profile of yogurts produced using wheat bran (Triticum aestivum) as cell immobilization carrier. Process Biochemistry, 55, 1–10.
Arslan-Tontul, S., & Erbas, M. (2017). Single and double layered microencapsulation of probiotics by spray drying and spray chilling. LWT - Food Science and Technology, 81, 160–169.
Huang, S., Vignolles, M.-L., Chen, X. D., Le Loir, Y., Jan, G., Schuck, P., & Jeantet, R. (2017). Spray drying of probiotics and other food-grade bacteria: a review. Trends in Food Science & Technology, 63, 1–17.
Rastall, R. A., & Gibson, G. R. (2015). Recent developments in prebiotics to selectively impact beneficial microbes and promote intestinal health. Current Opinion in Biotechnology, 32, 42–46.
Wang, P., Wu, Z., Wu, J., Pan, D., Zeng, X., & Cheng, K. (2016). Effects of salt stress on carbohydrate metabolism of Lactobacillus plantarum ATCC 14917. Current Microbiology, 73(4), 491–497.
Terpou, A., Bosnea, L., Kanellaki, M., Plessas, S., Bekatorou, A., Bezirtzoglou, E., & Koutinas, A. A. (2018). Growth capacity of a novel potential probiotic Lactobacillus paracasei K5 strain incorporated in industrial white brined cheese as an adjunct culture. Journal of Food Science, 83(3), 723–731.
Singleton, V. L., & Rossi, J. (1965). Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. American Journal of Enology and Viticulture, 16, 144–158.
Miller, N. J., & Rice-Evans, C. A. (1996). Spectrophotometric determination of antioxidant activity. Redox report : communications in free radical research, 2(3), 161–171.
Gentile, C., Reig, C., Corona, O., Todaro, A., Mazzaglia, A., Perrone, A., Gianguzzi, G., Agusti, M., & Farina, V. (2016). Pomological traits, sensory profile and nutraceutical properties of nine cultivars of loquat (Eriobotrya japonica Lindl.) fruits grown in Mediterranean area. Plant Foods for Human Nutrition (Dordrecht, Netherlands), 71(3), 330–338.
Tomaro-Duchesneau, C., Jones, M. L., Shah, D., Jain, P., Saha, S., & Prakash, S. (2014). Cholesterol assimilation by lactobacillus probiotic bacteria: an in vitro investigation. BioMed Research International, 2014, 9.
Charalampopoulos, D., Pandiella, S. S., & Webb, C. (2003). Evaluation of the effect of malt, wheat and barley extracts on the viability of potentially probiotic lactic acid bacteria under acidic conditions. International Journal of Food Microbiology, 82(2), 133–141.
Terpou, A., Gialleli, A.-I., Bekatorou, A., Dimitrellou, D., Ganatsios, V., Barouni, E., Koutinas, A. A., & Kanellaki, M. (2017). Sour milk production by wheat bran supported probiotic biocatalyst as starter culture. Food and Bioproducts Processing, 101, 184–192.
Bhat, B., Gupta, M., Andrabi, S. T., & Bajaj, B. K. (2017). Growth and viability of probiotic Weissella kimchi R-3 in fruit and vegetable beverages. Indian Journal of Biochemistry and Biophysics, 54, 191–199.
Mano, M. C. R., Neri-Numa, I. A., da Silva, J. B., Paulino, B. N., Pessoa, M. G., & Pastore, G. M. (2018). Oligosaccharide biotechnology: an approach of prebiotic revolution on the industry. Applied Microbiology and Biotechnology, 102(1), 17–37.
Sánchez-Rubio, M., Taboada-Rodríguez, A., Cava-Roda, R., López-Gómez, A., & Marín-Iniesta, F. (2016). Combined use of thermo-ultrasound and cinnamon leaf essential oil to inactivate Saccharomyces cerevisiae in natural orange and pomegranate juices. LWT - Food Science and Technology, 73, 140–146.
Iglesias, M. B., Abadias, M., Anguera, M., Sabata, J., & Viñas, I. (2017). Antagonistic effect of probiotic bacteria against foodborne pathogens on fresh-cut pear. LWT - Food Science and Technology, 81, 243–249.
Jessie Lau, L. Y., & Chye, F. Y. (2018). Antagonistic effects of Lactobacillus plantarum 0612 on the adhesion of selected foodborne enteropathogens in various colonic environments. Food Control, 91, 237–247.
Foster, R. K., & Marriott, H. E. (2006). Alcohol consumption in the new millennium - weighing up the risks and benefits for our health. Nutrition Bulletin, 31(4), 286–331.
Sabokbar, N., & Khodaiyan, F. (2016). Total phenolic content and antioxidant activities of pomegranate juice and whey based novel beverage fermented by kefir grains. Journal of Food Science and Technology, 53(1), 739–747.
Coda, R., Lanera, A., Trani, A., Gobbetti, M., & Di Cagno, R. (2012). Yogurt-like beverages made of a mixture of cereals, soy and grape must: microbiology, texture, nutritional and sensory properties. International Journal of Food Microbiology, 155(3), 120–127.
Đorđević, T. M., Šiler-Marinković, S. S., & Dimitrijević-Branković, S. I. (2010). Effect of fermentation on antioxidant properties of some cereals and pseudo cereals. Food Chemistry, 119(3), 957–963.
Valero-Cases, E., Nuncio-Jáuregui, N., & Frutos, M. J. (2017). Influence of fermentation with different lactic acid bacteria and in vitro digestion on the biotransformation of phenolic compounds in fermented pomegranate juices. Journal of Agricultural and Food Chemistry, 65(31), 6488–6496.
Di Cagno, R., Filannino, P., & Gobbetti, M. (2017). Lactic acid fermentation drives the optimal volatile flavor-aroma profile of pomegranate juice. International Journal of Food Microbiology, 248, 56–62.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mantzourani, I., Terpou, A., Alexopoulos, A. et al. Production of a Potentially Synbiotic Pomegranate Beverage by Fermentation with Lactobacillus plantarum ATCC 14917 Adsorbed on a Prebiotic Carrier. Appl Biochem Biotechnol 188, 1096–1107 (2019). https://doi.org/10.1007/s12010-019-02977-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-019-02977-4