Abstract
Grifola frondosa is an edible fungus with a variety of potential pharmacological activities. This study investigates the hypoglycemic, anti-diabetic nephritic, and antioxidant properties of G. frondosa polysaccharides in diet-streptozotocin-induced diabetic rats. After a 4-week treatment with 100 mg/kg of metformin and 200 mg/kg of one of four different G. frondosa polysaccharide mixtures (especially GFPS3 and GFPS4), diabetic rats had enhanced body weight and suppressed plasma glucose, indicating the hypoglycemic activities of the G. frondosa polysaccharides. G. frondosa polysaccharides regulated the level of serum creatinine, blood urea nitrogen, N-acetyl-β-d-glucosaminidase, and albuminuria; inhibited the serum levels of interleukin (IL)-2, IL-6, and TNF-α; and enhanced the serum levels of matrix metalloproteinase 9 and interferon-α, confirming their anti-diabetic nephritic activities. G. frondosa polysaccharides ameliorated the pathological alterations in the kidneys of diabetic rats. Moreover, G. frondosa polysaccharides modulated the serum levels of oxidant factors such as superoxide dismutase, glutathione peroxidase, catalase, malondialdehyde, and reactive oxygen species, revealing their antioxidant properties. Furthermore, the administration of G. frondosa polysaccharides inhibited nuclear factor kappa B activities in the serum and kidneys. All of the data revealed that the activation of nuclear factor kappa B plays a central role in G. frondosa polysaccharide-mediated anti-diabetic and anti-nephritic activities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As a metabolic disease, diabetes mellitus affects millions of people’s health and causes nearly 2.2% of all deaths worldwide (1). The imbalance of lipids, carbohydrates, and proteins, and hyperglycemia are recognized as pathological symptoms of diabetes (2, 3). Persistent hyperglycemia associated with insulin secretion deficiency in diabetes patients leads to various complications including nephropathy and retinopathy (1, 4). As reported, diabetic nephropathy is more common in type 2 diabetic patients than in type 1 diabetic patients.
One of the most common complications, diabetic nephropathy, is a chronic inflammation with high mortality and morbidity (5). The increased activation of intracellular signaling molecules is responsible for the development of diabetic nephropathy; in particular, nuclear factor-kappa B (NF-κB) plays a central role by regulating the levels of inflammatory cytokines including interleukin (6). During this process, the phosphorylation of the inhibitor kappa B (IκB) is required for NF-κB p65 activation (7). The oxidative system is reported to serve as a therapeutic target for diabetic nephropathy (8). Superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px) protect cell structure and membrane function (9). p65 is the initial point for the arousal of the downstream antioxidant effect of NF-κB (10). The inhibition of NF-κB may provide an effective treatment for diabetes (11). Most agents used clinically to treat diabetes only control blood glucose levels and fail to improve complications (12). Some agents display undesirable adverse effects during diabetes therapy such as insulin resistance and hypoglycemia (13). Recently, many studies have examined the use of herbal medicines as safe and efficient agents for treating diabetes mellitus.
Due to their limited adverse effects and “systemic therapeutic properties,” herbal products are recognized as a valuable reservoir for researchers seeking novel drugs (14). Cordyceps militaris water and ethanol extracts have demonstrated excellent anti-diabetic and anti-nephropathic activities in type 2 diabetic rats (15). Paecilomyces tenuipes has anti-hyperglycemic, anti-hyperlipidemic effects through the modulation of anti-oxidative factors in diabetic mice (16, 17). Grifola frondosa, an edible fungus, has various effects on anti-tumor, anti-oxidation, and immunoregulation (18). It has been confirmed that G. frondosa regulates fat metabolism in animal models (19). In type 2 diabetic mice models, an alpha-glucan from the fruit body of G. frondosa reduces the levels of blood sugar and cholesterol and improves insulin resistance (20). Additionally, G. frondosa polysaccharides display antioxidant action that reduces the memory impairment of old rats (21). In previous research, a range of bioactive G. frondosa polysaccharides have been isolated and shown to possess various biological activities such as anti-oxidative, immunomodulatory, and anti-tumor activities (18). However, the anti-diabetic effects of the polysaccharides obtained from the G. frondosa mycelium via submerged fermentation and their oxidation-related mechanisms have not yet been systematically reported.
