Abstract
A cosmid metagenomic library containing 1.3 × 105 clones was created from a soil sample. A novel gene (fae-xuan) encoding a feruloyl esterase was identified through functional screening. Primary sequence analysis showed that the gene consisted of 759 base pairs and encoded a protein of 252 amino acids. The gene was expressed in Escherichia coli BL21 (DE3) and the corresponding purified recombinant enzyme exhibited a molecular weight of 29 kDa. The FAE-Xuan showed high activity (40.0 U/mg) toward methyl ferulate with an optimal temperature and pH of 30 °C and 5.0, respectively. Besides methyl ferulate, FAE-Xuan can also hydrolyze methyl sinapate and methyl p-coumarate. The substrate utilization preferences and phylogenetic analysis indicated that FAE-Xuan belongs to type A FAE. FAE-Xuan was quite stable over a broad pH range from 3.0 to 10.0. The activity reduced remarkably in presence of Cu2+. FAE-Xuan can enhance the quantity of ferulic acid from de-starched wheat bran in presence of xylanase. The work presented here highlighted the effectiveness of metagenomic strategy in identifying novel FAEs with diverse properties for potential use in industrial production.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Feruloyl esterases (FAEs; E.C. 3.1.1.73), also referred to as cinnamoyl esterases, are a subclass of the carboxylic ester hydrolases which catalyze the hydrolysis of the ester linkages between polysaccharides (arabinoxylan, pectin, etc.) and phenolic acids (ferulic acid and p-coumaric) present in plant cell walls, thus facilitating the cell walls biodegradation [1, 2]. The released phenolic acids exhibited excellent biological activities, e.g., antioxidant, anti-inflammatory, antimicrobial, and anti-cancer and are hence effective components in food and pharmaceutical industries [3, 4]. Besides hydrolysis, feruloyl esterases are also widely used in paper pulp industry [5] and feed industry [6]. Given the broad applications of feruloyl esterases, much more attention has been recently paid to the enzyme.
Over the past few years, studies have revealed that many fungi and bacteria can produce FAE [7,8,9,10,11] and a large number of FAE-encoding genes have also been identified [12,13,14]. Unfortunately, the traditional enzyme screening approaches mainly depended on cultured microorganisms which constituted only a small proportion of the microbial kingdom [15] thus greatly limited the resource exploitation of novel FAEs. In fact, more than 99% of bacteria present in environment cannot be readily cultured using current cultivation technology [15]. The uncultured bacteria promise to be a large, although challenging, reservoir of unexplored FAEs. Recently, more interest has been fueled on the culture-independent metagenomic methods which proved to be a powerful tool in mining new FAEs. According to the metagenomic strategy, DNA is extracted directly from environmental samples, and after which, the DNA is cloned into a model host such as Escherichia coli. The resulting clones are screened by either sequence-driven or activity-driven procedures [16]. The uncultured bacteria are more readily accessible by metagenomic strategy, resulting in the number of discovered enzymes increasing rapidly. For example, several novel enzymes including FAE, xylanase, esterase, chitosanase, and protease were discovered by metagenomic methods [17,18,19,20,21] and they exhibited higher activity than those isolated from cultured microorganisms. Given that each gram of soil involves thousands of bacterial species [22, 23], we concentrated on the soil environments in search of novel FAEs using a metagenomic strategy.
In this study, a cosmid metagenomic library was constructed from soil samples and a novel FAE gene (fae-xuan) was discovered through functional screening. The gene was heterologously expressed in E. coli BL21 (DE3). The purified recombinant FAE-Xuan showed high activity (40.0 U/mg) toward methyl ferulate; it retained a broad pH applicability, especially being with strong activity and high stability in acidic conditions, all of which made FAE-Xuan an excellent candidate for industrial applications.
Materials and Methods
Bacterial Strains, Plasmids, and Enzymes
E. coli EPI100 and pWEB (Epicentre, USA) were used as the host and vector for cosmid library construction, respectively. E. coli DH5α (Novoprotein, Shanghai, China) and PUC118 (Takara, Dalian, China) were employed for subcloning. E. coli BL21 (DE3) and pET28a (Yuanye, Shanghai, China) were used for expression of recombinant protein. T4 DNA ligase, DNA polymerase, and all the restriction enzymes were purchased from Takara (Dalian, China). Primers were synthesized from General Biosystems (Chuzhou, China). Standard chemicals were obtained from Solarbio (Shanghai, China).
