Abstract
The phenylalanine ammonia-lyase (AvPAL) from Anabaena variabilis catalyzes the amination of substituent trans-cinnamic acid (t-CA) to produce racemic d,l-enantiomer arylalanine mixture owing to its low stereoselectivity. To produce high optically pure d-arylalanine, a modified AvPAL with high d-selectivity is expected. Based on the analyses of catalytic mechanism and structure, the Asn347 residue in the active site was proposed to control stereoselectivity. Therefore, Asn347 was mutated to construct mutant AvPAL-N347A, the stereoselectivity of AvPAL-N347A for d-enantiomer arylalanine was 2.3-fold higher than that of wild-type AvPAL (WtPAL). Furthermore, the residual l-enantiomer product in reaction solution could be converted into the d-enantiomer product through stereoselective oxidation by PmLAAD and nonselective reduction by reducing agent NH3BH3. At optimal conditions, the conversion rate of t-CA and optical purity (enantiomeric excess (eeD)) of d-phenylalanine reached 82% and exceeded 99%, respectively. The two enzymes displayed activity toward a broad range of substrate and could be used to efficiently synthesize d-arylalanine with different groups on the phenyl ring. Among these d-arylalanines, the yield of m-nitro-d-phenylalanine was highest and reached 96%, and the eeD exceeded 99%. This one-pot synthesis using AvPAL and PmLAAD has prospects for industrial application.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
d-Arylalanines are useful intermediates in the production of pharmaceuticals, including β-lactam antibiotics, small peptide hormones, and pesticides. The types of pharmaceuticals synthesized from d-arylalanines include analgesics, antistress agents, antidiabetics (e.g., nateglinide), and anticoagulants [1, 2]. With the increasing demand for d-arylalanies, chemical [3], fermentative [4], and enzymatic methods [5], have already been developed to synthesize d-arylalanines. In contrast with the chemical and fermentative methods, which suffer from process complexity, high cost, low yield, and environmental pollution, enzymatic methods are most suitable for industrial manufacture with regard to their optical purity and productivity and offer an efficient, highly specific and environmentally friendly alternative to chemical and fermentation methods. Various enzymatic approaches have been developed to achieve scalable processes for preparing d-arylalanines, including the use of d-amino acid aminotransferase (DAAT) [6, 7], l-amino acid oxidase (LAAO) [8, 9], and hydantoinase [10]. However, the operation process using aminotransferase is complex due to presence of an external cofactor (PLP) regeneration system, and the product consists of α-keto acids and d-arylalanines; the separation of this mixture presents a major limitation of the use of the DAAT-catalyzed synthesis. The LAAO-catalyzed method has been used to produce optical d-arylalanines through the asymmetric resolution of the racemic dl-arylalanines, and the method afforded a 50% theoretical yield. The hydantoinase method is the primary method used for the commercial production of d-amino acids, and the synthetic process followed is shown in Scheme S1 (Electronic Supplementary Material) [11, 12]. In this process, the dl-5-monosubstituted hydantoin as feedstock is hydrolyzed using hydantoinase, and the resulting N-carbamoyl-d-amino acid is subsequently hydrolyzed by carbamoylase to yield the d-arylalanines stereospecifically. However, the carbamoylase reaction is the rate-determining step in the process and suffers from low enzyme activity and instability in application [13]. Moreover, the feedstock, dl-5-monosubstituted hydantoin, which is produced through the enzymatic racemization of l-5-monosubstituted hydantoin, is not accessibly obtained. The racemization rate of the l-5-monosubstituted hydantoin by hydantoin racemase is very low. For industrial production, significant consideration must be given to the availability of feedstock, the optical purity of products, and the enzymatic catalysis steps. From an industrial point of view, the availability of cheap feedstock and the development of enzyme catalysts suitable for feedstock are the most important considerations. Since trans-cinnamic acid (t-CA) is commercially produced at low costs [14], their direct use as feedstock, would lead to a simple and efficient d-arylalanine synthesis process, which would be expected to improve yields and be economically beneficial.
