Abstract
Itaconic acid (IA; a building block platform chemical) is currently produced industrially from glucose by fermentation with Aspergillus terreus. In order to expand the use of IA, its production cost must be lowered. Lignocellulosic biomass has the potential to serve as low-cost source of sugars for IA production. It was found that the fungus cannot produce IA from dilute acid pretreated and enzymatically saccharified wheat straw hydrolysate even at 100-fold dilution. The effects of typical compounds (acetic acid, furfural, HMF and Mn2+, enzymes, CaSO4), culture conditions (initial pH, temperature, aeration), and medium components (KH2PO4, NH4NO3, CaCl2·2H2O, FeCl3·6H2O) on growth and IA production by A. terreus NRRL 1972 using mixed sugar substrate containing glucose, xylose, and arabinose (4:3:1, 80 g L−1) mimicking the wheat straw hydrolysate were investigated. Acetic acid, furfural, Mn2+, and enzymes were strong inhibitors to both growth and IA production from mixed sugars. Optimum culture conditions (pH 3.1, 33 °C, 200 rpm) and medium components (0.8 g KH2PO4, 3 g NH4NO3, 2.0 g CaCl2·2H2O, 0.83–3.33 mg FeCl3·6H2O per L) as well as tolerable levels of inhibitors (0.4 g acetic acid, < 0.1 g furfural, 100 mg HMF, < 5.0 ppb Mn2+, 24 mg CaSO4 per L) for mixed sugar utilization were established. The results will be highly useful for developing a bioprocess technology for IA production from lignocellulosic feedstocks.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Itaconic acid (IA; methylene succinic acid or methylene butanedioic acid) is a five-carbon (C5) unsaturated dicarboxylic acid with one carboxyl group conjugated to the methylene group. It is produced industrially from glucose by submerged fermentation using the filamentous fungus A. terreus [1,2,3]. In recent years, IA has gained importance as a fully sustainable building block chemical (platform chemical) for its broad applications in the manufacture of various synthetic resins, coatings, and polymers. It has applications in super-absorbents, phosphate-free detergents, and bioactive compounds particularly in the pharmaceutical industry and agriculture [4]. IA is regarded as α-substituted acrylic or methyl methacrylic acid (also known as Plexiglass) and has potential to replace fossil-based acrylic acid, and use of its derivatives for production of plastics is high. It is estimated that the conversion of IA to methyl methacrylate provides a giant market potential of up to 3.2 million tons per year [5]. IA can be used for synthesis of 3-methyltetrahydrofuran, a potential biofuel [5].
The market of IA is about 80,000 tons per year and the selling price is $2.00 kg−1 ($ 0.91 lb.−1) [6]. The USA represents the single largest market for IA, which is all imported from overseas. However, the production cost must be lowered in order to expand the market of IA [7]. It is one of the 12 building block molecules identified as potential new platform chemicals that can be produced from lignocellulosic sugars [8]. Recently, only a handful of research articles are available on the IA production by A. terreus strains using lignocellulosic hydrolysates. Tippkötter et al. [9] reported that A. terreus NRRL 1960 could not grow on untreated organosolv beech wood hydrolysate. They used several detoxification methods to remove the fermentation inhibitors. First, the fiber fraction was washed with 0.5 M NaOH at 50 °C overnight to remove lignin-derived phenolics prior to hydrolysis. The hydrolysate was then treated with zeolite for complete removal of monomeric phenolic compounds. The hydrolysate was finally treated with an equal mixture of anion and cation exchangers to remove metal ions. The fungal strain grew in the form of small pellets and produced 7.2 g IA from the detoxified hydrolysate containing 20.5 g glucose and 4.0 g xylose (total 24.5 g) per liter at a productivity of 0.1 g L−1 h−1 (41.7% of theoretical IA yield). Jimenz-Quero et al. [10] performed liquid state fermentation of acid and enzymatic hydrolysates of wheat bran and corn cob with a 10-times dilution and glucose added by two A. terreus strains (DSM 826 and 62071). No IA was formed by either of the two A. terreus strains tested. Pedrosa et al. [11] reported that A. terreus ATCC 10020 produced 1.9 g IA (yield of 49 mg g−1 sugar utilized) from H3PO4 pretreated and detoxified rice husk hydrolysate (41.0 g total sugars) utilizing 38.9 g sugar per L under optimized conditions. The detoxification of the hydrolysate was carried out by active carbon and CaO treatments. Krull et al. [12] pretreated wheat chaff with NaOH at room temperature, washed, and enzymatically saccharified with a commercial enzyme preparation. The hydrolysate as such gave only 0.6 g IA per L by fermentation with A. terreus DSM 23081. They obtained 27.7 g IA per L with a yield of 0.41 g g−1 total sugars from the enzymatically saccharified hydrolysate after purifying it through heat denaturation of protein, evaporation, and subsequently treating it with a cation exchanger. The concentrations of all cations were reduced by > 90%, and in particular, the Mn2+ concentration decreased from 1.72 mg L−1 to 0.5 μg L−1. It is evident that more research needs to be performed in order to cost-effectively utilize lignocellulosic hydrolysates as substrate for IA production.