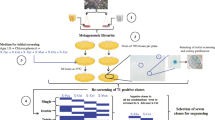
Metagenomic Library Construction
DNA from a soil sample was isolated and purified as previously described [24]. The purified soil DNA was subjected to metagenomic library construction using the pWEB™ Cosmid Cloning Kit (Epicentre, USA). Typically, the DNA was randomly sheared, end-repaired, and ligated to the pWEB vector. Cosmid clones were packaged with lambda phage packaging extracts, transfected into E. coli EPI100, and selected on LB agar containing 100 μg/mL ampicillin.
Functional Screening of FAE and Subcloning
Primary screening was performed on the LB-methyl ferulate agar plates and the transformants were cultured at 37 °C for 48 h. Feruloyl esterase-positive clones were identified by the formation of a clear halo surrounding the colony margins [25]. The identified positive clones were further analyzed for FAE activity by high-performance liquid chromatography (HPLC) equipped with a Zorbax SB-C18 column (Agilent Technologies, USA). A gradient elution was used with 25% water (containing 1% acetic acid) and 75% methanol at a flow rate of 0.8 mL/min and detected at 320 nm. Briefly, the positive clones were transferred to LB broth containing 100 μg/mL ampicillin and shaken on a rotary shaker at 37 °C overnight. The cells were harvested by centrifugation at 8000g for 10 min and resuspended in deionized water. The cell suspension was then inoculated to LB broth supplemented with methyl ferulate. The fermentation broth was centrifugated and concentrated and a small fraction of supernatant was later analyzed by HPLC. The culture supernatant from E. coli EPI100 harboring pWEB vector without insertion was used as negative control. The detected FA indicated FAE production.
The restriction enzyme BamHI was used to partially digest cosmid DNA that was extracted from an identified positive clone. The resulting DNA fragments (1–5 kb) were gel-purified, ligated into BamHI-digested dephosphorylated pUC118 and introduced into E. coli DH5α. The transformants were rescreened against FAE activity and confirmed by DNA sequencing. In this study, the putative FAE, named FAE-Xuan, has been deposited at the NCBI GenBank under the accession number MG874041.
Bioinformatic Analysis
Sequences were annotated using the online server ORF Finder to analyze open reading frames (ORFs) (https://www.ncbi.nlm.nih.gov/orffinder/). The similarity of amino acid sequences was investigated by the BLAST program (https://blast.ncbi.nlm.nih.gov/Blast.cgi).[26]. Multiple homology alignments were carried out using the Clustal Omega program (https://www.ebi.ac.uk/Tools/msa/clustalo/) [27]. The phylogenetic tree was constructed using the neighbor joining algorithm with MEGA 6.0 (http://www.megasoftware.net/) [28]. Theoretical molecular weight and isoelectric point were computed using ExPASy server (https://web.expasy.org/protparam/). The prediction of the tertiary structure was performed by a SWISS-MODEL server (https://www.swissmodel.expasy.org/) [29].
Expression and Purification of Recombinant FAE
The putative FAE gene was amplified with the following primers: fae-f, 5′-CATGCCATGGGCATGCGTGCAGGGGGGAG-3′ and fae-r, 5′-CCCAAGCTTCCGGCCGCTCAGCCAGT-3′ (the restriction enzyme sites were underlined for NcoI and HindIII, respectively). The amplified PCR products were double digested with NcoI and HindIII, followed by ligation into the pET28a expression vector digested with the corresponding enzymes to fuse a hexahistidine. The recombinant plasmid was transformed into E. coli BL21 (DE3).
The recombinant E. coli was cultured at 37 °C in LB broth supplemented with 50 μg/mL kanamycin. When the cell concentration reached an OD600nm of 0.6, they were induced by the addition of 0.3 mM isopropyl-β-d-thiogalactopyranoside (IPTG) for 12 h at 37 °C with shaking at 180 rpm. The cells were harvested by centrifugation at 12000g for 15 min at 4 °C and resuspended in 1× PBS buffer (pH 7.2) followed by addition of 200 μg/mL lysozyme. The harvested cells were lysed by sonication and the supernatant was collected by centrifugation at 12000g for 30 min at 4 °C. The recombinant enzyme was purified by Ni-NTA-Sefinose Column (Sangon Biotech, China) according to the manufacturer’s instructions. Enzyme purity and molecular weight were examined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE).[30].
Enzyme Activity
FAE activity was tested by measuring the release of FA from methyl ferulate (MFA).[31, 32]. The reaction mixture was carried out in citric acid-sodium citrate buffer (pH 5.0), which contained properly diluted enzyme as well as 0.5 mM MFA as the substrate, at 30 °C for 10 min. The released FA was monitored at 320 nm by UV-2102PC (UNICO, China). The reactions were performed in triplicate. One unit of FAE activity was defined as the amount of enzyme required to release 1 μmol FA per min from MFA [33].