Phenylalanine ammonia lyase (PAL) can catalyze the reversible addition of ammonia to t-CA at high concentration of NH4+ to produce phenylalanine. PAL is a member of the 4-methylene-imidazol-5-one (MIO)-dependent enzyme family, which includes PAL, histidine ammonia-lyase [15] (HAL), tyrosine ammonia-lyase [16] (TAL), and phenylalanine and tyrosine aminomutases [17, 18] (PAM and TAM, respectively). MIO is a highly electrophilic prosthetic group that is formed post-translationally and autocatalytically by condensation of a highly conserved Ala-Ser-Gly motif. The catalytic process was proposed as E1cB mechanism, the MIO electrophilically attacks an NH4+ group of the substrate to form a covalent adduct MIO-NH2, which facilitates the addition of NH4+ group at Cα and protonation of Cβ by a nearby tyrosine base located at 78 sites in the active site of the enzyme [19, 20] (Electronic Supplementary Material, Fig. S2). Since PAL from Anabaena variabilis exhibits higher stability and broader substrate scope than other PALs [21, 22], it can use t-CA or its derivatives as readily available starting materials [23,24,25], does not rely on cofactor recycling system, and achieves a 100% theoretical yield, a synthetic process using AvPAL has excellent prospects in industry. However, the reaction product is a racemic dl-type mixture resulting from the low stereoselectivity of AvPAL, which exhibits nearly equal l-selectivity and d-selectivity. A modified AvPAL with high d-setreoselectivity is expected. Although two excellent mutants H359Y and H359K with high d-selectivity were obtained using high-throughput screening method [26], we aim to find other key amino acids which manipulate the stereoselectivity of AvPAL through structure-and mechanism-based analysis and synthesize highly pure d-arylalanines using one-pot enzymatic synthesis process. The synthesis course was referred to method of Parmeggiani [26], the residual l-enantiomer was converted into the d-enantiomer through stereoselective oxidation by PmLAAD and nonselective reduction by the reducing agent NH3BH3. However, the MIO as an electrophilic group was inactivated by the nucleophile, such as sodium borohydride and NH3BH3, the t-CA or t-CAs with electron-donating group (such as methyl, hydroxyl) in phenyl ring could not be converted to phenylalanine in the presence of NH3BH3. Only t-CAs with electron-withdrawing groups (such as nitro, fluoro) can be converted to corresponding d-phenylalanine derivatives in the presence of NH3BH3 [26]. Until now, it is not clear why the inactivated MIO by borohydride can catalyze the t-CAs with electron-withdrawing groups in phenyl ring. Therefore, in order to expand the application in synthesis of highly pure d-arylalanines, we modified the process of Parmeggiani [26] to catalyze t-CA and its derivatives with electron-donating group in phenyl ring to produce d-arylalanines. In this work, the t-CA and derivatives were first converted to racemic arylalanines using AvPAL, and then, the PmLAAD and NH3BH3 were added into reaction system, the l-products were sequentially converted to d-products, which resolve the deactivation of MIO by NH3BH3. As a result, the conversion ratio of t-CA reached 82% and the optical purity (eeD) of d-phenylalanine exceeded 99%. Furthermore, the one-pot method can be used to catalyze the synthesis of d-arylalanines from trans-cinnamic acid with different groups in the phenyl ring, the process was effective in producing high purity d-arylalanines.
Experimental Methods
Vectors, Strains, Genes, and Materials
Escherichia coli JM109, E. coli BL21, and expression vector pET28a were purchased from Novagen. The phenylalanine ammonia lyase gene from Anabaena variabilis and the l-amino acid deaminase gene from Proteus mirabilis were synthesized commercially by Sangon Biotech (Shanghai, China). trans-Cinnamic acid and its derivatives were obtained from Sigma-Aldrich. All restriction enzymes (BamHI, NotI, NdeI), T4 DNA ligase, and DNA polymerase were purchased from TaKaRa (Japan). The commercial kits for plasmid extraction, gel extraction, and DNA extraction were purchased from Sangon Biotech (Shanghai, China).
Plasmid Construction
The PAL gene (pal) from Anabaena variabilis (NCBI, LF643444.1) and the PmLAAD gene (laad) from Proteus mirabilis (NCBI, EU669819.1) were synthesized commercially and inserted into the pUC19 vector to produce cloned pUC19-pal and pUC19-laad plasmids. The pal construct was designed with flanking restriction sites (BamHI and NotI) for subsequent subcloning and expression in the pET28a vector. The pUC-pal and pET28a vectors were digested simultaneously with BamHI and NotI. Then, the pal gene was ligated into the pET28 vector using T4 DNA ligase at 16 °C for 12 h to produce the expression plasmid pET28a-pal. Because PmLAAD is a membrane-bound protein and to achieve soluble expression in E. coli, the gene sequence of laad from P. mirabilis was modified by removing one N-terminal transmembrane region (from 21st to 87th nucleotide), the plasmids pET28a-laad was constructed by the previous described methods [27, 28]. The two expression plasmids pET28a-pal and pET28a-laad were transformed into E. coli JM109 and subsequently grown on Luria-Bertani (LB) agar plates containing kanamycin (50 μg/mL). The two plasmids were then isolated from the cells and successful subcloning was confirmed by sequencing.
Site-Directed Mutagenesis
The mutants were generated using PCR. The PCR reaction was conducted using the PrimeSTAR HS DNA Polymerase (Takara, Japan) and the pET28a-pal plasmid as the template DNA. The primer sequences (altered nucleotides underlined in parentheses) are 5′-AACTCAGT CACCGATAAC(GCC)CCACTAATT-3′ and 5′-ATCAACATCAATTAGTGG(GGC)GTTATCGG T-3′. After PCR, the template DNA was digested using DpnI (Takara, Japan). The PCR products (pET28a-pal-N347A) were first transformed into E. coli JM109 cells for sequencing and then into E. coli BL21 cells after plasmid preparation for enzyme expression.