Recently, 100 A. terreus strains were evaluated for growth and IA production from glucose, xylose, and arabinose [13]. Twenty strains showed good production of IA from all three sugars. Six strains (NRRL 1960, 1961, 1962, 1972, and 66125 and DSM 23081) were then chosen for investigation on growth and IA production from lignocellulosic hydrolysates. However, these strains could not even grow on dilute acid pretreated and enzymatically saccharified wheat straw hydrolysates (DAWSH) containing ~80 g mixed sugars per L [14]. In order to find out the reasons for such failure, the effects of typical inhibitory compounds and metal ions present in DAWSH on growth and IA production from mixed sugar substrate mimicking DAWSH by one selected A. terreus strain (NRRL 1972) were investigated. In addition, the effects of culture conditions and medium components for utilization of mixed sugars and production of IA are reported.
Materials and Methods
Materials
Wheat straw (WS) was purchased from a local farmer. It was dried in a forced-air oven at 55 °C for 24 h and milled in a hammer mill (W. J. Fitzpatrick Company, Troy, MI) to pass through a 1.27-mm screen. The milled WS was stored at room temperature. Celluclast 1.5 L (cellulase) and Novozyme 188 (β-glucosidase) were purchased from Brenntag Great Lakes, Milwaukee, WI, USA. Fiberzyme (hemicellulase) was supplied by Dyadic Corp., Jupiter, FL, USA. Aminex HPX 87P column (300 × 7.8 mm), Aminex HPX 87H column (300 × 7.8 mm), De-ashing cartridge (30 × 4.6 mm), Carbo-P micro-guard cartridge (30 × 4.6 mm), Cation H micro-guard cartridge (30 × 4.6 mm), and Dowex 50-X8 cation exchange resin (100/200 mesh) were purchased from Bio-Rad Laboratories Inc., Hercules, CA, USA. Membrane Filter Unit (0.2 mm) was purchased from Nalge Co., Rochester, NY, USA. All HPLC standards, sugars, medium components, furfural, hydroxymethyl furfural (HMF), phenolics, and organic acids were obtained from Sigma Chemical Co., St. Louis, MO, USA.
Fungal Strain and Inoculum Preparation
A. terreus NRRL 1972 obtained from ARS Culture Collection (Peoria, IL, USA) was used. The strain was stored in the form of spores at − 80 °C as 70% (v/v) glycerol stock. The stock culture was maintained on Czapek-Dox agar (Sigma) plates. The conidiospores were collected from the 7-day culture on Czapek-Dox-agar plates incubated at 30 °C by shaving and extracting spores using sterile water with 0.04% (v/v) Tween 80. The spore suspension was adjusted by dilution with sterile deionized water such that the final inoculum concentration was 106 spores mL−1 of the medium.