Characterization of FAE-Xuan
The optimum temperature was studied by measuring the enzyme activity in 50 mM citric acid-sodium citrate (pH 5.0) at different temperatures ranging from 4 to 50 °C. To determine thermostability, the enzyme was preincubated at different temperatures between 30 and 50 °C, and the residual activity was determined under optimal conditions (pH 5.0, 30 °C) at 1 h intervals. The optimal pH for enzyme activity was identified at 30 °C in various buffers: 50 mM citric acid-sodium citrate (pH 3.0–pH 6.0), 50 mM Tris-HCl (pH 6.0–pH 8.0) and 50 mM glycine-NaOH (pH 8.0–pH 10.0). To estimate pH stability, the enzyme was preincubated in various buffers (pH 3.0–pH 10.0) at 4 °C for 4 h, and the residual activity was determined under optimal conditions (pH 5.0, 30 °C).
Methyl p-coumarate (MpCA), methyl caffeate (MCA), and methyl sinapate (MSA) were utilized to test the substrate specificity of the purified FAE. The activity was measured as described above. In addition, enzyme kinetic parameters (Km, Vmax, kcat, kcat/Km) were also determined at different substrate concentrations ranging from 0.02 to 0.5 mM.
The effects of metal ions (5 mM) and chemical reagents including 5 mM EDTA, 1% SDS (w/v), 15% DMSO, 15% acetone, 15% isopropanol, and 15% DMF on the enzyme activity were investigated and the purified enzyme was preincubated with various chemicals. The residual enzyme activity was then determined and the activity of the enzyme without any additive was defined as 100% activity. The results were analyzed according to Duncan’s multiple comparison test (p < 0.05), using version V8 of the SAS software package. All assays were performed in triplicate.
Release of Ferulic Acid from De-starched Wheat Bran
The ability of the purified FAE-Xuan to release ferulic acid (FA) was examined using de-starched wheat bran (DSWB) as the hydrolysis substrate. Wheat bran was incubated in 0.3% potassium acetate at 95 °C for 30 min followed by discarding supernatant and washing with water repeatedly until the starch was removed completely [34]. The DSWB was dried at 80 °C for 10 h, milled to power followed by passing a 60-mesh sieve. The reaction mixture was performed in citric acid-sodium citrate buffer (pH 5.0) at 30 °C for 12 h. FAE-Xuan (100 U) was inoculated to the 50 mg DSWB solely or combined with commercial xylanase (200 U), and the buffer without enzyme was used as the control. The released FA was determined by HPLC as described above.
Results and Discussion
Metagenomic Library Construction and FAE Screening
In the current investigation, a cosmid metagenomic library was constructed from a soil sample to screen feruloyl esterases. The library contained 1.3 × 105 clones with an average DNA insert size of 40 kb and the content represented around 5.2 Gbp genomic DNA. A positive clone was primarily identified by the presence of a clear halo around the colony margin (Fig. 1) and its FAE activity was further confirmed by HPLC analysis. As shown in Fig. 2, the positive clone was able to partially hydrolyze methyl ferulate to release FA, and its fermentation broth displayed a significant peak compared with that of control. These results indicated that the positive clone possessed FAE activity. The subcloning library consisted of around 2000 clones with the average insert size was 3 kb. Only one positive subclone was obtained after screening and sequenced for further study.
To date, functional screening is an excellent strategy in the discovery of novel small molecules and enzymes, which mainly relies on easily observable phenotypic traits, such as pigmentation and transparent circles [35, 36]. For example, Hu et al. discovered a cold-active esterase using functional metagenomics [37]. The same pipeline was also used to characterize a new β-galactosidases from a soil metagenomic library [38].
Sequence Analysis of FAE-Xuan
FAE-Xuan contained a 759-bp ORF encoding a protein of 252 amino acids with a predicted theoretical MW of 27 kDa and a pI of 10. A BLASTp analysis showed that FAE-Xuan displayed the highest identity (49%) with the carboxylesterase from Gluconacetobacter sp. SXCC-1 (GenBank accession number WP_007396101) and 48% identity with the carboxylesterase from Magnetospirillum gryphiswaldense (GenBank accession number WP_024080221) at the amino acid level.
The multi-alignment analysis of the FAE-Xuan amino acid sequences with the most homologous sequences are shown in Fig. 3. The results suggested that FAE-Xuan contained a classical conserved pentapeptide signature motif G-X-S-X-G (amino acid positions 82–86). The region has been discovered in many other feruloyl esterases, such as EstF27 from a soil metagenomic library [39], FaeC from Aspergillus niger N402 [32], and Fae6 from a leachate metagenomic library [17]. The serine (S, 84) residue within the motif potentially acts as a catalytic nucleophile and together with aspartate (D, 203) and histidine (H, 224) residues constitute a typical catalytic triad of esterase [40].