Based on previous research, this study, performed on a high-fat diet–streptozotocin (STZ)-induced type 2 diabetic rat model, investigated the anti-hyperglycemic and anti-diabetic nephropathic activities of G. frondosa. Serum biochemical indexes including oxidant factors and inflammatory factors were detected to verify the positive effects of G. frondosa. The activities of NF-κB and IκBα were also analyzed to reveal the possible mechanisms of this activity. Our data provide evidence supporting the use of G. frondosa as a functional food for type 2 diabetic mellitus adjuvant therapy.
Methods and Materials
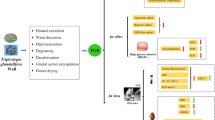
Extraction Preparation and Polysaccharide Separation
G. frondosa mycelium was obtained via submerged fermentation in a rotary shaker incubator (10 L, Biostat B; Germany). G. frondosa mycelium was extracted twice at 80 °C for 3 h using 30-fold double-distilled (D.D.) water. After centrifuging at 6000 rpm for 10 min, the supernatant was sequentially concentrated in an evaporator under reduced pressure. Later, the existing protein in the crude extracts was precipitated using Sevag reagent (V (n-butanol)/V (chloroform) = 1:4, 50 mL). Absolute ethanol was added to the supernatant to ensure the final ethanol concentrations were 50, 60, 70, and 80% and named, respectively, GFPS1, GFPS2, GFPS3, and GFPS4 (Fig. 1). The precipitations were collected, washed with ethanol, and dried to remove residual ethanol. According to the anthrone–sulfuric acid method (22), the extraction rates (GFPS weight (g)/mycelium weight (g)) of GFPS1, GFPS2, GFPS3, and GFPS4 were 0.18, 0.89, 2.43, and 2.78%, and the purities (polysaccharide weight (g)/GFPS weight (g)) of GFPS1, GFPS2, GFPS3, and GFPS4 were 79.52, 75.91, 80.27, and 83.13%, respectively.
The LC-10ATvp high-performance liquid chromatography system (Shimadzu, Japan) equipped with a TSK-GEL G4000PWXL column (Tosho Co., Japan) and an Alltech 2000ES Evaporative Light Scattering Detector (ELSD) (Shimadzu, Japan) were used to evaluate the molecular weights of GFPS1 to GFPS4, as described in previous studies (23). The dextran standards (molecular weights 5, 20, 50, 100, 200, and 400 kDa) were formulated into a 2.0 mg/mL solution. D.D. water driven by double pumps (Waters 150; Millipore, USA) provided the mobile phase with a flow rate of 0.45 mL/min. The aerosol level was 60%, the drift tube temperature was 120 °C, and the column temperature was 40 °C. The dextran standards were used to create a calibration curve, as previously described (24).
Diabetic Rat Model Establishment
The experimental protocol was approved by the Lab Animal Center of Jilin University. Male Wistar rats weighing 160–180 g were maintained on a 12-h dark/light cycle at 22 ± 1 °C with water and food available ad libitum.
Eighty-five rats were fed with a defined high-fat high-sucrose diet (HFHSD; 20% sucrose, 12% lard, 0.5% cholesterol) for 8 weeks and then injected with 25 mg/kg of STZ for 5 days (i.p.; once per day). Of the 85 experimental rats, 60 with fasting blood glucose levels over 11 mmol/L were used as diabetic rats in the further experiments (25). Another 10 rats that were orally treated with D.D. water for weeks and injected with citrate buffer for 5 days served as the control group.
Drug Treatment Process
The control rats (CTRL) were orally treated with physiological saline for 4 weeks. The diabetic rats were randomly divided into six groups and orally treated with physiological saline (Model group), 100 mg/kg of metformin (Met group), and 200 mg/kg of either GFPS1, GFPS2, GFPS3, or GFPS4 (G. frondosa polysaccharides groups) for 4 weeks. During the whole drug treatment period, bodyweight and fasting blood glucose levels were measured every week. At the end of the experimental period, blood and 24-h urine were collected for biochemical criteria detection. Then, the rats were euthanized by injection with 200 mg/kg pentobarbital, and the kidneys were collected for further experiments.