Medium Composition and Cultivation of A. terreus NRRL 1972
The optimized medium described by Hevekerl et al. [15] containing 0.8 g KH2PO4, 3 g NH4NO3, 1 g MgSO4·7H2O, 5 g CaCl2·2 H2O, 1.67 mg FeCl3·6H2O, 8 mg ZnSO4·7H2O, and 15 mg CuSO4·7H2O per L was used, unless otherwise stated. To mimic the typical sugars and their concentrations obtained from dilute acid pretreated (0.75% v/v, 160 °C, 10 min) and enzymatically saccharified (pH 4.5, 45 °C, 72 h) WS (150 g L−1) hydrolysate [16], a sugar mixture containing glucose, xylose, and arabinose (4:3:1) was used as substrate at 80 g L−1. Mixed sugars and all other medium components were added from sterile stock solutions. The pH of the medium without CaCl2 was adjusted at 3.1 with 0.5 M H2SO4 before inoculating the spore preparation. Unless otherwise stated, shake flask cultivations were performed with 25-mL medium in 125-mL Erlenmeyer flasks at 33 °C in a rotary shaker at 200 rpm for 7 days. The pH was not controlled during fermentation. Each experiment was performed in triplicate. For all experiments, the sugar mixture was dissolved in deionized water and passed through a column (440 × 45 mm) of Dowex 50-X8 cation exchange resin (100/200 mesh) to remove Mn2+, if any [17].
Analytical Methods
Cell mass was quantified using dry weight of twice rinsed cell pellet which was dried at 60 °C until constant weight was obtained. Fermentation broth after centrifugation (10,000×g, 10 min) was kept at − 20 °C before analysis of sugars (glucose, xylose, arabinose), IA, and byproducts (succinic acid, α-ketoglutaric acid, malic acid, cis-aconitic acid, and trans-aconitic acid) using high-performance liquid chromatography (HPLC). A Shimadzu Prominence Series (Shimadzu America Inc., Columbia, MD) HPLC system was used. Two columns (Aminex HPX-87P column with De-ashing and Carbo-P microguard cartridges and Aminex HPX 87H column with Cation H microguard cartridge) were used for analysis of sugars and organic acids, respectively. The Aminex HPX 87P column was maintained at 85 °C, and the sugars were eluted with Milli-Q (Millipore Corp., Bedford, MA, USA) filtered water at a flow rate of 0.6 mL min−1. The Aminex HPX 87H column was maintained at 65 °C, and the sugars and organic acids were eluted with 5 mM H2SO4 prepared using Milli-Q filtered water at a flow rate of 0.5 mL min−1. The detection was performed using a refractive index detector for sugars and UV detector at 210 nm for organic acids. Propionic acid (1%, w/v) was used as internal standard in order to estimate the liquid lost during 7-day aerobic fermentation at 33 °C. Protein was estimated by the protein-dye binding method using bovine serum albumin as standard [18]. The elemental analysis was performed using Optima 7000DV inductively coupled plasma-optical emission spectrometer (ICP-OES; Perkin-Elmer Company, Waltham, MA) by the procedure described by Bakota et al. [19]. Phosphate and sulfate contents were determined by anion HPLC using a Dionex Ion Chromatography System, ICS 2000 (Thermo Scientific Dionex, Sunnyvale, CA) and Chromeleon software. Elution of anions was accomplished on an IonPac AS18 column with IonPac AG18 guard column using an isocratic 32-mM KOH eluent at a flow rate of 1 mL min−1. Column and detector temperatures were maintained at 30 °C with a postcolumn suppression system AERS 500 at 80 mA. Peaks were detected by conductivity (DS6). Injection volume was 25 μL with a lower limit of detection at 1 μM.
Results
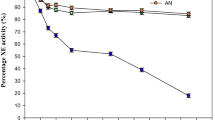
WS (150 g L−1) upon pretreatment with dilute acid and enzymatic saccharification using a cocktail of three commercial enzyme preparations (50 μL each of cellulase, ß-glucosidase, and hemicellulase preparation per g straw) generated 76.4 ± 0.8 g total sugars, 3.1 ± 0.1 g acetic acid, 202 ± 25 mg HMF, and 0.93 ± 0.01 g furfural per L. The DAWSH medium also contained 514 ± 22 mg Ca, 295 ± 8 mg Mg, 638 ± 18 mg Na, 1658 ± 85 mg K, 1796 ± 44 mg S, 17 ± 1 mg Fe, 11.6 ± 0.1 mg Mn, and 3.2 ± 0.1 mg Cu per L as determined by ICP-OES (Table 1). The strain NRRL 1972 could not grow on the DAWSH medium. It was able to grow on 100-fold diluted DAWSH, but it did not produce any IA. Even at 1000-fold diluted hydrolysate, the strain produced only 50% of IA relative to control fermentation. These data indicate the severity of inhibitory compounds present in DAWSH which caused the total inhibition of IA production even in the presence of 100-fold diluted DAWSH.