Multialignment analysis of the FAE-Xuan amino acid sequences with the most homologous sequences. The exact same residues were marked with an asterisk and in gray background. The conserved pentapeptide motif G-X-S-X-G and the putative catalytic triad (S84, D203, and H224) were indicated by a red solid box and dots, respectively
The FAE-Xuan tertiary structure was predicted by the SWISS-MODEL program using homology modeling with TtEst as a template [41]. As shown in Fig. 4, a typical α/β hydrolase fold is observed in the three-dimensional structure of FAE-Xuan, which consists of a cap domain and a catalytic domain [42]. The cap domain is formed by α-helice regions that are located at the upper of the central β-sheet and functions as the entrance to the substrate binding pocket, whereas the catalytic domain is composed of a β-sheet flanked by α-helices on both sides.
Three-dimensional of FAE-Xuan. A solid ribbon diagram of FAE-Xuan showed a classical α/β hydrolase fold, which consists of a cap domain and a catalytic domain. The α-helices, β-strands, and random coils were shown in red, green, and gray respectively. The putative catalytic triad (S84, D203, and H224) was indicated as ball-and-stick representations
Phylogenic Relationships and Classification of the FAE-Xuan
Based on primary sequence similarity, as well as substrate specificity on four model substrates (MFA, MpCA, MCA, and MSA), FAEs were categorized into four subclasses (types A–D) [43]. Generally, type A FAEs are active against MFA, MSA, and MpCA, but inactive against MCA, whereas type B FAEs prefer substrates MFA, MCA, and MpCA, but not MSA. Notably, types C and D retain broader substrate specificity with activities on the four model substrates.
A phylogenetic tree was generated using FAE-Xuan amino acid sequences with other FAEs of the known types A to D by the neighbor joining method (Fig. 5). The different types of FAE were clustered into four groups, with each type forming different clades. FAE-Xuan belonged to the cluster A, which showed that our new FAE was clearly a member of type A FAEs.
Members of the same subclass exhibited similar substrate specificity toward four methyl hydroxycinnamates, indicating that FAE-Xuan only selectively hydrolyzed MFA, MSA, and MpCA, but had no activity against MCA, resembling the other type A FAEs of the cluster A. Many type A FAEs were obtained from different culture-dependent sources [34, 44, 45], and to the best of our knowledge, only one type A FAE EstF27 was discovered from the soil metagenomic library [39]. FAE-Xuan was the second new FAE found by the same procedure. EstF27 was constituted by 291 amino acids and displayed optimal activity at 40 °C and pH 6.8. FAE-Xuan only contained 256 amino acids with the maximum activity at 30 °C and pH 5.0, especially, the activity of FAE-Xuan was 5.5, 3.7, and 4.3 times higher than that of EstF27 against MFA, MSA, and MpCA respectively.
Purification of the Expressed FAE-Xuan
FAE-Xuan was purified with the Ni-NTA column and analyzed by SDS-PAGE (Fig. 6). The purified protein fusing with polyhistidine tag showed a MW of 29 kDa, which was in accordance with the theoretical MW of 27 kDa.
Biochemical Characterization of FAE-Xuan
The biochemical characterization of purified FAE-Xuan was carried out using MFA as the substrate. Previous researches have revealed that FAEs had enzyme activity at temperatures ranging from 20 to 75 °C. But basically, they are active below 50 °C [31]. In this study, activity was examined over a temperature from 5 to 50 °C (Fig. 7a). The result showed that the activity of FAE-Xuan increased continuously as temperature increases from 5 to 30 °C and exhibited a maximum activity at 30 °C, followed by a decrease from 30 to 50 °C and almost inactive at 50 °C. The optimal temperature was similar to many FAE identified, such as AcFAE from Aspergillus clavatus [46] and FAE from Streptomyces olivochromogenes [47]. Figure 7b showed that FAE-Xuan activity was stable only at 30 °C.
The influences of pH on the enzyme activity and stability were also studied. FAE-Xuan was stable over a quite broad range of pH 3.0–10.0 (Fig. 7d). It exhibited an optimum pH at 5.0 (Fig. 7c), which is comparable to FAEA from A. niger [48] and AoFaeA from Aspergillus oryzae [34], and kept over 65% of the initial activity around pH 4.0–7.0; especially, it still retained 40% activity when the pH was as low as 3.0. However, the activity reduced rapidly above pH 8.0 and fully deactivated at pH 10.0. These characteristics suggested that the FAE-Xuan is an acidic enzyme. The strong activity in acidic conditions and the high stability in both acidic and alkaline conditions made FAE-Xuan a promising candidate for applying in industry.