Biochemical Criteria Measurement
In the blood samples, the levels of blood urea nitrogen (BUN), serum creatinine (Scr), N-acetyl-β-d-glucosaminidase (NAG), malondialdehyde (MDA), SOD, GSH-Px, reactive oxygen species (ROS), and catalase (CAT) were detected using commercial assay kits (Nanjing Jianchen Biotechnology Co. Ltd., Jiangsu, China). The levels of interleukin (IL)-2, interleukin-2 receptor (IL-2R), IL-6, tumor necrosis factor-α (TNF-α), matrix metalloproteinase 9 (MMP-9), interferon-α (IFN-α), and phosphor-NF-κB p65 were determined by enzyme-linked immunosorbent assay (ELISA) kits (RND, USA). In the urine samples, the levels of albuminuria were measured by commercial assay kits (Nanjing Jianchen Biotechnology Co. Ltd., Jiangsu, China).
Western Blot
The kidney tissues were homogenized as described in previous research (23). The protein concentrations were determined by the Bradford method, and 30 μg of proteins was separated using a 10% SDS-PAGE gel and transferred onto nitrocellulose membranes (0.45 μm; Bio Basic, Inc. USA). After blocking via 5% BSA solution, the membranes were blotted with primary antibodies of phosphor (P)-NF-κB p65, total (T)-NF-κB p65, P-IκBα, T-IκBα, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Santa Cruz, USA) (dilution of 1:2000) at 4 °C overnight, and then incubated with horseradish peroxidase-conjugated secondary antibodies (Santa Cruz, USA). Chemiluminescence was detected using ECL detection kits (GE Healthcare, UK). Image J software was applied to quantified the bands’ intensities.
Histopathological Observation of Kidneys
The kidney samples were fixed with 4% neutral paraformaldehyde for 48 h, dehydrated by passing successfully through different mixtures of ethyl alcohol–water, cleaned in xylene, and embedded in paraffin. Then they were sliced into 6-μm-thick sections. After staining with hematoxylin and eosin (HE) and periodic acid Schiff (PAS), histologic examinations were visualized using an IX73 inverted microscope (Olympus, Japan) (26).
Statistical Analysis
All of the values were expressed as mean ± SEM. One-way analysis of variance (ANOVA) followed by Dunn’s tests were used to detect statistical significance. A value of P < 0.05 was considered significant.
Results
Hypoglycemic Effect of G. frondosa Polysaccharides
The extraction rates, purity, peak retention time, and molecular weight of the GFPSs are shown in Table 1.
Compared with normal rats, the 8-week HFHSD fed and 5-day STZ injection groups had low growth rates and high blood glucose levels throughout the whole drug administration period (P < 0.05; Tables 2 and 3). Doses of 200 mg/kg of GFPS2, GFPS3, and GFPS4 had effects similar to Met (100 mg/kg), which strongly increased body weight by more than 13.8% (P < 0.05; Table 2) and reduced fasting blood glucose levels by over 30.2% (P < 0.05; Table 3) compared to diabetic rats.
Renal Protection of G. frondosa Polysaccharides
In diabetic rats, abnormally high levels of BUN, Scr, and NAG in serum, and high concentrations of albuminuria in urine were noted, which are all considered hallmarks of diabetic nephropathy (27). The 4-week administration of GFPS4 strongly down-regulated all of these factors (P < 0.05; Table 4), unlike Met, which failed to influence any of these levels (Table 5). GFPS3 suppressed the high levels of Scr, albuminuria, and NAG more than 25% compared to diabetic rats (P < 0.05; Table 4). However, GFPS2 only significantly inhibited the serum levels of NAG (P < 0.05; Table 4).
In diabetic rats, high levels of IL-2, IL-2R, IL-6, TNF-α, and IFN-α, and low levels of MMP-9 were observed, which is consistent with previous research findings that hyperglycemia causes kidney damage by disturbing multiple inflammatory factors (P < 0.05; Table 5). Met significantly reduced the levels of IL-6, TNF-α, and IFN-α (P < 0.05; Table 5), but failed to influence IL-2, IL-2R, or MMP-9 levels. Both GFPS1 and GFPS2 had no detectable effects on any of these factors. Although it did not affect IL-2R and IFN-α, GFPS3 resulted in a more than 14% reduction in IL-2, IL-6, and TNF-α, and a 19% reduction in MMP-9 (P < 0.05; Table 5). Furthermore, GFPS4 normalized all of the inflammation-related factors except for IL-2R in diabetic rats (P < 0.05; Table 5).