Effect of Inhibitory Compounds on Itaconic Acid Production From Mixed Sugars
The effect of acetic acid (0–1.0 g L−1) on growth and production of IA from mixed sugars (4:3:1) by A. terreus NRRL 1972 was evaluated (Fig. 1a). It is clear that acetic acid was a strong inhibitor of growth and IA production, even at the concentration of 0.8 g acetic acid per L. Thus, acetic acid alone could be responsible for total inhibition of growth of A. terreus NRRL 1972 on DAWSH which contained 3.1 ± 0.1 g acetic acid per L.
Effects of acetic acid (a), furfural (b), hydroxylmethyl furfural (c), manganese (d), CaSO4 (e), and enzymes (f) on cell growth, total sugars utilized, and itaconic acid production from mixed sugars (80 g L−1; glucose, xylose, arabinose, 4:3:1) at pH 3.1, 33 °C, and 200 rpm grown for 7 days. The data presented are means ± standard deviations for triplicate experiments. First bar, cell mass; second bar, sugars utilized; third bar, itaconic acid
The effect of furfural (0–0.8 g L−1) on mixed sugar utilization and production of IA is presented in Fig. 1b. Furfural inhibited the growth, sugar utilization, and IA production completely at 0.8 g L−1. At 0.4 g L−1 furfural concentration, the growth, sugar utilization, and IA production were decreased by 26, 68, and 61%, respectively. Thus, furfural alone at the concentration present in DAWSH (0.93 ± 0.01 g L−1) can completely inhibit the growth and IA production by the fungus.
HMF, on the other hand, did not have any negative influence on IA production from mixed sugars up to 200 mg L−1 concentration (Fig. 1c). However, at 400 mg L−1 concentration, the sugar utilization and IA production were decreased by 57 and 67%, respectively. These data indicate that HMF at the concentration present in DAWSH (202 ± 25 mg L−1) is not responsible for inhibition of IA production by the fungal strain.
Mixed phenolic compounds containing 50 mg each of vanillin, 2-furoic acid, p-coumaric acid, and ferulic acid per liter (total, 200 mg L−1) did not have any effect on growth, sugar utilization, and IA production from mixed sugars by the A. terreus strain NRRL 1972 (data not shown).
The effect of Mn2+ (0–50 ppb) on the growth, sugar utilization, and IA production by the fungus is shown in Fig. 1d. Mn2+ at ≤ 5 ppb had no effect on sugar utilization and IA production. Mn2+ at the 10-ppb level did not have an effect on sugar utilization, but the IA production decreased by 28%. At 50 ppb Mn2+ concentration, sugar utilization and IA production decreased by 54 and 97%, respectively. However, growth of the fungal strain was enhanced in the presence of Mn2+ with 2.7-, 3.3-, 4.1-, and 3.7-fold increase at 10, 20, 40, and 50 ppb Mn2+ concentrations, respectively.
CaSO4 was generated in the DAWSH after neutralization with Ca(OH)2 (lime). It was of interest to investigate the effect of CaSO4 on sugar utilization and IA production by the fungal strain. The result is presented in Fig. 1e. CaSO4 at 0.12 g L−1 inhibited the sugar utilization by 26% and the IA production was decreased also by 22%. At 0.24 g L−1 concentration, the sugar utilization and IA production decreased by 49 and 58%, respectively. After this concentration, no further decrease in both sugar utilization and IA production was observed. Thus, neutralizing the pretreated WS with lime can contribute towards inhibition of sugar utilization and IA production by the fungal strain. Na2SO4 tested up to 2.4 g L−1 did not affect sugar utilization and IA production by the strain (data not shown).