To examine the substrate specificity of the purified FAE-Xuan, four synthetic methyl hydroxycinnamates including MFA, MCA, MpCA, and MSA were used. As shown in Table 1, FAE-Xuan exhibited significant activity toward all four substrates except for MCA, which further suggested that it is indeed a type A FAE based on functional classification [43], and this is in agreement with phylogenetic analysis. Specifically, the maximum activity was as high as 40.0 U/mg when using MFA as a substrate and was much higher than that of EstF27 from a soil metagenomic library (7.3 U/mg) [39], TsFaeB from Talaromyces stipitatus (12.2 U/mg) [49], and AnidFAE from A. nidulans (21.7 U/mg) [50]. From the values of Km, FAE-Xuan possessed the highest substrate affinity for MFA, followed by MSA and MpCA. Besides, MFA was proved to be more efficient according to the calculated highest catalytic turnover (kcat) and catalytic efficiencies (kcat/Km).
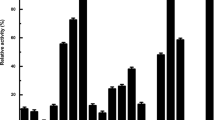
For further characterization of the FAE-Xuan, the effects of metal ions and chemical reagents on the enzyme activity were studied. As indicated in Fig. 8, the activity of FAE-Xuan was not affected by addition of Ca2+, but strongly inhibited by the presence of Ba2+ and Zn2+. Especially, the enzyme activity significantly reduced by addition of Cu2+, which was consistent with the properties of FAEs from Lactobacillus johnsonii [51], Clostridium stercorarium [52], and a soil metagenomic library [39]. In addition, a slight inhibition to the FAE-Xuan activity was observed in the presence of Fe2+ and Fe3+. Significant enzyme activity was enhanced by addition of K+, Na+, and Mg2+ to 120, 131, and 116% of the maximum activity respectively, suggesting that these metal ions assisted in increasing the FAE-Xuan activity. Further, EDTA, SDS, DMSO, and acetone had little effect on enzyme activity, whereas isopropanol and DMF strongly inhibited its activity.
Synergistic Interaction Between FAE-Xuan and Xylanase in Releasing FA from DSWB
The ability of the purified FAE-Xuan to release FA was examined using DSWB as the hydrolysis substrate. As shown in Fig. 9, no FA or very few FA was detected without enzymes or with xylanase alone. However, the quantity of released FA reached 1.51 μg/mL in the presence of FAE-Xuan. Furthermore, a significant increase of the released FA (4.66 μg/mL) were observed under the simultaneous presence of FAE-Xuan and xylanase, suggesting a synergistic interaction between the two enzymes in releasing FA from DSWB, which was consistent with the previous reports [34, 39, 53].
The low identity of FAE-Xuan with other known FAEs indicated that it is a novel FAE. Although the thermostability of FAE-Xuan is not comparable to that of FAEs from T. tengcongensis [54] and termite gut [55], it can retain high activity in acidic conditions and has a wide range of pH, all of which make it a valuable and potential candidate in industry.
In conclusion, the gene fae-xuan, which encoded a novel FAE, was successfully screened from a soil metagenomic library. The enzyme exhibited maximal activity at the pH of 5.0 and was quite stable over a wide pH range, especially, it retained high activity at a very low pH. Moreover, FAE-Xuan was non-sensitive to most of mental ions and chemical reagents. The quantity of FA released from DSWB was remarkably improved in the presence of xylanase, compared with using FAE-Xuan alone. The results from the current research highlighted the advantages of metagenomics approach for the discovery of new enzymes from soils, and this method could be particularly useful in mining new biocatalysts for application in modern agriculture, pharmaceutical, and food industries.
Change history
26 February 2019
The original version of this article unfortunately contained a mistake in the image and caption of Fig. 6. The corrected version of the image and caption is shown here.
References
Faulds, C. B. (2010). What can feruloyl esterases do for us? Phytochemistry Reviews, 9(1), 121–132.
Wong, D. W. (2006). Feruloyl esterase: a key enzyme in biomass degradation. Applied Biochemistry & Biotechnology Part A Enzyme Engineering & Biotechnology, 133(2), 87–112.
Ou, S., & Kwok, K. C. (2004). Ferulic acid: pharmaceutical functions, preparation and applications in foods. Journal of the Science of Food and Agriculture, 84(11), 1261–1269.
Kumar, N., & Pruthi, V. (2014). Potential applications of ferulic acid from natural sources. Biotechnology Reports, 4, 86–93.
Record, E., Asther, M., Sigoillot, C., Pagès, S., Punt, P. J., Delattre, M., Haon, M., Ca, V. D. H., Sigoillot, J. C., & Lesagemeessen, L. (2003). Overproduction of the Aspergillus niger feruloyl esterase for pulp bleaching application. Applied Microbiology and Biotechnology, 62(4), 349–355.