Expression and Purification
The three recombinant plasmids pET28a-pal, pET28a-pal-N347A, and pET28a-laad were transformed into E. coli BL21 and grown in Luria-Bertani medium with kanamycin (50 μg/mL). The culture was incubated at 37 °C until the OD600 reached 0.4–0.6. Then, the expression was induced by addition of 0.4 mmol/L IPTG to the cells followed by incubation at 24 °C for 24 h. The cells were collected by centrifugation at 4 °C and resuspended using 50 mmol/L Tris-HCl buffer (containing 10 mmol/L imidazole and 150 mmol/L NaCl at pH 7.5) and disrupted by sonication on ice. The extract was centrifuged at 15,000×g for 10 min, and the supernatant was used for further purification. The soluble supernatant was purified by His-tag purification according to the manufacturer’s protocol. The proteins were loaded onto a HisTrap FF column, and the column was washed using the elution buffer (50 mmol/L Tris-HCl buffer containing 250 mmol/L imidazole, 150 mmol/L NaCl). The resulting eluate was dialyzed overnight against 1000 mL of 50 mmol/L Tris-HCl buffer. The purity of the sample was determined by SDS-PAGE.
Molecular Docking
The crystal structure of AvPAL from Anabaena variabilis (PBD ID: 2NYN) was used as template, and the Asn 347 was replaced by Ala to generate the structure of mutant AvPAL-N347A with the maximum overlap; the RMSD (mean square deviation) was less than 2 Å. Docking of t-CA into the active pockets of AvPAL-N347A and PAL was performed using Autodock4.2. The t-CA as ligand was downloaded from the PubChem database on NCBI. A grid-centered box was set to a binding pocket to accommodate the ligand, and the grid-centered box consisted of conserved amino acids (N223, N347, R317, MIO, Y78, N451, E448, and Q451) to provide enough space for rotational movement of the ligand. The Lamarckian genetic algorithm was used in the program. The selection of docking poses was based on the following criteria: the carboxylate of t-CA should be placed in the corresponding binding pocket; the carboxylate group of t-CA should form salt a bond with the highly conserved Arg325, and the MIO and 78Tyr should point toward Cα and Cβ of the ligand. The results were visualized using PyMol.
The Amination Reaction Using AvPAL-N347A
The pure AvPAL-N347A (10 U) and trans-cinnamic acid (10 mmol/L) were added into Tris-HCl buffer (50 mmol/L, pH 7–11) containing 1–6 mol/L NH4OH, and the reaction was shaken at 200 r/min at 37 °C for 12 h, and then, 0.5 mL of the sample was extracted for analysis by HPLC.
The Oxidation Reaction Using PmLAAD
The pure PmLAAD (10 U) and dl-phenylalanine (10 mmol/L) were added into Tris-HCl buffer (50 mmol/L, pH 7–11), containing 1–6 mol/L NH4OH; the reaction was carried out in a similar manner to the amination condition.
The Effect of NH4 + on Stability of PmLAAD
The PmLAAD (10 U) was incubate in Tris-HCl buffer (50 mM, pH 8.5) containing 1–8 mol/L NH4+ and 10 mM dl-phenylalanine at 30 °C for 12 h; 0.5 mL of the sample was extracted to detect the relative activity using HPLC.
The Effect of Reducing Agent NH3BH3 on the Cascade Conversion Using AvPAL-N347A and PmLAAD
The effect of the reducing agent NH3BH3 on the cascade conversion using AvPAL-N347A and PmLAAD was assayed in Tris-HCl buffer (50 mmol/L, pH 8.5) containing 10 U AvPAL, 10 mmol/L t-CA and 4 mol/L NH4OH. The reaction was incubated for 12 h at 35 °C, the t-CA was aminated to racemic phenylalanine. Then, 10–60 mmol/L NH3BH3 and PmLAAD (10 U) were added to continue the reaction for 12 h. The l-phenylalanine was converted d-phenylalanine through oxidation by PmLAAD and reduction by NH3BH3. The samples were analyzed every 2 h using HPLC.
One-Pot Synthesis of d-Arylalanines
The substituted trans-cinnamic acids (a → n) were used as substrates to synthesize the d-arylalanines in reaction containing 40 mmol/L NH3BH3, 4 mol/L NH4OH, 10 U AvAL-N347A, and PmLAAD. In this process, the AvAL-N347A was first added into the reaction synthesis for 12 h, and then, the NH3BH3 and PmLAAD were added to continue the reaction for 12 h. The one-pot synthetic reaction was carried out at 35 °C, pH 8.5. The sample was extracted to analyze the conversion rate and eeD of d-arylalanines using HPLC.
Activity Assays of AvPAL and PmLAAD
The enzymatic activity of AvPAL was assayed in a reaction mixture (0.5 mL) containing 225 μL of Tris-HCl buffer (25 mM, pH 8.5), 250 μL of m-nitro-t-CA (10 mM), and an appropriate quantity of the enzyme. The reaction was carried out at 35 °C for 1 h and stopped by the addition of 0.5 mL methanol. The formation of m-nitro-Phe was measured by HPLC. One enzyme activity unit was defined as the amount of enzyme that produced 1 mmol m-nitro-Phe per hour at 35 °C. The activity of PmLAAD was performed according to the method described by Baek [29].