The enzyme cocktail used for hydrolysis of pretreated WS contained Celluclast, Novozyme 188, and Fiberzyme at a loading of 50 μL of each enzyme preparation per g straw which is equivalent to 7.5 mL of each enzyme preparation (total, 22.5 mL) per L of DAWSH. The protein content of Celluclast, Novozyme 188, and Fiberzyme were 69.7 ± 3.4, 94.5 ± 0.5, and 70.8 ± 6.1 mg mL−1. As shown in Fig. 1f, all three enzyme preparations at 7.5 mL L−1 of medium inhibited IA production with ß-glucosidase inhibiting 100%, cellulase inhibiting 95%, and hemicellulase preparation inhibiting at 75% at the concentration used for saccharification of DAWSH. The combination of the three enzyme preparations (enzyme cocktail) which is typically used for hydrolysis of pretreated lignocellulosic biomass inhibited 100% IA production. This result indicates that enzyme cocktail itself is responsible for inhibition of IA production by the fungal strain. However, the growth and sugar utilization were not inhibited by any of the three enzyme preparations or their combinations. In fact, the growth of the fungal strain was doubled with the addition of cellulase preparation. As shown in Table 1, the enzyme cocktail (at the concentration present in DAWSH) contained 67 ± 0 ppb Mn2+. At this concentration, IA production can be totally inhibited (Fig. 1d).
Effect of Culture Conditions on IA Production From Mixed Sugars
Culture conditions such as initial pH, temperature, and aeration affected the growth, sugar utilization, and production of IA from mixed sugars by A. terreus NRRL 1972. The effects of initial pHs (2.0–5.0) on the fermentation of glucose, xylose, arabinose, and mixed sugars (4:3:1) were studied. The data on mixed sugar fermentation is shown in Fig. 2a. The final pH values of the fermentation broth after 7 days were 1.81 ± 0.01, 1.88 ± 0.01, 1.99 ± 0.13, 1.79 ± 0.03, and 1.96 ± 0.06 at initial pH values of 2.0, 2.5, 3.1, 4.0, and 5.0, respectively. The sugar utilization and IA production were decreased at initial pHs 4.0 and 5.0, and there was no IA production at initial pH 5.0. It was found that the fungus produced IA optimally at pH 2.0 to 3.1. Cell mass was also highest under these conditions, and at initial pH 3.1, the sugar utilization was highest.
Effects of initial pH at 33 °C and 200 rpm (a), temperature at pH 3.1 and 200 rpm (b), and aeration at pH 3.1 and 33 °C (c) on cell growth, total sugars utilized, and itaconic acid production from mixed sugars (80 g L−1; glucose, xylose, arabinose, 4:3:1) for 7 days. The data presented are means ± standard deviations for triplicate experiments. First bar, cell mass; second bar, sugars utilized; third bar, itaconic acid
The fungus utilized all sugar substrates (glucose, xylose, arabinose, and mixed sugars) and produced IA optimally at 33 °C. The data on mixed sugar fermentation at temperatures 29, 33, 37, and 43 °C is shown in Fig. 2b. The cell mass was much higher at both 37 and 41 °C being optimal at 37 °C. The sugar utilization was lower at 29 and 41 °C.
The effects of aeration on growth, sugar utilization, and IA production from glucose, xylose, arabinose, and mixed sugars were investigated. It (100–400 rpm) had similar effect on the fermentation of glucose, xylose, and mixed sugars—optimally fermenting the sugars at 200 rpm except for arabinose where it was fermented optimally at 300 rpm (data not shown). The data on cell mass, sugar utilization, and IA production at 100–400 rpm from mixed sugars are presented in Fig. 2c. Lower sugar utilization and IA production were observed at 100 rpm.