Lynchj, P., Prema, D., Van Hamme, D., Church, J. S., & Beauchemin, K. A. (2014). Fiber degradability, chemical composition and conservation characteristics of alfalfa haylage ensiled with exogenous fbrolytic enzymes and a ferulic acid esterase-producing inoculant. Revue Canadienne De Science Animale, 94, 697–704.
Hassan, S., & Hugouvieux-Cotte-Pattat, N. (2011). Identification of two feruloyl esterases in Dickeya dadantii 3937 and induction of the major feruloyl esterase and of pectate lyases by ferulic acid. Journal of Bacteriology, 193(4), 963–970.
Koseki, T., Takahashi, K., Fushinobu, S., Iefuji, H., Iwano, K., Hashizume, K., & Matsuzawa, H. (2005). Mutational analysis of a feruloyl esterase from Aspergillus awamori involved in substrate discrimination and pH dependence. Biochimica Et Biophysica Acta General Subjects, 1722(2), 200–208.
Rashamuse, K., Burton, S., & Cowan, D. (2007). A novel recombinant ethyl ferulate esterase from Burkholderia multivorans. Journal of Applied Microbiology, 103(5), 1610–1620.
Rumbold, K., Biely, P., Mastihubová, M., Gudelj, M., Gübitz, G., Robra, K. H., & Prior, B. A. (2003). Purification and properties of a feruloyl esterase involved in lignocellulose degradation by Aureobasidium pullulans. Applied and Environmental Microbiology, 69(9), 5622–5626.
Zeng, W., & Chen, H. Z. (2009). Air pressure pulsation solid state fermentation of feruloyl esterase by Aspergillus niger. Bioresource Technology, 100(3), 1371–1375.
Blum, D. L., Kataeva, I. A., Li, X. L., & Ljungdahl, L. G. (2000). Feruloyl esterase activity of the Clostridium thermocellum cellulosome can be attributed to previously unknown domains of XynY and XynZ. Journal of Bacteriology, 182(5), 1346–1351.
Dalrymple, B. P., & Swadling, Y. (1997). Expression of a Butyrivibrio fibrisolvens E14 gene (cinB) encoding an enzyme with cinnamoyl ester hydrolase activity is negatively regulated by the product of an adjacent gene (cinR). Microbiology, 143(4), 1203–1210.
Li, J., Cai, S., Luo, Y., & Dong, X. (2011). Three feruloyl esterases in Cellulosilyticum ruminicola H1 act synergistically to hydrolyze esterified polysaccharides. Applied and Environmental Microbiology, 77(17), 6141–6147.
Mark, B., Petra, H., Davidp, W., Jesals, P., Flash, B., Timothys, H., & Uwet, B. (2008). Characterization of lipases and esterases from metagenomes for lipid modification. Journal of the American Oil Chemists Society, 85, 47–53.
Handelsman, J. (2004). Metagenomics: application of genomics to uncultured microorganisms. Microbiology and Molecular Biology Reviews: MMBR, 68(4), 669–685.
Rashamuse, K., Sanyika, W., Ronneburg, T., & Brady, D. (2012). A feruloyl esterase derived from a leachate metagenome library. BMB Reports, 45(1), 14–19.
Cheng, F., Sheng, J., Dong, R., Men, Y., Gan, L., & Shen, L. (2012). Novel xylanase from a Holstein cattle rumen metagenomic library and its application in xylooligosaccharide and ferulic acid production from wheat straw. Journal of Agricultural and Food Chemistry, 60(51), 12516–12524.
Elend, C., Schmeisser, C., Leggewie, C., Babiak, P., Carballeira, J. D., Steele, H. L., Reymond, J. L., Jaeger, K. E., & Streit, W. R. (2006). Isolation and biochemical characterization of two novel metagenome-derived esterases. Applied Biochemistry and Biotechnology, 169, 3637–3645.
Li, H., Fei, Z., Gong, J., Yang, T., Xu, Z., & Shi, J. (2015). Screening and characterization of a highly active chitosanase based on metagenomic technology. Journal of Molecular Catalysis B: Enzymatic, 111, 29–35.
Lee, D. G., Jeon, J. H., Jang, M. K., Kim, N. Y., Lee, J. H., Lee, J. H., Kim, S. J., Kim, G. D., & Lee, S. H. (2007). Screening and characterization of a novel fibrinolytic metalloprotease from a metagenomic library. Biotechnology Letters, 29(3), 465–472.
Torsvik, V., Daae, F. L., Sandaa, R. A., & Ovreås, L. (1998). Novel techniques for analysing microbial diversity in natural and perturbed environments. Journal of Biotechnology, 64(1), 53–62.