HPLC Analysis
The d-enantiomers and l-enantiomers were detected by HPLC on a C18 column (4.6 mm × 75 mm, Hitachi, Japan) at 210 nm according to the method described by Fukuhara [30]. The mobile phase contained 20% (v/v) methanol and a complex of optically active l-Pro-Cu(II) (1.5 mmol/L l-Pro and 0.75 mmol/L CuSO4). The retention times of d-enantiomers and l-enantiomers are shown in Table S1 (Electronic Supplementary Material), respectively. The eeD of d-enantiomers was calculated by the following equation:
where eeD is the enantiomeric excess of d-enantiomers, lphe, is the concentration of l-enantiomers; dphe is the concentration of d-enantiomers.
Results and Discussion
Selection of Mutation Sites Based on Analysis of Structure of AvPAL
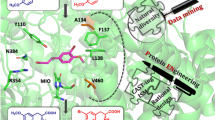
PAL and PAM are belonged to MIO-dependent enzyme family, which acts as similar mechanism. The TcPAMs from Taxus chinensis and PaPAM from Pantoea agglomerans exhibit different stereoselectivity and produce R-β-phenylalanine and S-β-phenylalanine, respectively [31, 32]. The TcPAM catalyzes the isomerization of S-α-phenylalanine to R-β-phenylalanine through exchanging the position of the amine group (Cα → Cβ) and pro-3S hydrogen proton (Cβ → Cα) with retention of the configuration at the reaction termini [33], which requires reorientation after deamination of S-α-phenylalanine to t-CA in which the re-face of the Cβ and the si-face of the Cα carton atoms are positioned for amine re-addition and re-protonation [33, 34]. To achieve the retention of the configuration, the C1–Ca and Cβ–Cipso of t-CA are demonstrated to rotate 180° prior to the rebound of the amino group to Cβ and the hydrogen proton to Cα. In order to produce d-phenylalanine using AvPAL, the C1–Ca and Cβ–Cipso of t-CA in active site might be necessary to rotate. Therefore, the binding orientation of t-CA in active site was resolved using molecular docking. According to the structure and results of docking (Fig. 1), the carboxylate binding pocket in the active site consists of three highly conserved Asn (N233, N347, N451) residues and an Arg residue at position 317, the carboxylate group of t-CA forms a salt bond with highly conserved Arg317, and the three Asn residues are involved in formation of a hydrogen bond network. The hydrogen bond network might control the accommodation of t-CA with different binding orientations. As shown in Fig. 1a, b, the si-face of Ca points toward the MIO-NH2 adduct and the 78Tyr-H proton points toward the re-face of Cβ; this binding mode prefers to produce l-phe. To produce d-phe, the binding orientation needs to be switched to point the re-face of Ca toward the MIO-NH2 adduct and the 78Tyr-H proton toward the si-face of Cβ, as shown in Fig. 1c, d. To achieve the switch in binding modes, the C1–Cα and Cβ–Cipso bond were considered to rotate to expose the re-face to the MIO-NH2 and the si-face to the 78Tyr-H proton, which would be constricted by the key residue N347 in the hydrogen bond network. Therefore, the N347 was mutated to Ala to perturb the hydrogen bond network and facilitate the rotation to improve d-selectivity.
The binding mode in active site between trans-cinnamic acid with active site. The substrate trans-cinnamic acid (t-CA, blue) was docked into WtPAL and mutant AvPAL-N347A, in which two binding orientations were found. a The MIO-NH2 adducts and Tyr78 point to Si-face of Ca and Re-face of Cβ of t-CA; c MIO-NH2 adducts and Tyr78 point to Re-face of Ca and Si-face of Cβ of t-CA. b, d The hydrogen bond network of two binding orientations between residues in active site with t-CA. The hydrogen bond network was drawn using ChemDraw according to the a, c part in this figure. The hydrogen bond and salt bridge were showed as black and yellow dotted lines, respectively. The key residues in active site are colored by atom as follows: C (green), O (red), and N (blue)
Effects of Mutation at Residue 347 Site on Stereoselectivity
The mutant and wild-type genes were expressed in E. coli BL21 (DE3). After purification, the mutant AvPAL-N347A and wild-type AvPAL (WtPAL) proteins appeared as a single band of approximately 50 kDa on SDS-PAGE (Fig. 2), in agreement with the molecular weight calculated from the amino acid sequence. Consequently, the mutant AvPAL-N347A exhibits high stereoselectivity for d-m-nitro-phe. As shown in Fig. 3, the activities of WtPAL for l-m-nitro-phe and d-m-nitro-phe were 5.6 and 3.1 U/mg, respectively. However, the activity of mutant AvPAL-N347A for d-m-nitro-phe was 2.3-fold higher (7.3 U/mg) than WtPAL. Although, the activity for d-selectivity of AvPAL-N347A is lower than that of mutants H359Y (11.35 U/mg) and H359K (10.76 U/mg) [26], the N347 is another key amino acid to manipulate the stereoselectivity. Furthermore, CD was used to investigate the structures of WtPAL and mutant AvPAL-N347A. The results showed that the increased activity for d-m-nitro-phe might result from the perturbance of the hydrogen bond network and improved the rotation of the C1–Cα bond and not change the structure. The CD spectrum of the mutant was similar to that of the wild type (Fig. 4).