Effect of Medium Components on IA Production From Mixed Sugars
The effect of KH2PO4 (0.0–3.2 g L−1) on the production of IA by the strain NRRL 1972 was investigated using mixed sugars. The data is presented in Fig. 3a. It shows that without KH2PO4, the fungus could utilize only 11% of supplied sugars and produced 0.5 g IA per L in 7 days. It produced 30.3 ± 0.8 g IA utilizing 56.6 ± 2.1 g sugars and 30.7 ± 2.0 g IA utilizing 60.6 ± 2.8 g sugars in 7 days at 0.1 and 0.4 g KH2PO4 per L concentrations, respectively. At 0.8 g L−1 concentration, the fungal strain utilized 76.6 ± 2.2 g sugars and produced 40.7 ± 0.7 g IA per L in 7 days. At 1.6 to 3.2 g L−1, the utilization of sugars and production of IA were very similar to 0.8 g KH2PO4 per L.
Effects of KH2PO4 (a), NH4NO3 (b), CaCl2·2H2O (c), and FeCl3·6H2O (d) on cell growth, total sugars utilized, and itaconic acid production from mixed sugars (80 g L−1; glucose, xylose, arabinose, 4:3:1) at pH 3.1 and 33 °C grown for 7 days. The data presented are means ± standard deviations for triplicate experiments. First bar, cell mass; second bar, sugars utilized; third bar, itaconic acid
The fermentation of mixed sugars and optimal production of IA occurred at 3 g NH4NO3 in the medium per L (Fig. 3b). Further increase of NH4NO3 concentration to 4.5 g L−1 decreased the IA production by 28.6% even though sugar utilization was not affected. However, the cell mass was increased by 63% in comparison to that at 3 g NH4NO3 per L. NH4NO3 concentration at 6.0 g L−1 did not have further detrimental effect on IA production.
The effect of CaCl2·2H2O (0.05–10.0 g L−1) on the cell growth, sugar utilization, and IA production from mixed sugars by the fungal strain is presented in Fig. 3c. The data indicates that CaCl2·2H2O at 2.0 g L−1 gave the maximum IA production although the sugar utilization was optimal at 2.0–5.0 g L−1 of CaCl2·2H2O. The standard medium has 5.0 g CaCl2·2H2O per L. At 10.0 g L−1, growth, sugar utilization, and IA production decreased by 50.6, 52.0, and 64.5%, respectively.
The effect of FeCl3·6H2O on the growth, sugar utilization, and IA production from mixed sugars is presented in Fig. 3d. FeCl3·6H2O at the concentrations used enhanced the growth of the fungus and did not have any effect on sugar utilization. However, the IA production was decreased by 22.8 and 57.4% at 16.7 and 33.4 mg L−1 concentration, respectively. Without FeCl3.6H2O, poor growth, sugar utilization, and IA production were observed. Optimal production of IA occurred between 0.83 and 3.33 mg FeCl3·6H2O L−1 instead of 1.67 mg L−1 used in standard medium for IA production from glucose. The fungal strain produced six minor byproducts (total, ~2.0 g L−1) in addition to IA from mixed sugars. These byproducts were malic acid, α-ketoglutaric acid, succinic acid, cis-aconitic acid, and trans-aconitic acid.
Discussion
Lignocellulosic biomass, upon diluting acid pretreatment at high temperature and enzymatic hydrolysis, generates a mixture of sugars including glucose, xylose, arabinose, and galactose depending on the feedstock [20]. At the same time, a number of inhibitory compounds such as acetic acid, furfural, and HMF were typically generated depending on the severity of acid, duration, and temperature of pretreatment. Acetic acid at 0.8 g L−1 was a strong inhibitor of IA production from mixed sugars by A. terreus NRRL 1972. The presence of acetic acid at 1.0 g L−1 was recently shown to completely inhibit growth of A. terreus strain DSM 23081 [12]. However, A. terreus strain NRRL 1972 was found to be more tolerant to furfural and HMF than A. terreus DSM 23081 [12]. A concentration of 0.1 g of HMF or furfural per L led to 70% decrease in IA production by the strain (DSM 23081), whereas the IA production was not affected by 0.1 g HMF per L and it was decreased by 17% in the presence of 0.1 g furfural per L by the strain NRRL 1972.