Torsvik, V., Goksøyr, J., & Daae, F. L. (1990). High diversity in DNA of soil bacteria. Applied and Environmental Microbiology, 56(3), 782–787.
Brady, S. F. (2007). Construction of soil environmental DNA cosmid libraries and screening for clones that produce biologically active small molecules. Nature Protocols, 2(5), 1297–1305.
Donaghy, J., Kelly, P. F., & Mckay, A. M. (1998). Detection of ferulic acid esterase production by Bacillus spp. and lactobacilli. Applied Microbiology and Biotechnology, 50(2), 257–260.
Altschul, S. F., Wootton, J. C., Gertz, E. M., Agarwala, R., Morgulis, A., Schäffer, A. A., & Yu, Y. K. (2005). Protein database searches using compositionally adjusted substitution matrices. FEBS Journal, 272(20), 5101–5109.
Sievers, F., Wilm, A., Dineen, D., Gibson, T. J., Karplus, K., Li, W., Lopez, R., Mcwilliam, H., Remmert, M., & Söding, J. (2014). Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Molecular Systems Biology, 7(1), 539–544.
Saitou, N., & Nei, M. (1987). The neighbor-joining method: a new method for reconstructing phylogenetic trees. Molecular Biology and Evolution, 4(4), 406–425.
Biasini, M., Bienert, S., Waterhouse, A., Arnold, K., Studer, G., Schmidt, T., Kiefer, F., Cassarino, T. G., Bertoni, M., & Bordoli, L. (2014). SWISS-MODEL: modelling protein tertiary and quaternary structure using evolutionary information. Nucleic Acids Research, 42, 252–258.
Laemmli, U. K. (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature, 227(5259), 680–685.
Dilokpimol, A., Mäkelä, M. R., Aguilarpontes, M. V., Benoitgelber, I., Hildén, K. S., & Vries, R. P. (2016). Diversity of fungal feruloyl esterases: updated phylogenetic classification, properties, and industrial applications. Biotechnology for Biofuels, 9(1), 231–248.
Dilokpimol, A., Mäkelä, M. R., Mansouri, S., Belova, O., Waterstraat, M., Bunzel, M., Vries, R. P. D., & Hildén, K. S. (2017). Expanding the feruloyl esterase gene family of Aspergillus niger by characterization of a feruloyl esterase, FaeC. New Biotechnology, 37(Pt B), 200–209.
Shin, H. D., & Chen, R. R. (2007). A type B feruloyl esterase from Aspergillus nidulans with broad pH applicability. Applied Microbiology and Biotechnology, 73(6), 1323–1330.
Zeng, Y., Yin, X., Wu, M. C., Yu, T., Feng, F., Zhu, T. D., & Pang, Q. F. (2014). Expression of a novel feruloyl esterase from Aspergillus oryzae in Pichia pastoris with esterification activity. Journal of Molecular Catalysis B: Enzymatic, 110, 140–146.
Handelsman, J., Rondon, M. R., Brady, S. F., Clardy, J., & Goodman, R. M. (1998). Molecular biological access to the chemistry of unknown soil microbes: a new frontier for natural products. Chemistry & Biology, 5, 245–249.
Iqbal, H. A., Feng, Z., & Brady, S. F. (2012). Biocatalysts and small molecule products from metagenomic studies. Current Opinion in Chemical Biology, 16(1-2), 109–116.
Hu, X. P., Heath, C., Taylor, M. P., Tuffin, M., & Cowan, D. (2011). A novel, extremely alkaliphilic and cold-active esterase from Antarctic desert soil. Extremophiles Life Under Extreme Conditions, 16, 79–86.
Wang, K., Li, G., Yu, S. Q., Zhang, C. T., & Liu, Y. H. (2010). A novel metagenome-derived β-galactosidase: gene cloning, overexpression, purification and characterization. Applied Microbiology and Biotechnology, 88(1), 155–165.
Sang, S. L., Li, G., Hu, X. P., & Liu, Y. H. (2011). Molecular cloning, overexpression and characterization of a novel feruloyl esterase from a soil metagenomic library. Journal of Molecular Microbiology and Biotechnology, 20(4), 196–203.
Arpigny, J. L., & Jaeger, K. (1999). Bacterial lipolytic enzymes: classification and properties. The Biochemical Journal, 343(1), 177–183.
Sayer, C., Isupov, M. N., Bonchosmolovskaya, E., & Littlechild, J. A. (2015). Structural studies of a thermophilic esterase from a new Planctomycetes species, Thermogutta terrifontis. FEBS Journal, 282(15), 2846–2857.