Furthermore, HE and PAS staining revealed that compared to the control rats, the kidneys of the diabetic rats had degenerated glomeruli, mesangial expansion, and thickened basement membranes (Fig. 2a, b). GFPS significantly ameliorated the incidence of glomerular basement membrane thickening or mesangial proliferation and inflammatory infiltrate injuries (Fig. 2a, b). In the kidneys of the diabetic rats, a large number of glycogen-filled proximal tubules and extracellular matrix deposit were found through PAS-positive areas, which were all strongly relieved by GFPSs, especially GFPS3 and GFPS4 (Fig. 2b).
Anti-Oxidative Effects of G. frondosa Polysaccharides
The imbalance of oxidative factors is not only responsible for hyperglycemia but also for inflammation, which may be directly associated with SOD, GSH, and ROS (28). Compared with diabetic rats, the GFPS3 and GFPS4 groups had strongly enhanced levels of SOD, GSH-Px, and CAT, and reduced levels of MDA and ROS (P < 0.05; Table 6). GFPS2 only enhanced the serum levels of GSH-Px and CAT (P < 0.05; Table 6). Furthermore, neither the Met nor the GFPS1 group showed any effects on the chosen oxidative factors (Table 6).
Regulatory Effects of G. frondosa Polysaccharides on NF-κB
NF-κB has been found to regulate multiple inflammatory factors including interleukin. The regulation of NF-κB transcriptional activity is related to the phosphorylation of IκB and the NF-κB p65 subunit. In serum, high levels of phosphor-NF-κB and p65 were noted in diabetic rats, and were significantly suppressed in the Met, GFPS3, and GFPS4 groups (P < 0.05; Fig. 3a). Similar results for NF-κB p65 and IκBα were noted in the kidneys by the Western blot test. GFPSs treatment strongly suppressed the high expression levels of P-NF-κB p65 and P-IκBα in the kidneys (P < 0.05; Fig. 3b).
Diabetic rats were treated with G. frondosa polysaccharides for 4 weeks, and a the levels of NF-κB in serum and b the activity of NF-κB p65 and IκBα in kidney were detected via ELISA and western blot, respectively. Quantification data of the expression of P-NF-κB were normalized by corresponding T-NF-κB. Data are expressed as mean ± SEM (n = 10) and analyzed by using a one-way ANOVA. ##P < 0.01 vs. CTRL, *p < 0.05 vs. diabetic rats
Discussion
A high-fat diet combined with SZT is commonly used to establish diabetic animal models, especially models for type 2 diabetes mellitus (29). Compared with diabetic rats, the treated rats had improved body weight and suppressed fasting blood glucose levels, verifying the hyperglycemic activity of G. frondosa polysaccharides. Although the dysfunction of β cell caused by insufficient insulin has been shown to be responsible for hyperglycemia, this study fails to detect whether G. frondosa polysaccharides influence insulin synthesis or secretion. Further experiments will focus on the ability of G. frondosa activities to protect against β-cell damage.
As one of the most common microvascular complications of diabetes, the development of diabetic nephropathy is related to inflammatory and immunosuppressive factors (30). The levels of Scr, BUN, and albuminuria, which are considered indexes for kidney function, were all normalized by the administration of G. frondosa polysaccharides, confirming their anti-nephropathy properties. The imbalance of the oxidant system in diabetes favors the dysfunction of endothelial and controls the apoptosis of podocyte during diabetic nephropathy (31). In diabetic patients, the disequilibrium of physical function, especially hyperglycemia, results in the accumulation of intracellular ROS, which mediates the metabolic defects associated with the diabetic state (32). SOD and GSH-Px, primary enzymes of enzymatic antioxidants, can directly eliminate excess levels of ROS in diabetics (33). G. frondosa polysaccharides enhanced the levels of SOD, GSH-Px, and CAT and also reduced the serum levels of MDA, another oxidant factor producing lipid peroxidation, indicating the reduction in lipid peroxidation and weaker oxidant stress (34). It has been confirmed that antioxidant agents successfully restored the alloxan-induced β-cell damages (35). C. militaris has been shown to promote hypoglycemic and anti-diabetic nephritic activity by regulating the oxidative system (25). In this study, the anti-oxidative properties of GFPSs contributed to their anti-diabetic and anti-nephritic activities in diabetic rats.