SDS-PAGE of recombinant enzyme expressed in E. coli BL21. M, molecular weight marker; a1, the cell extracts of E.coli BL21 (DE3) harboring pET28a-laad; a2, the purified PmLAAD; b1, the cell extracts of E. coli BL21 (DE3) harboring pET28a-pal; b2, the purified AvPAL; b3, the cell extracts of E. coli BL21 (DE3) harboring pET28a-pal-N347A; b4, the purified AvPAL-N347A
The Synthesis Route of d-Arylalanine
Although the activity of AvPAL-N347A for d-m-nitro-phe was increased, its activity for l-m-nitro-phe remained at 2.4 U/mg, and the product is still d,l-mixture. To obtain highly pure d-enantiomer, it was necessary to convert the l-enantiomer to d-enantiomer. Therefore, the synthesis route was referred to previous reports [26, 35], which is shown in Scheme 1. The l-enantiomer product was transformed to the intermediate amino acid through oxidation by PmLAAD [28], and then, the amino acid was reduced to d-enantiomer and l-enantiomer by amine borane (NH3BH3) [35], the d-enantiomer was continually accumulated in reaction. However, in the cascade process of Parmeggiani [26, 35], the PAL, LAAD, and NH3BH3 were simultaneously added into reaction system, the result is that only t-CAs with electron-withdrawing groups (such as nitro, fluoro) can be converted to corresponding d-product in the presence of NH3BH3, and the t-CA or t-CAs with electron-donating group (such as methyl, hydroxyl) in phenyl ring could not be converted in the presence of NH3BH3, which might result from the deactivation of AvPAL by NH3BH3. In this work, the cascade process was modified, which the AvPAL, PmLAAD, and NH3BH3 were successively added into the reaction system to resolve the deactivation of AvPAL by NH3BH3 and convert the t-CA and its derivatives with electron-donating groups (such as methyl and hydroxyl).Thus, the d-enantiomer could be constantly accumulated.
The PmLAAD was prepared through heterologous expression. In order to achieve soluble expression in E. coli, the gene sequence of laad from P. mirabilis was modified by removing one N-terminal transmembrane region, the methods was referred to the references [27, 28].The open reading frame of the PmLAAD gene is 1350 bp long, encoding 450 amino acids. The recombinant expression vector pET-28a-laad was constructed and then transformed into E. coli BL21 (DE3) for PmLAAD expression. SDS-PAGE analysis showed that the recombinant PmLAAD polypeptide was approximately 50 kDa (Fig. 2), which is agreement with the molecular weight calculated from the amino acid sequence. The recombinant PmLAAD was purified by His-tag-purification using an AkTA-purifier; PmLAAD appeared as a single band of approximate 50 kDa on an SDS-PAGE.
The Conditions of AvPAL-N347A-Catalyzed Amination and PmLAAD-Catalyzed Oxidation
The amination and oxidation conditions are different due to the different enzymatic properties of the two enzymes. To obtain optimal reaction conditions, the amination and oxidation were assayed as shown in Fig. 5; the maximal amination rate of trans-cinnamic acid was achieved at conditions of 4–5 mol/L NH4OH, 40–50 °C, pH 9–10, reaching 93% amination. However, the maximal oxidation rate of l-phenylalanine was obtained at conditions of 1–4 mol/L NH4OH, 30–40 °C, and pH 7–8. Therefore, in order to reach a high amination rate and oxidation rate simultaneously, the optimal reaction conditions were determined as 4 mol/L NH4OH, 35 °C, and pH 8.5.
The Effects of Ammonia on Stability of PmLAAD
The PmLAAD (10 U) was incubate in solution containing 1–8 mol/L NH4+ for 12 h. The enzyme was stable at 1–5 mol/L NH4+, no activity decrease was observed under 1–2 mol/L NH4+. The activity maintained about 90% at 5 mol/L NH4+, and about 60% of activity remained after incubation at 8 mol/L NH4+ (Fig. 6). The result was similar to the previous works, which optimal concentration is 5 mol/L NH4+ [26].
The Effect of Reducing Agent NH3BH3 on the Cascade Conversion Using AvPAL-N347A and PmLAAD
The conversion reaction was carried out over 24 h at 35 °C, and pH 8.5, the reaction system consists of 10–60 mmol/L NH3BH3, 4 mol/L NH4OH, and 10 U AvPAL-N347A and PmLAAD. Samples were analyzed to calculate the conversion of trans-cinnamic acid to d-phenylalanine. The results are shown in Fig. 7 and the conversion rate increased with the increasing concentration of NH3BH3 and exceeded 80% at 40 mmol/L NH3BH3. However, if the concentration of NH3BH3 was further increased, the conversion rate was gradually decreased, which might be a result of the inhibitory activity of PmLAAD at high concentrations reduction agent.