Kaparaju et al. [21] identified vanillin, 2-furoic acid, p-coumaric acid, and ferulic acid as four main phenolic compounds present in hydrothermally pretreated WS hydrolysate at concentration of 100 mg L−1 (value expressed at 100 g WS per L). This strain is different from A. terreus DSM 23081 with respect to inhibition of IA production by vanillin. The production of IA decreased slowly with an increase of vanillin concentration. Vanillin at 60 mg L−1 caused a decrease in IA production by A. terreus DSM 23081 by 24.3%, whereas vanillin at 50 mg L−1 did not have any effect on IA production by strain NRRL 1972.
Karaffa et al. [17] reported that a deficiency of Mn2+ (< 3 ppb) is needed to achieve high IA production by A. terreus NRRL 1960. About 11,560 ± 200 ppb Mn2+ was present in the WSH medium per L, which is 231-fold higher than 50 ppb (Table 1). This indicates that Mn2+ alone could be responsible for the total inhibition of IA production by the fungus. Krull et al. [12] reported that crude wheat chaff hydrolysate prepared by alkali pretreatment contained 1721 ± 5 ppb Mn2+ L−1. The enzyme preparations used for hydrolysis of dilute acid pretreated WS inhibited the IA production by A. terreus NRRL 1972. Krull et al. [12] reported that with the increase in enzyme concentration (Biogazyme 2×) to 7.5 g L−1 during simultaneous saccharification and fermentation of alkali pretreated wheat chaff by A. terreus DSM 23081, the spores were minimally germinated, no compact pellets were formed, and IA was not produced. The enzyme preparation at 4.6 g L−1 contained 46 ppb Mn2+ as determined by using ICP-OES.
The optimal initial pH, temperature, and aeration for production of IA from mixed sugars were found to be pH 3.1, 33 °C, and 200 rpm, respectively. Rychtera and Wase [22] reported that an initial pH of 3.1 ± 0.1 appeared to be optimal for IA production from glucose by A. terreus NRRL 1960. Aeration plays an important role for successful IA production from glucose by A. terreus in submerged fermentation [3, 23]. The strain NRRL 1972 performed optimally at 0.8 g KH2PO4, 3 g NH4NO3, and 2 g CaCl2·2H2O and between 0.83 and 3.33 mg FeCl3·6H2O per L for mixed sugar fermentation to IA.
Conclusions
The research clearly shows the severity of the inhibitory factors present in DAWSH for A. terreus fermentation to IA. In addition to typical fermentation inhibitors such as furfural and acetic acid, the fungal fermentation was very sensitive to manganese ion and enzyme cocktail used for saccharification of pretreated WS. The inhibitory compounds must be removed from DAWSH in order to produce IA from it. The data presented will be very valuable for developing a process technology for production of IA from lignocellulosic hydrolysate.
References
Nubel, R. C., & Ratajak, E. J. (1962). Process for producing itaconic acid. US Patent, 3, 044,941.
Batti, M., & Schweiger, L. B. (1963). Process for the production of itaconic acid. US Patent, 3, 078,217.
Willke, T., & Vorlop, K.-D. (2001). Biotechnological production of itaconic acid. Applied Microbiology and Biotechnology, 56(3-4), 289–295.
Saha, B. C. (2017). Emerging biotechnologies for production of itaconic acid and its applications as a platform chemical. Journal of Industrial Microbiology & Biotechnology, 44(2), 303–315.
Choi, S., Song, C. W., Shin, J. H., & Lee, S. Y. (2015). Biorefineries for the production of top building block chemicals and their derivatives. Metabolic Engineering, 28, 223–239.
Okabe, M., Lies, D., Kanamasa, S., & Park, E. Y. (2009). Biotechnological production of itaconic acid and its biosynthesis in Aspergillus terreus. Applied Microbiology and Biotechnology, 84(4), 597–606.
Klement, T., & Büchs, J. (2013). Itaconic acid—a biotechnological process in change. Bioresource Technology, 135, 422–431.
Werphy, T., & Peterson, G. (2004). Top value added chemicals from biomass: volume 1—results of screening for potential candidates from sugars and synthetic gas. US Department of Energy, pp. 1–76. http://www1.eere.energy.gov/bioenergy/pdfs/35523.pdf.