G. frondosa polysaccharides regulated the levels of IL-2, IL-6, TNF-α, IFN-α, and MMP-9 in serum. The overexpression of IL-2 is responsible for the regulation of endogenous cytokines, which leads to deteriorating glomerular damages (36). The elevation of pro-inflammatory cytokines, especially IL-6 and TNF-α, is associated with renal damage and B-cell development (37). Elevated MMP-9 levels were noted during the enhanced inflammatory state in type 2 diabetics (38). As reported, NF-κB, a nuclear transcription factor, protects kidneys by controlling the levels of inflammatory cytokine (39,40,41). The regulation of NF-κB transcriptional activity is related to the phosphorylation of IκB and the NF-κB p65 subunit. Under hyperglycemic conditions, the increased phosphorylation of IκBα leads to the activation of the NF-κB p65 subunit (42). However, excess ROS may lead to the nuclear translocation of NF-κB by phosphorylating IκB (43). The changing expression of phosphor-NF-κB was noted in cobalt chloride-mediated oxidative stress and inflammation in human renal proximal tubular epithelial cells (44). In cationic bovine serum albumin (C-BSA)-induced membranous glomerulonephritis rat models, C. militaris fruit body extracts displayed significant renal protection by attenuating oxidative stress and inflammation (23). Together, these results suggest that NF-κB may play a central role in GFPSs-mediated antioxidant and anti-inflammation activities in diabetic rats.
This study has several limitations. Previous studies have shown that molecular weight, chemical composition, glycosidic linkages, and degree of branching can strongly influence the bioactivities of polysaccharides (45). However, although we observed that GFPS3 and GFPS4 were more effective than the other polysaccharides, we failed to determine the relationship between their activities and characteristics in the results of this study. The GFPSs obtained from G. frondosa mycelium were not pure enough to systematically identify their structures.
In conclusion, in diet-STZ-induced type 2 diabetic rats, the hypoglycemic, anti-diabetic nephritic, and antioxidant properties of G. frondosa polysaccharides were successfully confirmed. Specifically, they suppressed fasting plasma glucose levels, regulated serum biochemical indices, and balanced the state of the oxidative system. We also found that NF-κB plays a central role in these effects. Although further investigation is needed, this study supports the use of G. frondosa as an anti-diabetic and anti-nephritic agent or functional food.
References
Sheikh, B. A., Pari, L., Rathinam, A., & Chandramohan, R. (2015). Trans-anethole, a terpenoid ameliorates hyperglycemia by regulating key enzymes of carbohydrate metabolism in streptozotocin induced diabetic rats. Biochimie, 112, 57–65.
Kerner, W., & Bruckel, J. (2014). Definition, classification and diagnosis of diabetes mellitus. Experimental and Clinical Endocrinology & Diabetes, 122(7), 384–386.
Putakala, M., Gujjala, S., Nukala, S., & Desireddy, S. (2017). Beneficial effects of Phyllanthus amarus against high fructose diet induced insulin resistance and hepatic oxidative stress in male Wistar rats. Applied Biochemistry and Biotechnology, 183(3), 744–764.
Jiang, P., Dong, Z., Ma, B., Ni, Z., Duan, H., Li, X., Wang, B., Ma, X., Wei, Q., Ji, X., & Li, M. (2016). Effect of Vanadyl rosiglitazone, a new insulin-mimetic vanadium complexes, on glucose homeostasis of diabetic mice. Applied Biochemistry and Biotechnology, 180(5), 841–851.
Zhu, K., Kakehi, T., Matsumoto, M., Iwata, K., Ibi, M., Ohshima, Y., Zhang, J., Liu, J., Wen, X., Taye, A., Fan, C., Katsuyama, M., Sharma, K., & Yabe-Nishimura, C. (2015). NADPH oxidase NOX1 is involved in activation of protein kinase C and premature senescence in early stage diabetic kidney. Free Radical Biology & Medicine, 83, 21–30.