One-Pot Preparation of d-Arylalanines Using AvPAL-N347A Coupling PmLAAD
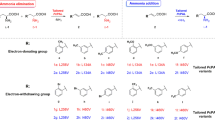
A series of substituted trans-cinnamic acids (a → n, Table 1) were used as substrates to synthesize d-arylalanines at conditions of 40 mmol/L NH3BH3, 4 mol/L NH4OH, and 10 U AvPAL-N347A and PmLAAD. The one-pot synthetic reaction was carried out at 35 °C, pH 8.5, and for 16 h. As shown in Table 1, substrates with fluoro, nitro, and methyl substituent groups are accommodated by AvPAL-N347A and PmLAAD; the conversion rate and eeD were found to be varied based on the electronic properties and position of the substituent group. The conversions tend to follow the following pattern: meta-substituted trans-cinnamic acid exhibits higher total conversion rate than ortho- and para-substituted trans-cinnamic acids. This might be a result of a narrow and long hydrophobic pocket containing highly conservative residues (such as 104Leu, 107Phe, 108Leu, 219Leu, 222Met, and 448E) (Fig. 8), in which it might exist close to the para and ortho position and the width might be insufficient to accommodate the ortho- and para-substituent substrates. The conversion was also found to be dominated by the electronic properties of ring-substituent group. The trans-cinnamic acids with strongly electron-withdrawing groups (such as nitro) at the phenyl ring are converted predominantly to d-arylalanines. This was especially, true of trans-cinnamic acid with the m-nitro group, which was transferred into 96% of d-product, and the eeD exceeded 99%. The results are similar to the previous works, and the highest conversion rate was 80% (eeD of 98%) [26]. In contrast with the process of Parmeggiani [26], the t-CA with strongly electron-donating groups (such as methyl or hydroxyl) could be converted in spite of low conversion rate (Table 1), which might result from low activity of AvPAL for substrate with electron-donating groups. Therefore, the AvPAL is needed to further enhance activity for t-CA with electron-donating group in phenyl ring using molecular modification and expand the application in synthesis of d-arylalanines.
Phenyl ring binding pocket of AvPAL. The binding pocket for the aromatic ring of t-CA is shown in orange and consists of hydrophobic residues (such as 104Leu, 107Phe, 108Leu, 219Leu, 222Met). The salt bridge between 317Arg with carboxylate group of t-CA is shown as black dotted lines. The oxygen, nitrogen, and carbon atoms are shown in red, blue, and gray, respectively
Conclusions
In this paper, we successfully generated a mutant PAL with high stereoselectivity for d-m-nitro-phe through molecular modification based on structure and mechanism. The stereoselectivity of the mutant for d-m-nitro-phe is approximately 2.3-fold higher than that of WtPAL. The AvPAL-N347A from A. variabilis exhibits activity toward a broad substrate scope, especially toward m-F and m-NO2. The PmLAAD from P. mirabilis showed high selectivity for aromatic amino acids. Using the AvPAL-N347A-catalyzed animation and PmLAAD-catalyzed oxidation, one-pot synthetic process for d-arylalanines was successfully constructed using t-CA and its derivatives as substrates. The conversion rate of t-CA reached 82% and eeD exceeded 99%. The t-CA with the electron-withdrawing nitro group at the meta-position proved to be the most suitable substrate, and the conversion rate reached 96% and eeD exceeded 99%. Moreover, the t-CA with strongly electron-donating groups (such as methyl or hydroxyl) could be converted in spite of low conversion rate and the conversion rate of substrate with m-Me reached 23%. In contrast with the hydantoinase method and previous cascade process, this modified one-pot enzyme synthesis using AvPAL-N347A, PmLAAD, and NH3BH3 afforded an alternative approach for synthesizing highly pure d-arylalanines.
References
Genchi, G. (2017). An overview on D-amino acids. Amino Acids, 49(9), 1521–1533.
Parmeggiani, F., Ahmed, S. T., Thompson, M. P., Weise, N. J., Galman, J. L., Gahloth, D., Dunstan, M. S., Leys, D., & Turner, N. J. (2016). Single-biocatalyst synthesis of enantiopure D-arylalanines exploiting an engineered D-amino acid dehydrogenase. Advanced Synthesis and Catalysis, 358(20), 3298–3306.
Fox, M. E., Jackson, M., Meek, G., & Willets, M. (2011). Large-scale synthesis of a substituted D-phenylalanine using asymmetric hydrogenation. Organic Process Research and Development, 15(5), 1163–1171.
Muller, U., van Assema, F., Gunsior, M., Orf, S., Kremer, S., Schipper, D., Wagemans, A., Townsend, C. A., Sonke, T., & Bovenberg, R. (2006). Metabolic engineering of the E. coli L-phenylalanine pathway for the production of D-phenylglycine (D-Phg). Metabolic Engineering, 8(3), 196–208.