Pereira, M. R., Maester, T. C., Mercaldi, G. F., Lemos, E. G. D. M., Hyvönen, M., & Balan, A. (2017). From a metagenomic source to a high-resolution structure of a novel alkaline esterase. Applied Microbiology and Biotechnology, 101, 1–15.
Crepin, V. F., Faulds, C. B., & Connerton, I. F. (2004). Functional classification of the microbial feruloyl esterases. Applied Microbiology and Biotechnology, 63(6), 647–652.
Zhang, S. B., Wang, L., Liu, Y., Zhai, H. C., Cai, J. P., & Hu, Y. S. (2015). Expression of feruloyl esterase A from Aspergillus terreus and its application in biomass degradation. Protein Expression and Purification, 115, 153–157.
Nieter, A., Haaseaschoff, P., Linke, D., Nimtz, M., & Berger, R. G. (2014). A halotolerant type A feruloyl esterase from Pleurotus eryngii. Fungal Biology, 118(3), 348–357.
Damásio, A. R. L., Braga, C. M. P., Brenelli, L. B., Citadini, A. P., Mandelli, F., Cota, J., Almeida, R. F. D., Salvador, V. H., Paixao, D. A. A., & Segato, F. (2013). Biomass-to-bio-products application of feruloyl esterase from Aspergillus clavatus. Applied Microbiology and Biotechnology, 97(15), 6759–6767.
Faulds, C. B., & Williamson, G. (1991). The purification and characterization of 4-hydroxy-3-methoxycinnamic (ferulic) acid esterase from Streptomyces olivochromogenes. Journal of General Microbiology, 137(10), 2339–2345.
Vries, R. P. D., Michelsen, B., Poulsen, C. H., Kroon, P. A., Heuvel, R. H. V. D., Faulds, C. B., Williamson, G., Hombergh, J. P. V. D., & Visser, J. (1997). The faeA genes from Aspergillus niger and Aspergillus tubingensis encode ferulic acid esterases involved in degradation of complex cell wall polysaccharides. Applied and Environmental Microbiology, 63(12), 4638–4644.
Garcia-Conesa, M. T., Crepin, V. F., Goldson, A. J., Williamson, G., Cummings, N. J., Connerton, I. F., Faulds, C. B., & Kroon, P. A. (2004). The feruloyl esterase system of Talaromyces stipitatus: production of three discrete feruloyl esterases, including a novel enzyme, TsFaeC, with a broad substrate specificity. Journal of Biotechnology, 108(3), 227–241.
Debeire, P., Khoune, P., Jeltsch, J. M., & Phalip, V. (2012). Product patterns of a feruloyl esterase from Aspergillus nidulans on large feruloyl-arabino-xylo-oligosaccharides from wheat bran. Bioresource Technology, 119, 425–428.
Lai, K. K. (2009). Biochemical properties of two cinnamoyl esterases purified from a Lactobacillus johnsonii strain isolated from stool samples of diabetes-resistant rats. Applied and Environmental Microbiology, 75(15), 5018–5024.
Donaghy, J. A., Bronnenmeier, K., Sotokelly, P. F., & Mckay, A. M. (2000). Purification and characterization of an extracellular feruloyl esterase from the thermophilic anaerobe Clostridium stercorarium. Journal of Applied Microbiology, 88(3), 458–466.
Topakas, E., Moukouli, M., Dimarogona, M., & Christakopoulos, P. (2012). Expression, characterization and structural modelling of a feruloyl esterase from the thermophilic fungus Myceliophthora thermophila. Applied Microbiology and Biotechnology, 94(2), 399–411.
Abokitse, K., Wu, M., Bergeron, H., Grosse, S., & Lau, P. C. (2010). Thermostable feruloyl esterase for the bioproduction of ferulic acid from triticale bran. Applied Microbiology and Biotechnology, 87(1), 195–203.
Rashamuse, K., Ronneburg, T., Sanyika, W., Mathiba, K., Mmutlane, E., & Brady, D. (2014). Metagenomic mining of feruloyl esterases from termite enteric flora. Applied Microbiology and Biotechnology, 98(2), 727–737.
Funding
This work was supported by the Fund for Qing Lan Project of Jiangsu Province, by the Fundamental Research Funds for the Central Universities (KYYJ201708), and by special funds of agro-product quality safety risk assessment of the Ministry of Agriculture of the People’s Republic of China (GJFP201701505).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and animal rights
This article does not contain any studies with human or animal subjects.
Rights and permissions
About this article
Cite this article
Li, X., Guo, J., Hu, Y. et al. Identification of a Novel Feruloyl Esterase by Functional Screening of a Soil Metagenomic Library. Appl Biochem Biotechnol 187, 424–437 (2019). https://doi.org/10.1007/s12010-018-2832-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-018-2832-1