Fornoni, A., Ijaz, A., Tejada, T., & Lenz, O. (2008). Role of inflammation in diabetic nephropathy. Current Diabetes Reviews, 4(1), 10–17.
Yuan, H. D., Huang, B., & Chung, S. H. (2011). Protective effect of cinnamaldehyde on streptozotocin-induced damage in rat pancreatic β-cells. Food Science and Biotechnology, 20(5), 1271–1276.
Elsherbiny, N. M., El-Sherbiny, M., & Said, E. (2015). Amelioration of experimentally induced diabetic nephropathy and renal damage by nilotinib. Journal of Physiology and Biochemistry, 71(4), 635–648.
Wu, J. Q., Kosten, T. R., & Zhang, X. Y. (2013). Free radicals, antioxidant defense systems, and schizophrenia. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 46, 200–206.
Khatiwala, R. V., Zhang, S., Li, X., Devejian, N., Bennett, E. and Cai, C. (2018) Inhibition of p16(INK4A) to rejuvenate aging human cardiac progenitor cells via the upregulation of anti-oxidant and NFkappaB signal pathways. Stem cell reviews.
Suryavanshi, S. V., & Kulkarni, Y. A. (2017). NF-κβ: a potential target in the management of vascular complications of diabetes. Frontiers in Pharmacology, 8.
Kania, D. S., Gonzalvo, J. D., & Weber, Z. A. (2011). Saxagliptin: a clinical review in the treatment of type 2 diabetes mellitus. Clinical Therapeutics, 33(8), 1005–1022.
Scheen, A. (2007). Antidiabetic agents in subjects with mild dysglycaemia: prevention or early treatment of type 2 diabetes? Diabetes & Metabolism, 33(1), 3–12.
Salihu Shinkafi, T., Bello, L., Wara Hassan, S., & Ali, S. (2015). An ethnobotanical survey of antidiabetic plants used by Hausa-Fulani tribes in Sokoto, Northwest Nigeria. Journal of Ethnopharmacology, 172, 91–99.
Liu, C., Song, J., Teng, M., Zheng, X., Li, X., Tian, Y., Pan, M., Li, Y., Lee, R. J., & Wang, D. (2016). Antidiabetic and antinephritic activities of aqueous extract of Cordyceps militaris fruit body in diet-streptozotocin-induced diabetic Sprague Dawley rats. Oxidative Medicine and Cellular Longevity, 2016, 9685257.
Liu, C., Zeng, X., Li, Y., Ma, H., Song, J., Li, Y., Zhou, Y., Lee, R. J., & Wang, D. (2017). Investigation of hypoglycemic, hypolipidemic and antinephritic activities of Paecilomyces tenuipes N45 in diet/streptozotocin induced diabetic rats. Molecular Medicine Reports, 15(5), 2807–2813.
Du, L., Liu, C., Teng, M., Meng, Q., Lu, J., Zhou, Y., Liu, Y., Cheng, Y., Wang, D., & Teng, L. (2016). Anti-diabetic activities of Paecilomyces tenuipes N45 extract in alloxan-induced diabetic mice. Molecular Medicine Reports, 13(2), 1701–1708.
He, X., Wang, X., Fang, J., Chang, Y., Ning, N., Guo, H., Huang, L., Huang, X., & Zhao, Z. (2017). Polysaccharides in Grifola frondosa mushroom and their health promoting properties: a review. International Journal of Biological Macromolecules, 101, 910–921.
Kubo, K., & Nanba, H. (1997). Anti-hyperliposis effect of maitake fruit body (Grifola frondosa). I. Biological & Pharmaceutical Bulletin, 20(7), 781–785.
Hong, L., Xun, M., & Wutong, W. (2007). Anti-diabetic effect of an alpha-glucan from fruit body of maitake (Grifola frondosa) on KK-ay mice. The Journal of Pharmacy and Pharmacology, 59(4), 575–582.