Breuer, M., Ditrich, K., Habicher, T., Hauer, B., Kesseler, M., Sturmer, R., & Zelinski, T. (2004). Industrial methods for the production of optically active intermediates. Angewandte Chemie, International Edition, 43(7), 788–824.
Kobayashi, J., Shimizu, Y., Mutaguchi, Y., Doi, K., & Ohshima, T. (2013). Characterization of D-amino acid aminotransferase from Lactobacillus salivarius. Journal of Molecular Catalysis B Enzymatic, 94, 15–22.
Liu, R. X., Liu, S. P., Cheng, S., Zhang, L., Ding, Z. Y., Gu, Z. H., & Shi, G. Y. (2015). Screening, characterization and utilization of D-amino acid aminotransferase to obtain D-phenylalanine. Applied Biochemistry and Microbiology, 51(6), 695–703.
Hossain, G. S., Li, J., Shin, H., Du, G., Liu, L., & Chen, J. (2014). L-Amino acid oxidases from microbial sources: types, properties, functions, and applications. Applied Microbiology and Biotechnology, 98(4), 1507–1515.
Singh, S., Gogoi, B. K., & Bezbaruah, R. L. (2011). Racemic resolution of some DL-amino acids using Aspergillus fumigatus L-amino acid oxidase. Current Microbiology, 63(1), 94–99.
Park, J. H., Kim, G. J., & Kim, H. S. (2000). Production of D-amino acid using whole cells of recombinant Escherichia coli with separately and coexpressed D-hydantoinase and N-carbamoylase. Biotechnology Progress, 16(4), 564–570.
Altenbuchner, J., Siemann-Herzberg, M., & Syldatk, C. (2001). Hydantoinases and related enzymes as biocatalysts for the synthesis of unnatural chiral amino acids. Current Opinion in Biotechnology, 12(6), 559–563.
Gao, X. Z., Ma, Q. Y., & Zhu, H. L. (2015). Distribution, industrial applications, and enzymatic synthesis of D-amino acids. Applied Microbiology and Biotechnology, 99(8), 3341–3349.
Foster, I. M., Dorrington, R. D., & Burton, S. G. (2003). Enhanced hydantoinase and N-carbamoylase activity on immobilisation of Agrobacterium tumefaciens. Biotechnology Letters, 25(1), 67–72.
Parmeggiani, F., Ahmed, S. T., Weise, N. J., & Turner, N. J. (2016). Telescopic one-pot condensation-hydroamination strategy for the synthesis of optically pure L-phenylalanines from benzaldehydes. Tetrahedron, 72(46), 7256–7262.
Sanchez-Murcia, P. A., Bueren-Calabuig, J. A., Camacho-Artacho, M., Cortes-Cabrera, A., & Gago, F. (2016). Stepwise simulation of 3,5-dihydro-5-methylidene-4H-imidazol-4-one (MIO) biogenesis in histidine ammonia-lyase. Biochemistry, 55(41), 5854–5864.
Watts, K. T., Mijts, B. N., Lee, P. C., Manning, A. J., & Schmidt-Dannert, C. (2006). Discovery of a substrate selectivity switch in tyrosine ammonia-lyase, a member of the aromatic amino acid lyase family. Chemistry & Biology, 13(12), 1317–1326.
Heberling, M. M., Masman, M. F., Bartsch, S., Wybenga, G. G., Dijkstra, B. W., Marrink, S. J., & Janssen, D. B. (2015). Ironing out their differences: dissecting the structural determinants of a phenylalanine aminomutase and ammonia lyase. ACS Chemical Biology, 10(4), 989–997.
Walter, T., King, Z., & Walker, K. D. (2016). A tyrosine aminomutase from rice (Oryza sativa) isomerizes (S)-alpha- to (R)-beta-tyrosine with unique high enantioselectivity and retention of configuration. Biochemistry, 55(1), 1–4.
Lovelock, S. L., Lloyd, R. C., & Turner, N. J. (2014). Phenylalanine ammonia lyase catalyzed synthesis of amino acids by an MIO-cofactor independent pathway. Angewandte Chemie, International Edition, 53(18), 4652–4656.
Pinto, G. P., Ribeiro, A. J. M., Ramos, M. J., Fernandes, P. A., Toscano, M., & Russo, N. (2015). New insights in the catalytic mechanism of tyrosine ammonia-lyase given by QM/MM and QM cluster models. Archives of Biochemistry and Biophysics, 582, 107–115.
Bencze, L. C., Filip, A., Banoczi, G., Tosa, M. I., Irimie, F. D., Gellert, A., Poppe, L., & Paizs, C. (2017). Expanding the substrate scope of phenylalanine ammonia-lyase from Petroselinum crispum towards styrylalanines. Organic & Biomolecular Chemistry, 15(17), 3717–3727.