Tippkötter, N., Duwe, A.-N., Wiesen, S., Sieker, T., & Ulber, R. (2014). Enzymatic hydrolysis of beech wood lignocellulose at high solid contents and its utilization as substrate for the production of biobutanol and dicarboxylic acids. Bioresource Technology, 167, 447–455.
Jimenez-Quero, A., Pollet, E., Zhao, M., Marchioni, E., Averous, L., & Phalip, V. (2016). Itaconic and fumaric acid production from biomass hydrolyzates by Aspergillus strains. Journal of Microbiology and Biotechnology, 26(9), 1557–1565.
Pedrosa, G. B., Montipo, S., Mario, D. A. N., Alves, S. H., & Martins, A. F. (2017). Building block itaconic acid from left-over biomass. Biomass Conversion & Biorefinery, 7(1), 23–35.
Krull, S., Eidt, L., Hevekerl, A., Kuenz, A., & Prüße, U. (2018). Itaconic acid production from wheat chaff by Aspergillus terreus. Process Biochemistry, 63, 169–176.
Saha, B. C., Kennedy, G. J., Qureshi, N., & Bowman, M. J. (2017). Production of itaconic acid from pentose sugars by Aspergillus terreus. Biotechnology Progress, 33(4), 1059–1067.
Saha, B. C., & Kennedy, G. J. (2018). Ninety six well microtiter plate as microbioreactors for production of itaconic acid by six Aspergillus terreus strains. Journal of Microbiological Methods, 144, 53–59.
Hervekerl, A., Kuenz, A., & Vorlop, K.-D. (2014). Filamentous fungi in microtiter plates—an easy way to optimize itaconic acid production with Aspergillus terreus. Applied Microbiology and Biotechnology, 98(16), 6983–6989.
Saha, B. C., Nichols, N. N., & Cotta, M. A. (2011). Ethanol production from wheat straw by recombinant Escherichia coli strain FBR5 at high solid loading. Bioresource Technology, 102(23), 10892–10897.
Karaffa, L., Diaz, R., Papp, B., Fekete, E., Sándor, E., & Kubicek, C. P. (2015). A deficiency of manganese ions in the presence of high sugar concentrations is the critical parameter for achieving high yields of itaconic acid by Aspergillus terreus. Applied Microbiology and Biotechnology, 99(19), 7937–7944.
Bradford, H. H. (1976). Rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72(1-2), 248–254.
Bakota, E. L., Dunn, R. O., & Seal, X. L. (2015). Heavy metals screening of rice bran oils and its relation to composition. European Journal of Lipid Science and Technology, 117(9), 1452–1462.
Saha, B. C. (2003). Hemicellulose bioconversion. Journal of Industrial Microbiology & Biotechnology, 30(5), 279–291.
Kaparaju, P., Serrano, M., Thomsen, A. B., Kongjan, P., & Angelidaki, I. (2009). Bioethanol, biohydrogen and biogas production from wheat straw in a biorefinery concept. Bioresource Technology, 100(9), 2562–2568.
Rychtera, M., & Wase, D. A. (1981). The growth of Aspergillus terreus and the production of itaconic acid in batch and continuous cultures. The influence of pH. Journal of Chemical Technology & Biotechnology, 31(1), 509–521.
Gyamerah, M. H. (1995). Oxygen requirement and energy relations of itaconic acid fermentation by Aspergillus terreus NRRL 1960. Applied Microbiology and Biotechnology, 44(3-4), 356–361.
Acknowledgements
The authors thank James Swezey, microbiologist (retired), for supplying Aspergillus terreus NRRL 1972 from ARS Culture Collection, Peoria, IL, and Kim Ascherl for the metal analysis by ICP-OES.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the US Department of Agriculture. USDA is an equal opportunity provider and employer.
Rights and permissions
About this article
Cite this article
Saha, B.C., Kennedy, G.J., Bowman, M.J. et al. Factors Affecting Production of Itaconic Acid from Mixed Sugars by Aspergillus terreus. Appl Biochem Biotechnol 187, 449–460 (2019). https://doi.org/10.1007/s12010-018-2831-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-018-2831-2