Chen, Z., Tang, Y., Liu, A., Jin, X., Zhu, J., & Lu, X. (2017). Oral administration of Grifola frondosa polysaccharides improves memory impairment in aged rats via antioxidant action. Molecular Nutrition & Food Research, 61(11).
Zhu, H., Sheng, K., Yan, E., Qiao, J., & Lv, F. (2012). Extraction, purification and antibacterial activities of a polysaccharide from spent mushroom substrate. International Journal of Biological Macromolecules, 50(3), 840–843.
Song, J., Wang, Y., Liu, C., Huang, Y., He, L., Cai, X., Lu, J., Liu, Y., & Wang, D. (2016). Cordyceps militaris fruit body extract ameliorates membranous glomerulonephritis by attenuating oxidative stress and renal inflammation via the NF-kappaB pathway. Food & Function, 7(4), 2006–2015.
Cui, H., Chen, Y., Wang, S., Kai, G., & Fang, Y. (2011). Isolation, partial characterisation and immunomodulatory activities of polysaccharide from Morchella esculenta. Journal of the Science of Food and Agriculture, 91(12), 2180–2185.
Dong, Y., Jing, T., Meng, Q., Liu, C., Hu, S., Ma, Y., Liu, Y., Lu, J., Cheng, Y., Wang, D., & Teng, L. (2014). Studies on the antidiabetic activities of Cordyceps militaris extract in diet-streptozotocin-induced diabetic Sprague-Dawley rats. BioMed Research International, 2014, 160980.
Wang, J., Song, J., Wang, D., Zhang, N., Lu, J., Meng, Q., Zhou, Y., Wang, N., Liu, Y., Wang, D., & Teng, L. (2016). The anti-membranous glomerulonephritic activity of purified polysaccharides from Irpex lacteus Fr. International Journal of Biological Macromolecules, 84, 87–93.
Williams, M. E. (2005). Diabetic nephropathy: the proteinuria hypothesis. American Journal of Nephrology, 25(2), 77–94.
Rubattu, S., Pagliaro, B., Pierelli, G., Santolamazza, C., Castro, S. D., Mennuni, S., & Volpe, M. (2014). Pathogenesis of target organ damage in hypertension: role of mitochondrial oxidative stress. International Journal of Molecular Sciences, 16(1), 823–839.
Mahendran, G., Thamotharan, G., Sengottuvelu, S., & Bai, V. N. (2014). Anti-diabetic activity of Swertia corymbosa (Griseb.) Wight ex C.B. Clarke aerial parts extract in streptozotocin induced diabetic rats. Journal of Ethnopharmacology, 151(3), 1175–1183.
Ellis, E. N., & Good, B. H. (1991). Prevention of glomerular basement membrane thickening by aminoguanidine in experimental diabetes mellitus. Metabolism, 40(10), 1016–1019.
Hua, W., Huang, H. Z., Tan, L. T., Wan, J. M., Gui, H. B., Zhao, L., Ruan, X. Z., Chen, X. M., & Du, X. G. (2015). CD36 mediated fatty acid-induced podocyte apoptosis via oxidative stress. PLoS One, 10(5), e0127507.
Rolo, A. P., & Palmeira, C. M. (2006). Diabetes and mitochondrial function: role of hyperglycemia and oxidative stress. Toxicology and Applied Pharmacology, 212(2), 167–178.
Tang, Z., Gao, H., Wang, S., Wen, S., & Qin, S. (2013). Hypolipidemic and antioxidant properties of a polysaccharide fraction from Enteromorpha prolifera. International Journal of Biological Macromolecules, 58, 186–189.
Lo, C. S., Shi, Y. X., Chenier, I., Ghosh, A., Wu, C. H., Cailhier, J. F., Ethier, J., Lattouf, J. B., Filep, J. G., Ingelfinger, J. R., Zhang, S. L., & Chan, J. S. D. (2017). Heterogeneous nuclear ribonucleoprotein F stimulates Sirtuin-1 gene expression and attenuates nephropathy progression in diabetic mice. Diabetes, 66(7), 1964–1978.
Sebai, H., Selmi, S., Rtibi, K., Souli, A., Gharbi, N., & Sakly, M. (2013). Lavender (Lavandula stoechas L.) essential oils attenuate hyperglycemia and protect against oxidative stress in alloxan-induced diabetic rats. Lipids in Health and Disease, 12(1), 189.