Jaliani, H. Z., Farajnia, S., Mohammadi, S. A., Barzegar, A., & Talebi, S. (2013). Engineering and kinetic stabilization of the therapeutic enzyme Anabeana variabilis phenylalanine ammonia lyase. Applied Biochemistry and Biotechnology, 171(7), 1805–1818.
Lovelock, S. L., & Turner, N. J. (2014). Bacterial Anabaena variabilis phenylalanine ammonia lyase: A biocatalyst with broad substrate specificity. Bioorganic and Medicinal Chemistry, 22(20), 5555–5557.
DreSsen, A., Hilberath, T., Mackfeld, U., Billmeier, A., Rudat, J., & Pohl, M. (2017). Phenylalanine ammonia lyase from Arabidopsis thaliana (AtPAL2): a potent MIO-enzyme for the synthesis of non-canonical aromatic alpha-amino acids. Part I: Comparative characterization to the enzymes from Petroselinum crispum (PcPAL1) and Rhodosporidium toruloides (RtPAL). Journal of Biotechnology, 258, 148–157.
DreSsen, A., Hilberath, T., Mackfeld, U., Billmeier, A., Rudat, J., & Pohl, M. (2017). Phenylalanine ammonia lyase from Arabidopsis thaliana (AtPAL2): a potent MIO-enzyme for the synthesis of non-canonical aromatic alpha-amino acids. Part II: Application in different reactor concepts for the production of (S)-2-chloro-phenylalanine. Journal of Biotechnology, 258, 158–166.
Parmeggiani, F., Lovelock, S. L., Weise, N. J., Ahmed, S. T., & Turner, N. J. (2015). Synthesis of D- and L-phenylalanine derivatives by phenylalanine ammonia lyases: a multienzymatic cascade process. Angewandte Chemie, International Edition, 54(15), 4608–4611.
Liu, L., Hossain, G. S., Shin, H. D., Li, J. H., Du, G. C., & Chen, J. (2013). One-step production of alpha-ketoglutaric acid from glutamic acid with an engineered L-amino acid deaminase from Proteus mirabilis. Journal of Biotechnology, 164(1), 97–104.
Motta, P., Molla, G., Pollegioni, L., & Nardini, M. (2016). Structure-function relationships in L-amino acid deaminase, a flavor protein belonging to a novel class of biotechnologically relevant enzymes. The Journal of Biological Chemistry, 291(20), 10457–10475.
Baek, J. O., Seo, J. W., Kwon, O., Seong, S. I., Kim, I. H., & Kim, C. H. (2011). Expression and characterization of a second L-amino acid deaminase isolated from Proteus mirabilis in Escherichia coli. Journal of Basic Microbiology, 51(2), 129–135.
Fukuhara, T. Y. S. (1990). Novel ligand-exchange chromatographic resolution of DL-amino acids using nucleotides and coenzymes. Journal of Chromatographic Science, 28(1), 114–117.
Wybenga, G. G., Szymanski, W., Wu, B., Feringa, B. L., Janssen, D. B., & Dijkstra, B. W. (2014). Structural investigations into the stereochemistry and activity of a phenylalanine-2,3-aminomutase from Taxus chinensis. Biochemistry, 53(19), 3187–3198.
Ratnayake, N. D., Wanninayake, U., Geiger, J. H., & Walker, K. D. (2011). Stereochemistry and mechanism of a microbial phenylalanine aminomutase. Journal of the American Chemical Society, 133(22), 8531–8533.
Mutatu, W., Klettke, K. L., Foster, C., & Walker, K. D. (2007). Unusual mechanism for an aminomutase rearrangement: retention of configuration at the migration termini. Biochemistry, 46(34), 9785–9794.
Feng, L., Wanninayake, U., Strom, S., Geiger, J., & Walker, K. D. (2011). Mechanistic, mutational, and structural evaluation of a Taxus phenylalanine aminomutase. Biochemistry, 50(14), 2919–2930.
Alexandre, F. R., Pantaleone, D. P., Taylor, P. P., Fotheringham, I. G., Ager, D. J., & Turner, N. J. (2002). Amine-boranes: effective reducing agents for the deracemisation of DL-amino acids using L-amino acid oxidase from Proteus myxofaciens. Tetrahedron Letters, 43(4), 707–710.
Acknowledgments
The authors gratefully acknowledged Prof. Zheming Zhou from the School of Biotechnology, Jiangnan University for the facilities and infrastructure. The authors also acknowledged the help rendered by Weifeng Sun from Xihua University in proofreading the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (21506172; 31671797), Natural Sciences Foundation supported by Anhui Province universities (KJ2016A801) and Anhui Polytechnic University Youth Talent Support Program (2016BJRC006).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Electronic Supplementary Material
ESM 1
(DOC 48 kb)
Rights and permissions
About this article
Cite this article
Zhu, L., Feng, G., Ge, F. et al. One-Pot Enzymatic Synthesis of d-Arylalanines Using Phenylalanine Ammonia Lyase and l-Amino Acid Deaminase. Appl Biochem Biotechnol 187, 75–89 (2019). https://doi.org/10.1007/s12010-018-2794-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-018-2794-3