Bertelli, R., Di Donato, A., Cioni, M., Grassi, F., Ikehata, M., Bonanni, A., Rastaldi, M. P., & Ghiggeri, G. M. (2014). LPS nephropathy in mice is ameliorated by IL-2 independently of regulatory T cells activity. PLoS One, 9(10), e111285.
Peters, T., Bloch, W., Wickenhauser, C., Tawadros, S., Oreshkova, T., Kess, D., Krieg, T., Muller, W., & Scharffetter-Kochanek, K. (2006). Terminal B cell differentiation is skewed by deregulated interleukin-6 secretion in beta2 integrin-deficient mice. Journal of Leukocyte Biology, 80(3), 599–607.
Eilenberg, W., Stojkovic, S., Piechota-Polanczyk, A., Kaider, A., Kozakowski, N., Weninger, W. J., Nanobachvili, J., Wojta, J., Huk, I., Demyanets, S., & Neumayer, C. (2017). Neutrophil gelatinase associated lipocalin (NGAL) is elevated in type 2 diabetics with carotid artery stenosis and reduced under metformin treatment. Cardiovascular Diabetology, 16(1), 98.
Li, S., Zhang, Y., & Zhao, J. (2007). Preparation and suppressive effect of astragalus polysaccharide in glomerulonephritis rats. International Immunopharmacology, 7(1), 23–28.
Zhang, S., Xin, H., Li, Y., Zhang, D., Shi, J., Yang, J., & Chen, X. (2013). Skimmin, a coumarin from Hydrangea paniculata, slows down the progression of membranous glomerulonephritis by anti-inflammatory effects and inhibiting immune complex deposition. Evidence-based complementary and alternative medicine. eCAM, 2013, 819296.
Pan, P., Wang, Y. J., Han, L., Liu, X., Zhao, M., & Yuan, Y. F. (2010). Effects of sodium houttuyfonate on expression of NF-kappaB and MCP-1 in membranous glomerulonephritis. Journal of Ethnopharmacology, 131(1), 203–209.
Manna, P., Das, J., Ghosh, J., & Sil, P. C. (2010). Contribution of type 1 diabetes to rat liver dysfunction and cellular damage via activation of NOS, PARP, IkappaBalpha/NF-kappaB, MAPKs, and mitochondria-dependent pathways: prophylactic role of arjunolic acid. Free Radical Biology & Medicine, 48(11), 1465–1484.
Rashid, S., Nafees, S., Siddiqi, A., Vafa, A., Afzal, S. M., Parveen, R., Ali, N., Hasan, S. K., Barnwal, P., Shahid, A., & Sultana, S. (2017). Partial protection by 18beta glycrrhetinic acid against cisplatin induced oxidative intestinal damage in Wistar rats: possible role of NFkB and caspases. Pharmacological Reports : PR, 69(5), 1007–1013.
Oh, S. W., Lee, Y. M., Kim, S., Chin, H. J., Chae, D. W., & Na, K. Y. (2014). Cobalt chloride attenuates oxidative stress and inflammation through NF-kappaB inhibition in human renal proximal tubular epithelial cells. Journal of Korean Medical Science, 29(Suppl 2), S139–S145.
Methacanon, P., Madla, S., Kirtikara, K., & Prasitsil, M. (2005). Structural elucidation of bioactive fungi-derived polymers. Carbohydrate Polymers, 60(2), 199–203.
Acknowledgements
This work is supported by the Project from Health and Family Planning Commission Project from Jiangsu province (no. H201536) and Scientific Research Program of the Affiliated Hospital of Jiangsu University in China (no. jdfyRC-2015004).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors have declared that there is no conflict of interest.
Rights and permissions
About this article
Cite this article
Kou, L., Du, M., Liu, P. et al. Anti-Diabetic and Anti-Nephritic Activities of Grifola frondosa Mycelium Polysaccharides in Diet-Streptozotocin-Induced Diabetic Rats Via Modulation on Oxidative Stress. Appl Biochem Biotechnol 187, 310–322 (2019). https://doi.org/10.1007/s12010-018-2803-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-018-2803-6